Pollution Characteristics and Risk Assessment of Heavy Metals in the Sediments of the Inflow Rivers of Dianchi Lake, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description and Sampling
2.2. Laboratory Analyses
2.2.1. Water Quality Index Analysis
2.2.2. Eutrophication Index Calculation
2.2.3. Digestion of Sediments
2.2.4. Sequential Extraction Process of the Sediment
2.2.5. Element Determination
2.3. Method for Pollution Risk Evaluation
2.3.1. Contamination Factor
2.3.2. Pollution Load Index
2.3.3. Potential Ecological Risk Index
2.4. Method for Source Identification
3. Results and Discussion
3.1. Water Quality and Chemical Properties
3.2. Heavy Metal Distribution and Chemical Properties
3.2.1. Content of the Total Heavy Metals
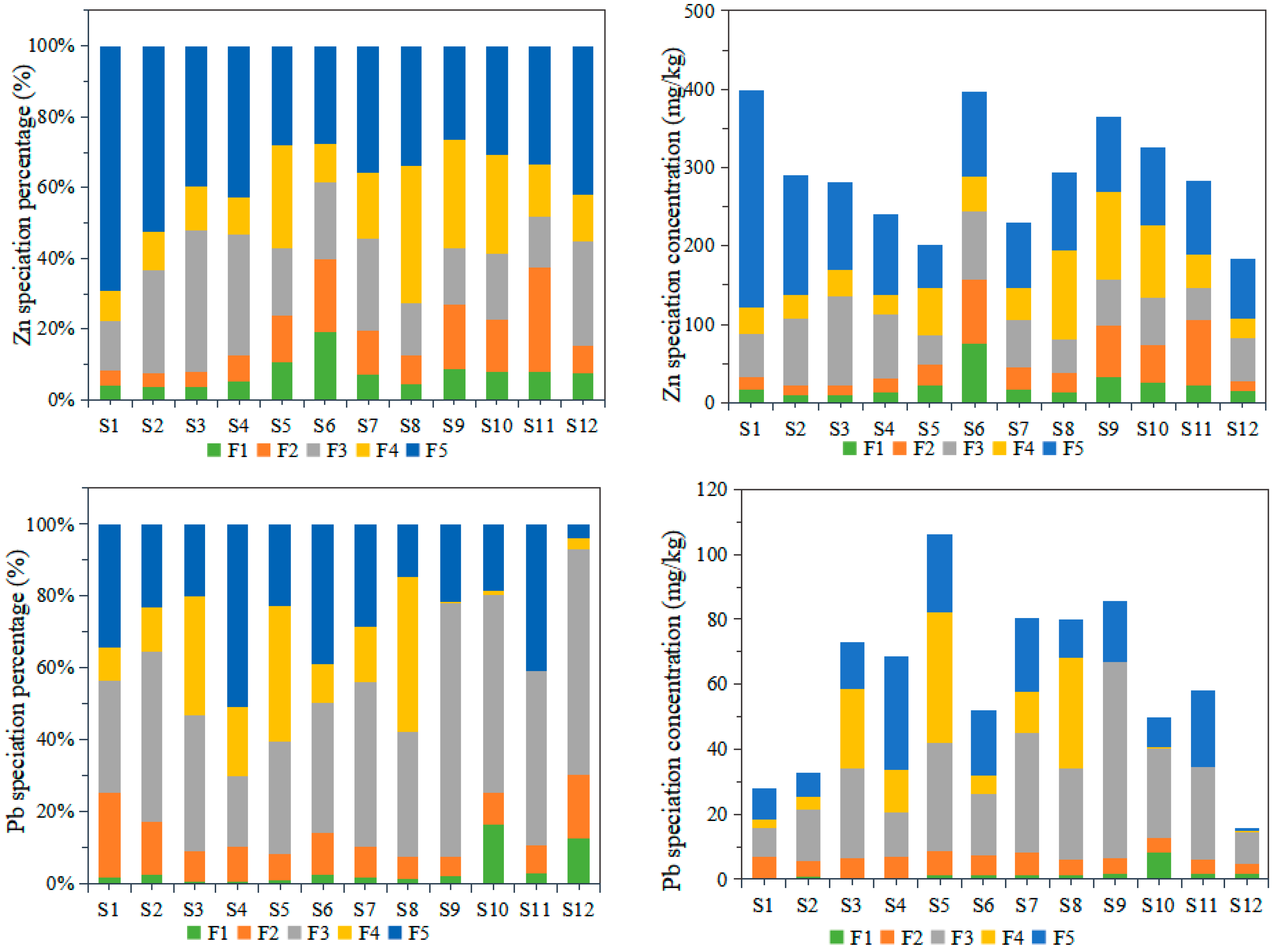
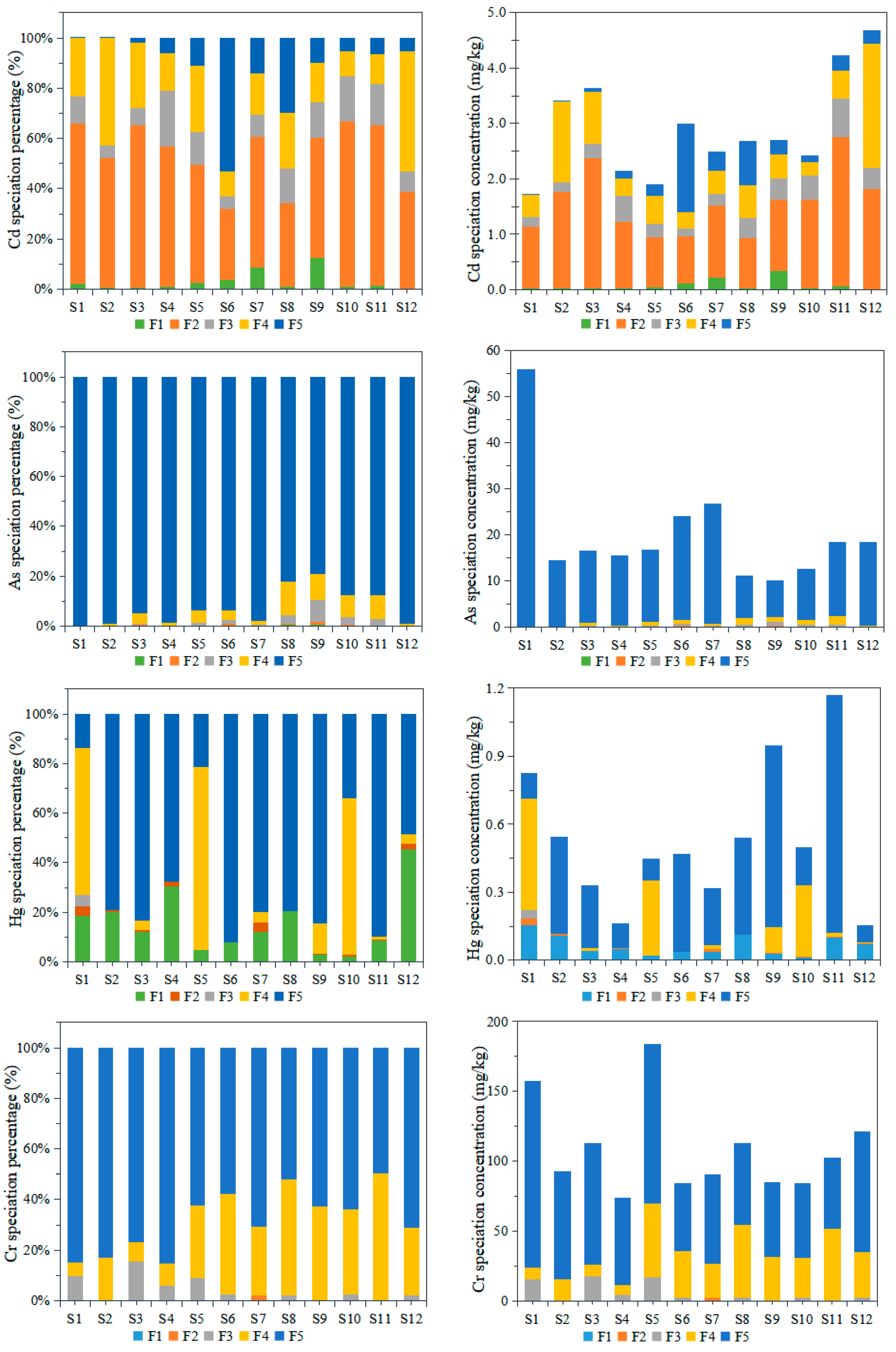
3.2.2. Chemical Speciation of the Heavy Metals
3.3. Pollution Assessment of the Heavy Metals
3.3.1. Contamination Factor and Pollution Load Index
3.3.2. Potential Ecological Risk Index
3.3.3. Comparison of the Results from Different Methods
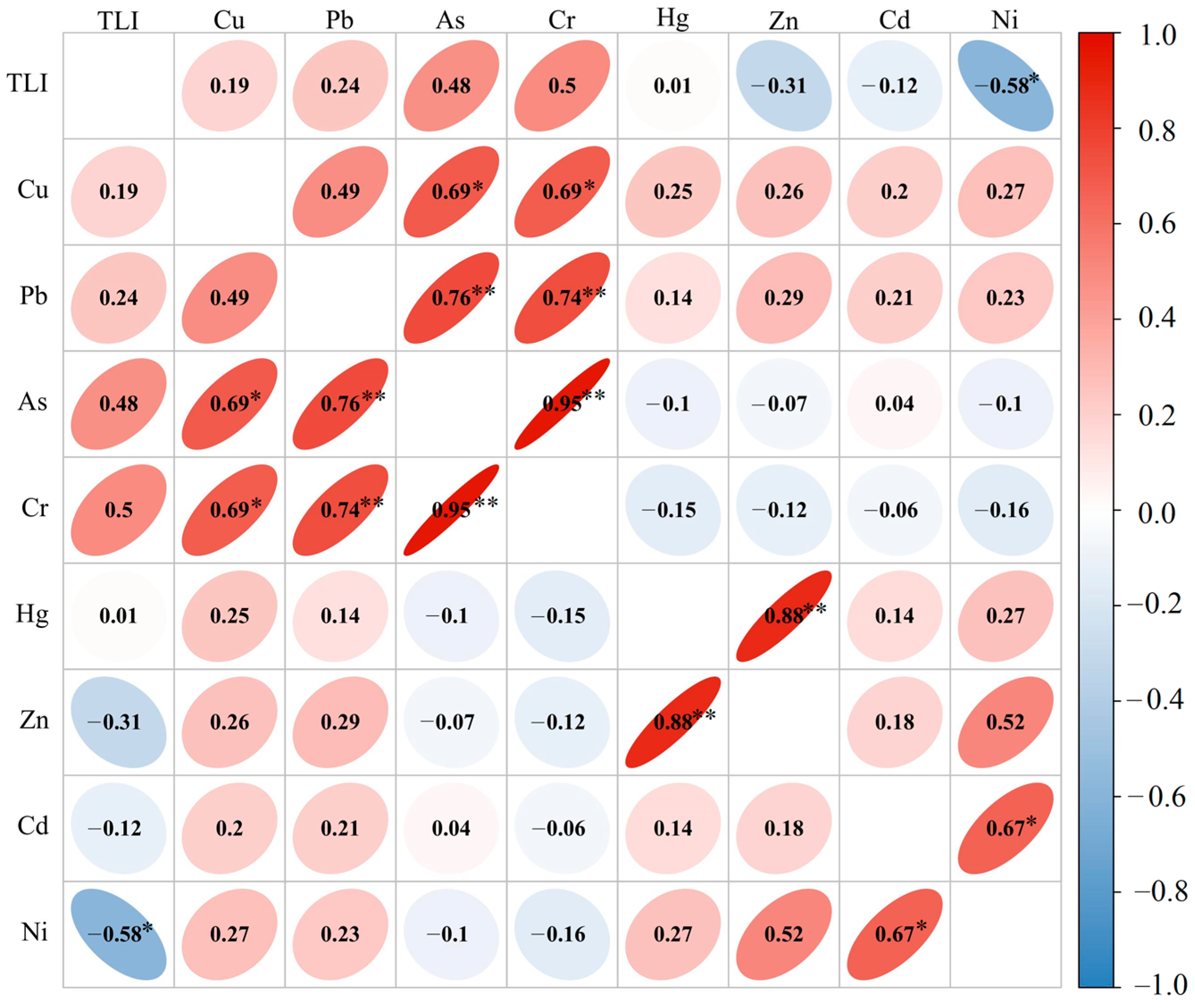
3.4. Source Identification of the Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, X.; Liang, J.; Shi, H.; Yan, T.; He, Z.; Li, L.; Hu, H. Health risk assessment of heavy metals based on source analysis and Monte Carlo in the downstream basin of the Zishui. Environ. Res. 2024, 245, 117975. [Google Scholar] [CrossRef] [PubMed]
- Le, T.V.; Nguyen, B.T. Heavy metal pollution in surface water bodies in provincial Khanh Hoa, Vietnam: Pollution and human health risk assessment, source quantification, and implications for sustainable management and development. Environ. Pollut. 2024, 343, 123216. [Google Scholar] [CrossRef] [PubMed]
- Khaleeq, A.; Ahmed, M.; Huma, R.; Mujtaba, A.; Noor, S.; Rehman, R.; Sheikh, T.; Qamar, S.; Iqbal, D.N.; Alharthy, R.D. Evaluation of trace and heavy metals in different varieties of sauces to characterize their impact on human health. J. Food Compos. Anal. 2022, 114, 104789. [Google Scholar] [CrossRef]
- Demirak, A.; Yilmaz, F.; Levent Tuna, A.; Ozdemir, N. Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 2006, 63, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Simionov, I.A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.L.; Faggio, C.; Petrea, S.M. Human health risk assessment of potentially toxic elements and microplastics accumulation in products from the Danube River Basin fish market. Environ. Toxicol. Pharmacol. 2023, 104, 104307. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Xia, W.; Qu, X.; Yuan, H.; Xie, S.; Lin, L. Eutrophication and heavy metal pollution patterns in the water suppling lakes of China’s south-to-north water diversion project. Sci. Total Environ. 2020, 711, 134543. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yu, H.; Liu, Q.; Lan, W.; Ye, Q.; Niu, Y.; Niu, Y. Effect of river-lake connectivity on heavy metal diffusion and source identification of heavy metals in the middle and lower reaches of the Yangtze River. J. Hazard. Mater. 2021, 416, 125818. [Google Scholar] [CrossRef]
- Oguma, A.Y.; Klerks, P.L. Pollution-induced community tolerance in benthic macroinvertebrates of a mildly lead-contaminated lake. Environ. Sci. Pollut. Res. 2017, 24, 19076–19085. [Google Scholar] [CrossRef]
- Simionov, I.A.; Cristea, D.S.; Petrea, S.M.; Mogodan, A.; Jijie, R.; Ciornea, E.; Nicoară, M.; TurekRahoveanu, M.M.; Cristea, V. Predictive Innovative Methods for Aquatic Heavy Metals Pollution Based on Bioindicators in Support of Blue Economy in the Danube River Basin. Sustainability 2021, 13, 8936. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lakewater bodies—From 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar]
- Wang, Y.; Yu, H.; Yi, M.; Zhou, R.; Li, H.; Xu, S.; Tang, J.; Wang, C. Spatial distribution, sources, and risks of heavy metals in soil from industrial areas of Hangzhou, eastern China. Environ. Earth Sci. 2023, 82, 95. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Karri, R.R.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Nizamuddin, S. Magnetic nanoparticles incorporation into different substrates for dyes and heavy metals removal—A review. Environ. Sci. Pollut. Res. 2020, 27, 43526–43541. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Huang, C.; Chen, F.; Chen, C.; Zhang, H.; Deng, W.; Ye, F. Anthropogenic influences on the sources and distribution of organic carbon, black carbon, and heavy metals in Daya Bay’s surface sediments. Mar. Pollut. Bull. 2023, 196, 115571. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Yang, X.; Dai, X.; Zhang, Q.; Malik, A.; Sadeghpour, A. Heavy metal pollution risk assessments and their transportation in sediment and overlay water for the typical Chinese reservoirs. Ecol. Indic. 2020, 112, 106166. [Google Scholar] [CrossRef]
- Ding, T.; Du, S.; Zhang, Y.; Wang, H.; Zhang, Y.; Cao, Y.; Zhang, J.; He, L. Hardness-dependent water quality criteria for cadmium and an ecological risk assessment of the Shaying River Basin, China. Ecotoxicol. Environ. Saf. 2020, 198, 110666. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, N.; Bing, Y.; Chen, S.; Zhang, Z.; Chang, S.; Chen, Y.; Xie, S. Denitrifier communities impacted by heavy metal contamination in freshwater sediment. Environ. Pollut. 2018, 242, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Debnath, A.; Singh, P.K.; Sharma, Y.C. Spatial distribution of heavy metals in the sediments of River Ganges, India: Occurrence, contamination, source identification, seasonal variations, mapping, and ecological risk evaluation. Mar. Pollut. Bull. 2024, 198, 115910. [Google Scholar] [CrossRef]
- Jiang, Q.; He, J.; Ye, G.; Christakos, G. Heavy metal contamination assessment of surface sediments of the East Zhejiang coastal area during 2012–2015. Ecotoxicol. Environ. Saf. 2018, 163, 444–455. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Yang, X.; Yang, H. Spatial distribution and ecological risk assessment of heavy metal pollution in surface sediments from shallow lakes in East China. J. Geochem. Explor. 2020, 213, 106490. [Google Scholar] [CrossRef]
- Barbosa, A.; Gutierrez-Galindo, E.A.; Daessle, L.W.; Orozco-Borbon, M.V.; Segovia-Zavala, J.A. Relationship between metal enrichments and a biological adverse effects index in sediments from Todos Santos Bay, northwest coast of Baja California, México. Mar. Pollut. Bull. 2012, 64, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Gao, B.; Hao, H.; Zhou, H.; Lu, J.; Sun, K. Lead contamination in sediments in the past 20 years: A challenge for China. Sci. Total Environ. 2018, 640, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Förstner, U. Lake sediments as indicators of heavy-metal pollution. Naturwissenschaften 1976, 63, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, S. Heavy metal enrichments in the Changjiang (Yangtze River) catchment and on the inner shelf of the East China Sea over the last 150years. Sci. Total Environ. 2016, 543, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pu, X.; Pan, J.; Wang, B. Heavy metal pollution status in surface sediments of Swan Lake lagoon and Rongcheng Bay in the northern Yellow Sea. Chemosphere 2013, 93, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, L.; Zhang, F.; Ding, Y.; Gao, J.; Chen, J.; Yan, H.; Shao, W. Heavy metal pollution and assessment in the tidal flat sediments of Haizhou Bay, China. Mar. Pollut. Bull. 2013, 74, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Gui, S.; Huang, H.; Zhang, H.; Wang, C.; Guo, W. Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe River protected area, China. Chemosphere 2017, 175, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Shen, Z.; Niu, J.; Tang, Z. Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J. Hazard. Mater. 2009, 166, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Fu, T.; Hu, B.; Shi, Z.; Zhou, L.; Zhu, Y. Identification of the potential risk areas for soil heavy metal pollution based on the source-sink theory. J. Hazard. Mater. 2020, 393, 122424. [Google Scholar] [CrossRef]
- Liao, J.; Ru, X.; Xie, B.; Zhang, W.; Wu, H.; Wu, C.; Wei, C. Multi-phase distribution and comprehensive ecological risk assessment of heavy metal pollutants in a river affected by acid mine drainage. Ecotoxicol. Environ. Saf. 2017, 141, 75–84. [Google Scholar] [CrossRef]
- Shen, J.; Liu, E.; Zhu, Y.; Hu, S.; Qu, W. Distribution and chemical fractionation of heavy metals in recent sediments from Lake Taihu, China. Hydrobiologia 2007, 581, 141–150. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Wang, R. The research of biodiversity for the species of phytoplankton in Dianchi Lake, Kunming, China. J. Yunnan Univ. 2005, 27, 170–175. [Google Scholar]
- Wan, X.; Pan, X.; Wang, B.; Zhao, S.; Hu, P.; Li, F.; Boulanger, B. Distributions, historical trends, and source investigation of polychlorinated biphenyls in Dianchi Lake, China. Chemosphere 2011, 85, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, T.; Zhang, Y.; Yu, T. Spatial distribution and risk assessment of heavy metals in sediments from a hypertrophic plateau lake Dianchi, China. Environ. Monit. Assess. 2014, 186, 1219–1234. [Google Scholar]
- Chen, M.; Liu, S.; Wu, W.; Yang, F.; Chen, J.; Pan, B. Adsorption and release of phosphate in the case of Dianchi sediments. J. Chem. Eng. Jpn. 2010, 43, 913–920. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Zhong, Z.; Chen, J.; Peng, B. Sediment accumulation of Dianchi Lake determined by Cs-137 dating. J. Geog. Sci. 2009, 19, 225–238. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, S.; Jiao, L.; Liu, W.; Tong, Z. Characteristics of Nitrogen Pollution and the Potential Mineralization in Surface Sediments of Dianchi Lake. Environ. Sci. 2015, 36, 471–480. (In Chinese) [Google Scholar]
- GB 11892-89; Water Quality—Determination of Permanganate Index. Environmental Protection Department (EPD): China, 1989. Available online: https://www.chinesestandard.net/China/Chinese.aspx/GBT11892-1989 (accessed on 24 April 2024).
- HJ 897-2017; Water Quality—Determination of Chlorophyll A—Spectrophotometric Method. Environmental Protection Department (EPD): China, 2017. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/method_standard2/201801/t20180105_429208.shtml (accessed on 24 April 2024).
- Rao, K.; Tang, T.; Zhang, X.; Wang, M.; Liu, J.; Wu, B.; Wang, P.; Ma, Y. Spatial-temporal dynamics, ecological risk assessment, source identification and interactions with internal nutrients release of heavy metals in surface sediments from a large Chinese shallow lake. Chemosphere 2021, 282, 131041. [Google Scholar] [CrossRef] [PubMed]
- Sut-Lohmann, M.; Ramezany, S.; Kästner, F.; Raab, T.; Heinrich, M.; Grimm, M. Using modified Tessier sequential extraction to specify potentially toxic metals at a former sewage farm. J. Environ. Manag. 2022, 304, 114229. [Google Scholar] [CrossRef]
- HJ 780-2015; Soil and Sediment-Determination of Inorganic Element—Wavelength Dispersive X-ray Fluorescence Spectrometry. Environmental Protection Department (EPD), 2015.
- GB/T 17141-1997; Soil Quality—Determination of Lead, Cadmium—Graphite Furnace Atomic Absorption Spectrophoto-Metry. Environmental Protection Department (EPD), 1997. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/199805/t19980501_82029.shtml (accessed on 24 April 2024).
- HJ 680-2013; Soil and Sedimen—Determination of Mercury, Arsenic, Selenium, Bismuth, Antimony—Microwave Dissolution/Atomic Fluorescence Spectrometry. Environmental Protection Department (EPD), 2013. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201312/t20131203_264304.shtml (accessed on 24 April 2024).
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standards for Surface Water. Environmental Protection Department (EPD), 2002. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/quality_standard/200710/t20071024_111792.shtml (accessed on 24 April 2024).
- Zhang, M.; He, P.; Qiao, G.; Huang, J.; Yuan, X.; Li, Q. Heavy metal contamination assessment of surface sediments of the subei shoal, china: Spatial distribution, source apportionment and ecological risk. Chemosphere 2019, 223, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, Y.; Zhang, Z.; Dai, J. Factorialkriging and stepwise regression approach to identify environmental factorsinfluencing spatial multi-scale variability of heavy metals in soils. J. Hazard. Mater. 2013, 261, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, M.; Zhao, C.; Yang, K.; Li, K.; Peng, M.; Yang, Z.; Liu, F.; Liu, Y.; Bai, R.; et al. Concentrations of toxic metals and ecological risk assessment for sediments of major freshwater lakes in China. J. Geochem. Explor. 2015, 157, 15–26. [Google Scholar] [CrossRef]
- Baran, A.; Tarnawski, M. Assessment of heavy metals mobility and toxicity in contaminated sediments by sequential extraction and a battery of bioassays. Ecotoxicology 2015, 24, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Rinklebe, J.; Shaheen, S.M. Assessing the mobilization of cadmium, lead, and nickel using a seven-step sequential extraction technique in contaminated floodplain soil profiles along the Central Elbe River, Germany. Water Air Soil Pollut. 2014, 225, 1–20. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Al-Mamun, M.H.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indicat. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Antoniadis, V.; Zanni, A.A.; Levizou, E.; Shaheen, S.M.; Anthoula, D.; Bolan, N.; Rinklebe, J. Modulation of hexavalent chromium toxicity on Origanum vulgare in an acidic soil amended with peat, lime, and zeolite. Chemosphere 2018, 195, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.B. Issues and recommendations for exploratory factor analysis and principal component analysis. Res. Soc. Adm. Pharm. 2021, 17, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Sprajc, P.; Bjegovi’c, M.; Vasi´c, B. Energy security in decision making and governance—Methodological analysis of energy trilemma index. Renew. Sustain. Energy Rev. 2019, 114, 109341. [Google Scholar] [CrossRef]
- Zhang, L.; Wong, M.H. Environmental mercury contamination in China: Sources and impacts. Environ. Int. 2007, 33, 108–121. [Google Scholar] [CrossRef] [PubMed]

| Item | Chl-a | CODMn | TN | TP | As | TLI∑ |
|---|---|---|---|---|---|---|
| μg/L | mg/L | mg/L | mg/L | μg/L | ||
| Mean | 32.6 | 4.41 | 3.23 | 0.136 | 1.28 | 43.3 |
| Median | 11.0 | 3.70 | 2.11 | 0.135 | 1.10 | 44.3 |
| CV% | 118% | 39.6% | 73.1% | 49.1% | 77.5% | 21.2% |
| Std. | 38.4 | 1.75 | 2.36 | 0.067 | 0.994 | 9.17 |
| Min | -- | 2.90 | 1.02 | 0.030 | -- | 25.1 |
| Max | 128 | 9.70 | 7.58 | 0.290 | 5.00 | 58.6 |
| Skewness | 1.228 | 1.596 | 0.832 | 0.531 | 2.342 | −0.448 |
| Kurtosis | 0.557 | 2.69 | −0.907 | 0.104 | 7.992 | −0.676 |
| Item | Unit | Average | Variance | MS | F | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | |||||
| Chl-a | μg/L | 32.7 | 32.5 | 1539 | 1540 | 0 | 0.00 | 0.9918 |
| CODMn * | mg/L | 5.50 | 3.33 | 3.70 | 0.11 | 28.38 | 14.92 | 0.0008 |
| TN * | mg/L | 5.11 | 1.38 | 3.99 | 0.029 | 72.84 | 36.23 | 6.94 × 10−6 |
| TP | mg/L | 0.13 | 0.14 | 0.00 | 0.00 | 0.00 | 0.20 | 0.6574 |
| As * | μg/L | 1.82 | 0.75 | 1.20 | 0.25 | 6.83 | 9.44 | 0.0056 |
| TLI∑ | 46.7 | 39.95 | 76.41 | 74.40 | 273.92 | 3.63 | 0.0698 | |
| Name | Hg | Pb | As | Cd | Cu | Zn | Ni | Cr |
|---|---|---|---|---|---|---|---|---|
| Mean | 0.71 | 63.07 | 21.94 | 1.41 | 104 | 307 | 6.06 | 114 |
| Median | 0.29 | 62.95 | 17.80 | 0.20 | 99.50 | 212 | 0 | 108 |
| CV% | 113% | 43.1% | 83.70% | 200% | 49.90% | 68.30% | 244% | 33.90% |
| Std. | 0.81 | 27.20 | 18.35 | 2.82 | 51.89 | 210 | 14.81 | 38.51 |
| Min | 0.06 | 25.80 | 6.70 | 0 | 25.80 | 61.70 | 0 | 61.00 |
| Max | 3.29 | 122 | 96.60 | 11.10 | 227 | 689 | 48.90 | 220 |
| Skewness | 1.77 | 0.22 | 3.16 | 2.85 | 0.50 | 0.69 | 2.37 | 1.10 |
| Kurtosis | 3.30 | −0.94 | 12.21 | 7.76 | −0.32 | −1.22 | 4.18 | 1.29 |
| Background value | 0.06 | 34.20 | 5.50 | 0.35 | 62.00 | 95.00 | 42.40 | 93.00 |
| Element | Average (mg/kg) | Variance | MS | F | p-Value | ||
|---|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | ||||
| As | 27.40 | 16.48 | 523.98 | 115.29 | 716 | 2.2405 | 0.1487 |
| Hg | 0.72 | 0.70 | 0.48 | 0.88 | 0.0013 | 0.0020 | 0.9650 |
| Cu | 101.78 | 106.20 | 1776 | 3845 | 117 | 0.0416 | 0.8402 |
| Pb | 68.63 | 57.51 | 491.56 | 985.75 | 741 | 1.0038 | 0.3273 |
| Zn | 326.33 | 288.48 | 40,036 | 51,428 | 8600 | 0.1880 | 0.6688 |
| Cd | 1.03 | 1.78 | 6.96 | 9.33 | 3.3750 | 0.4144 | 0.5264 |
| Cr | 109.27 | 109.05 | 635 | 2160 | 0.2841 | 0.0002 | 0.9888 |
| Ni | 10.95 | 1.17 | 396.29 | 10.12 | 574 | 2.8271 | 0.1068 |
| Site | CFi | PLI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Hg | Cu | Pb | Zn | Cd | Cr | Ni | ||
| S1 | 9.95 | 3.22 | 2.10 | 1.85 | 2.09 | 0.59 | 1.76 | 0.00 | 2.31 |
| S2 | 4.16 | 3.03 | 1.06 | 0.95 | 1.73 | 6.33 | 1.04 | 0.00 | 2.28 |
| S3 | 1.88 | 2.38 | 2.81 | 0.88 | 1.90 | 0.07 | 1.33 | 0.00 | 1.07 |
| S4 | 2.66 | 2.29 | 0.77 | 1.62 | 2.02 | 2.73 | 0.80 | 0.00 | 1.87 |
| S5 | 6.26 | 3.41 | 1.05 | 2.75 | 3.66 | 0.14 | 2.11 | 0.00 | 1.78 |
| S6 | 2.40 | 1.97 | 0.53 | 2.26 | 3.85 | 0.97 | 0.94 | 0.00 | 1.66 |
| S7 | 2.45 | 11.60 | 1.24 | 1.33 | 5.95 | 0.36 | 1.00 | 0.00 | 2.15 |
| S8 | 4.61 | 37.27 | 2.35 | 2.45 | 9.69 | 4.00 | 1.30 | 0.00 | 5.81 |
| S9 | 1.98 | 19.18 | 1.35 | 1.17 | 5.71 | 0.00 | 0.94 | 0.00 | 3.21 |
| S10 | 2.76 | 19.45 | 2.27 | 1.95 | 9.92 | 2.61 | 0.97 | 0.23 | 4.29 |
| S11 | 3.24 | 14.65 | 1.97 | 2.25 | 6.31 | 1.50 | 1.16 | 0.22 | 3.55 |
| S12 | 5.50 | 14.70 | 2.63 | 2.66 | 6.66 | 28.96 | 1.33 | 0.32 | 6.91 |
| Average | 3.99 | 11.10 | 1.68 | 1.84 | 4.96 | 4.02 | 1.22 | 0.06 | 3.07 |
| Site | PERI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hg | Pb | As | Cd | Zn | Ni | Cu | Cr | ||
| S1 | 128.8 | 9.3 | 99.5 | 17.6 | 2.1 | 0.0 | 10.5 | 3.5 | 271.1 |
| S2 | 121.3 | 4.7 | 41.6 | 189.9 | 1.7 | 0.0 | 5.3 | 2.1 | 366.6 |
| S3 | 95.3 | 4.4 | 18.8 | 2.1 | 1.9 | 0.0 | 14.0 | 2.7 | 139.3 |
| S4 | 91.6 | 8.1 | 26.6 | 81.9 | 2.0 | 0.0 | 3.9 | 1.6 | 215.7 |
| S5 | 136.6 | 13.7 | 62.6 | 4.3 | 3.7 | 0.0 | 5.3 | 4.2 | 230.4 |
| S6 | 78.8 | 11.3 | 24.0 | 29.1 | 3.8 | 0.0 | 2.6 | 1.9 | 151.5 |
| S7 | 464.1 | 6.7 | 24.5 | 10.7 | 6.0 | 0.0 | 6.2 | 2.0 | 520.1 |
| S8 | 1490.6 | 12.3 | 46.1 | 120.0 | 9.7 | 0.0 | 11.7 | 2.6 | 1693.0 |
| S9 | 767.2 | 5.8 | 19.8 | 0.0 | 5.7 | 0.0 | 6.7 | 1.9 | 807.2 |
| S10 | 778.1 | 9.8 | 27.6 | 78.4 | 9.9 | 0.5 | 11.4 | 1.9 | 917.6 |
| S11 | 585.9 | 11.3 | 32.4 | 45.0 | 6.3 | 0.4 | 9.8 | 2.3 | 693.4 |
| S12 | 587.8 | 13.3 | 55.0 | 868.7 | 6.7 | 0.6 | 13.2 | 2.7 | 1547.9 |
| Average | 443.8 | 9.2 | 39.9 | 120.6 | 5.0 | 0.1 | 8.4 | 2.4 | 629.5 |
| Component | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigenvalue | 2.805 | 2.151 | 1.191 |
| % of variance | 31.172 | 23.900 | 13.238 |
| Cumulative % | 31.172 | 55.072 | 68.310 |
| Hg | 0.456 | −0.080 | −0.200 |
| Pb | 0.160 | 0.265 | 0.100 |
| As | −0.059 | 0.384 | 0.089 |
| Cd | −0.136 | 0.066 | 0.547 |
| Cu | 0.209 | 0.089 | 0.095 |
| Zn | 0.437 | −0.056 | −1.00 |
| Ni | −0.084 | −0.059 | 0.511 |
| Cr | 0.005 | 0.372 | −0.039 |
| TLI∑ | −0.023 | 0.286 | −0.247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Chen, G.; Wang, X.; Shen, J.; Zhang, H.; Lin, Y.; Shen, Y.; Lang, F.; Gong, C. Pollution Characteristics and Risk Assessment of Heavy Metals in the Sediments of the Inflow Rivers of Dianchi Lake, China. Toxics 2024, 12, 322. https://doi.org/10.3390/toxics12050322
He L, Chen G, Wang X, Shen J, Zhang H, Lin Y, Shen Y, Lang F, Gong C. Pollution Characteristics and Risk Assessment of Heavy Metals in the Sediments of the Inflow Rivers of Dianchi Lake, China. Toxics. 2024; 12(5):322. https://doi.org/10.3390/toxics12050322
Chicago/Turabian StyleHe, Liwei, Guangye Chen, Xinze Wang, Jian Shen, Hongjiao Zhang, Yuanyuan Lin, Yang Shen, Feiyan Lang, and Chenglei Gong. 2024. "Pollution Characteristics and Risk Assessment of Heavy Metals in the Sediments of the Inflow Rivers of Dianchi Lake, China" Toxics 12, no. 5: 322. https://doi.org/10.3390/toxics12050322
APA StyleHe, L., Chen, G., Wang, X., Shen, J., Zhang, H., Lin, Y., Shen, Y., Lang, F., & Gong, C. (2024). Pollution Characteristics and Risk Assessment of Heavy Metals in the Sediments of the Inflow Rivers of Dianchi Lake, China. Toxics, 12(5), 322. https://doi.org/10.3390/toxics12050322








