Abstract
Several epidemiological studies have demonstrated that particulate matter (PM) in air pollution can be involved in the genesis or aggravation of different cardiovascular, respiratory, perinatal, and cancer diseases. This study assessed the in vitro effects of PM10 on the secretion of cytokines by a human monocytic cell line (THP-1). We compared the chemotactic, pro-inflammatory, and anti-inflammatory cytokines induced by PM10 collected for two years during three different seasons in five different Mexico City locations. MIP-1α, IP-10, MCP-1, TNF-α, and VEGF were the main secretion products after stimulation with 80 μg/mL of PM10 for 24 h. The THP-1 cells showed a differential response to PM10 obtained in the different sites of Mexico City. The PM10 from the north and the central city areas induced a higher pro-inflammatory cytokine response than those from the south. Seasonal pro-inflammatory cytokine secretion always exceeded anti-inflammatory secretion. The rainy-season-derived particles caused the lowest pro-inflammatory effects. We concluded that toxicological assessment of airborne particles provides evidence supporting their potential role in the chronic exacerbation of local or systemic inflammatory responses that may worsen the evolution of some chronic diseases.
1. Introduction
Air pollution is a well-known risk factor for adverse human health effects [1,2]. Exposure to high quantities of airborne particulate matter (PM) is associated with pregnancy complications [3,4,5,6] and increased morbidity and mortality from respiratory [7,8,9] and cardiovascular diseases [10,11,12,13]. PM’s proposed damage mechanisms involve the secretion of pro-inflammatory cytokines and direct cytotoxic and genotoxic effects [14,15,16]. Some of these effects are mediated by reactive oxygen species, as demonstrated by in vivo and in vitro studies [17,18,19]. Target organs and health outcomes have been related to three types of particles of different sizes and compositions. The PM10 (aerodynamic diameter (AED) < 10 μm) is mainly deposited in the upper airways. In comparison, PM2.5 (AED < 2.5 μm) and PM0.1 (AED < 0.1 μm) can reach the intravascular compartment from terminal bronchioles and alveoli [20,21]. All these size fractions of PM can induce or aggravate conditions with an inflammatory background. PM also significantly contributed to excess mortality during the COVID-19 pandemic [22,23].
PM composition depends on the sources, human activities, and geographical and meteorological local characteristics. PM can be anthropogenic derived from vehicle emissions, industry, soil erosion or produced naturally during dust storms, forest fires, or volcanic eruptions. Seasonal variations in air pollution and PM-related health effects have been demonstrated [10,24,25]. Indeed, PM’s toxic and inflammatory potential has been shown to vary due to the chemical diversity of its components based on local conditions and the time of the year [26,27,28]. A relationship has been demonstrated between elevated PM10 levels and increased mortality, showing evidence even from the assessment of seasonality together with temperature variability in Mexico City [29].
Mexico City is one of the most densely populated cities globally, with 5967 people per km2, and it is also among the most polluted cities in Latin America [30]. Given the multifactorial causes of pollution and that 99.5% of the population is urban, Mexico City residents have been exposed to high pollutant levels for decades, with notably high levels of O3, PM2.5, and PM10. Additionally, geographical conditions, including altitude and being located at a tropical latitude within a valley surrounded by mountains, make the city more prone to high air pollution levels. Studies conducted by the air-monitoring network of the Mexico City government showed that the permissible PM10 level of 150 μg/m3 was generally exceeded in some areas of the city, mainly in correlation with the location of industrial activity [31,32]. In addition, different studies examining PM in Mexico City have shown a seasonal variation in size and chemical composition [33,34].
Most public health recommendations about air pollutant exposure are based on epidemiological findings correlating air quality monitoring with health outcomes, and few efforts are available to evaluate the direct effect of air pollutants on biological responses and to use these results to identify geographical regions or seasonal timeframes with higher risks for health. Therefore, in this study, the human monocyte cell line THP-1 was evaluated as a biomonitor of inflammatory responses to PM10 collected in different areas and seasons of a megacity.
2. Materials and Methods
PM sampling. PM10 were collected in five Mexico City locations from March 2010 to February 2012. The ethics committees of Facultad de Medicina, UNAM (102-2009) and the University of Michigan Institutional Review Board (HUM00023514) approved the protocols for sample collection and analysis. Sampling sites were selected based on their proximity to Mexico City’s air monitoring stations in areas representing either dominant industrial, business, or residential activities. The chosen locations vary in traffic-related pollution, demographics, and urban infrastructure: an industrial region located in the north, a business one situated downtown or central, and residential areas in the south, the east, and the west [35].
High-volume air samplers (TE6070V-2.5, Tisch Environmental, Inc.; Hamilton, OH, USA, airflow rate 1.13 m3 min−1) [14] equipped with modified nitrocellulose membranes were used to collect PM10 for 24 h on Mondays, Wednesdays, and Fridays for 24 months. The PM collection periods corresponded to the three seasons observed at Mexico City’s latitude, namely warm-dry (WD): March–May; rainy (R): June–October; and cold-dry (CD): November–February.
PM sample preparation. PM was mechanically recovered from the membranes and pooled according to month and site, resulting in 18 samples from the WD season, 30 samples from the R season, and 24 samples from the CD season. Following measurements of their weight, the PM samples were stored individually in baked glass vials and preserved in the dark, at 4 °C, in desiccators. PM samples were sterilized by autoclaving before use for in vitro exposure experiments [33].
Cell culture. To evaluate the cell response to PM, we used THP-1 cells (human monocytic cell line) obtained from the American Type Culture Collection (TIB 202). The cell suspensions were grown in RPMI 1640 media (Sigma Chemical, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, containing penicillin (50 U/mL) and streptomycin (50 mg/mL).
One milliliter per well cell suspensions (550,000 cells/mL) were kept in 24-well plates at 37 °C in a 5% CO2/95% air atmosphere. Culture media was replaced by serum-free media and incubated for 24 h before exposure to PM. In previous work, 80 μg/mL was the optimal PM concentration to induce cytokine production with minimal cell viability loss (up to 10%) [33]. PM stock suspensions (1.0 mg/mL) were prepared in cell culture media, sonicated for 5 min, and vortexed before addition to cell cultures to reach a final concentration of 80 μg/mL. THP-1 cells were incubated for 24 h in the presence of PM, then centrifuged at 2000× g, and supernatants were recovered and maintained at −80 °C until use for cytokine quantification. Three independent experiments were carried out in triplicate with each PM sample. Non-exposed cells were used as negative controls, and their basal cytokine levels were subtracted from the experimental values.
Multiplex for cytokines/chemokines. A panel of fifteen cytokines (MAP human cytokine/chemokine magnetic bead panel kit; Millipore Corporation, Billerica, MA, USA) was used, including Eotaxin, interleukin (IL), IL-10, IL-17, IL-2, IL-6, IL-12p40, IL-1α, IL-1β, interleukin-1 receptor antagonist (IL-1RA), soluble interleukin-2 receptor alpha (sIL-2Rα), interferon-gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein (MIP-1α), tumor necrosis factor-alpha (TNF-α), and vascular endothelial growth factor (VEGF). Multiplex analyses were performed in samples kept at −80 °C for no more than 90 days, following the published protocol of the manufacturer, and concentrations are expressed in pg/mL.
Statistical Analysis. Cytokine concentrations in culture media of PM-stimulated THP-1 were averaged according to each triplicate. Descriptive statistics were calculated for each of the city’s five locations and seasons of the year. Natural log (ln)-transformation of cytokine data was performed to approximate normality. The Shapiro–Wilk test and Anderson–Darling test were used to assess continuous data for normality, and the Levene’s and Breusch–Pagan tests were used to evaluate heteroskedasticity. One-way analysis of variance (ANOVA) with post hoc test Tukey’s HSD was used to test differences across regions and seasons. A supervised clustering heatmap was performed separating cytokine families by regions and seasons. Concentrations were standardized, and the Spearman correlation distance measure was applied to cluster analysis.
Principal component analysis (PCA) was used to characterize a cytokine profile to indicate the level of inflammatory balance in the cellular response. An iterative process was used to reduce cytokines’ dimensionality and lead to the maximum variance between cytokine families and city-regions. To achieve this, data were normalized and centered with a mean of 0 and a standard deviation of 1. The Kaiser–Guttman criterion was used to choose the number of components to retain [36]. The cytokines selected for the final PCA explained the highest variance within two principal components; they defined the best profile that maximized the variance between the city sectors (one chemotactic, two pro-inflammatory, and one anti-inflammatory). Principal component scores were compared between city regions using the Kruskal–Wallis rank-sum test with Dunn’s multiple comparison test. A Mexico City region-level choropleth map indicating distinct areas according to the balance between the selected cytokines was created. To calculate the proinflammatory (IL-1α, IL-β, TNF-α, IL-6) ratio over the anti-inflammatory (IL-1RA, IL-10) in the different regions, simple ratios were calculated in each of them, according to the values reported in Table 1.

Table 1.
Descriptive statistics for cytokine production over the course of two years in response to PM exposure according to Sectors.
3. Results
Eotaxin, IL-2, IL-12p40, IL-17, and sIL-2Ra were not included in the analysis because their concentrations in the culture media were below the detection limit. MIP-1α, IP-10, MCP-1, TNF-α, and VEGF were the main secretion products of THP-1 cells upon PM10 stimulation. IL-6 had a minor contribution to THP-1 responses. Cytokines were grouped for analysis according to their main biological activity: chemotactic (MIP-1α, IP-10, and MCP-1), pro-inflammatory (IL-1α, IL-1β, TNF-α, and IL-6), and anti-inflammatory (IL-1RA and IL-10). THP-1 cells showed differential responses to PM10 obtained from different regions of Mexico City and during the two years of follow-up (Figure 1; Table 1).
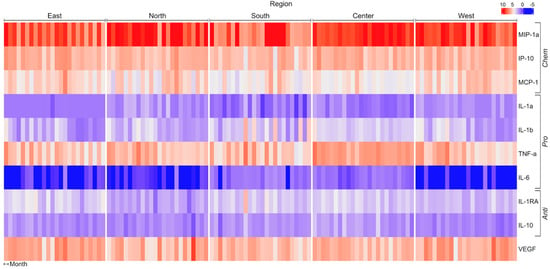
Figure 1.
Matrix showing change in pro-, anti-, and chemotactic cytokines when THP−1 cells are incubated with PM10 from different regions of Mexico City. Concentration units (pg/mL) were standardized, and the Spearman correlation distance measure was applied to cluster analysis. IL, interleukin; IP10, induced protein-10; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Chemokine averaged concentrations were higher with PM10 obtained in the north, central, and west areas and lowered with particles from the south (Figure 2; Table 1). In addition, the secretion of MIP-1α was twice as high when stimulated with PM10 from the north, central, and west compared with the response induced by particles from the south or east (Figure 2, Table 1 and Table 2).
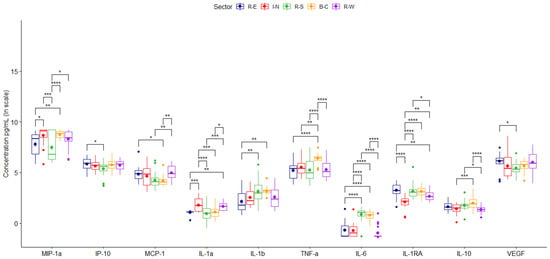
Figure 2.
Concentrations of cytokines secreted by TPH-1 cells stimulated by PM10. Comparison between the average values obtained for each of the cytokines secreted by samples collected during two years in different megacity regions. Data are presented as medians (middle line) and first and third quartiles (boxes) on a logarithm scale of pg/mL. Units in picograms per milliliter (pg/mL). Significance codes: * (p < 0.05), ** (p ≤ 0.01), *** (p ≤ 0.001) or **** (p ≤ 0.0001).

Table 2.
Differences across Sectors over the course of two years according to cytokine production in response to PM exposure.
PM10 induced differential location-based pro-inflammatory cytokine production. Particles from the south and central generated higher values of IL-1β and IL-6. However, IL-1α was mainly caused by particles from the north and west (Figure 2, Table 1 and Table 2), and particles from the central induced the highest levels of TNF-α.
Particles from the north caused less secretion of the anti-inflammatory cytokines IL-10 and IL-1RA. VEGF did not show differential responses (Figure 2).
City-averaged values of particle-induced chemokines showed only a significant increase in chemotactic (MCP-1) during the warm-dry season (WD) (Figure 3). Consistently higher values of pro-inflammatory cytokines, except for IL-6, were induced by particles collected during warm-dry and cold-dry seasons (Figure 3, Table 3 and Table 4). No major differences in anti-inflammatory cytokine secretion were found between seasons. VEGF was increased in the media culture of rainy season stimulated cells (Figure 3).
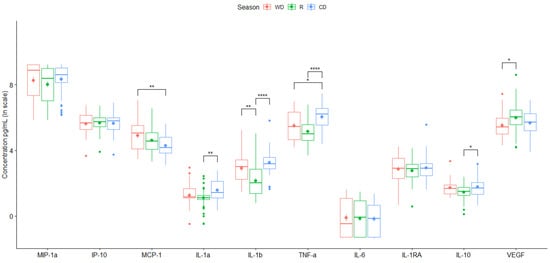
Figure 3.
Mean value of cytokine secretion by the THP-1 cell line induced by PM10 collected in the different monitoring stations for two years. Data are presented as medians (middle line) and first and third quartiles (boxes) in a natural logarithm scale of pg/mL. One-way analysis of variance (ANOVA) and Tukey’s HSD post hoc analysis were used to test differences across seasons. Significance codes: * (p ≤ 0.05), ** (p ≤ 0.01) or **** (p ≤ 0.0001).

Table 3.
Descriptive statistics for cytokine production over the course of two years in response to PM exposure according to Seasons.

Table 4.
Differences across Seasons over the course of two years according to cytokine production in response to PM exposure.
According to the function of the groups of cytokines previously mentioned, an iterative selection was made using principal component analysis which allowed us to simplify the data set for all cytokines secreted by THP-1 cells after being stimulated by particles from different regions during two years of observation. Representative cytokines with direct biological significance, including chemokine (MIP-1α), proinflammatory (IL-1α and IL-1β), and anti-inflammatory (IL-1RA), resulted from this analysis. Using this combination of cytokines, three clearly differentiated regions were identified: first the northern region, characterized by the highest proinflammatory particles; second the central and west; and third comprising the southern and eastern regions (Figure 4). Principal component scores reflecting the balance between proinflammatory and anti-inflammatory cytokines revealed that the northern zone induced the highest proinflammatory response and the southern and eastern the lowest (Figure 5, Table 5 and Table 6).
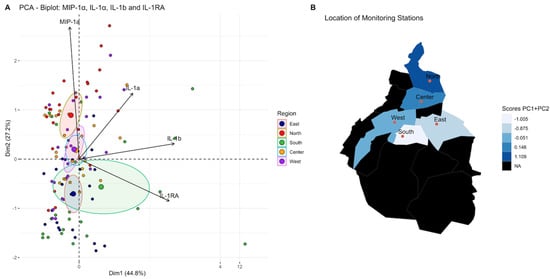
Figure 4.
Iterative principal component analysis of cytokines secreted by TPH−1 cells stimulated by PM10 from the different regions of the city selected four main contributors for variance: (A). Principal component 1(PC1, Dim 1) comprises IL-1b and IL-1RA. Principal component 2 (PC2, Dim 2) comprises MIP-1a and IL-1a. (B). Comparison of scores PC1 + PC2 in different regions of the megacity. The distribution pattern obtained is shown. With these cytokines, three clusters are identified: the first included the north, the second was central and west regions, and the last one was in the south and east regions. The black areas on the map are forest zones, almost not urbanized.
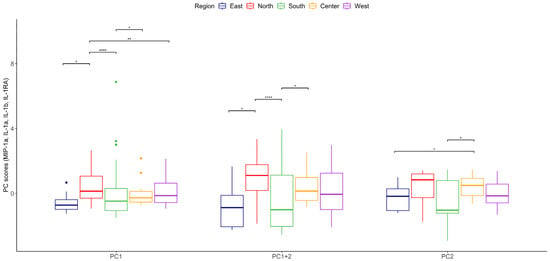
Figure 5.
Comparison of PC1 + PC2 scores among regions of the megacity, Kruskal–Wallis test. Statistically significant changes relative to PC1 + PC2 scores among regions are shown with * (p ≤ 0.05), ** (p ≤ 0.01) or **** (p ≤ 0.0001).

Table 5.
Eigenvalues for principal components selection extracted by principal component analysis of MIP-1α, IL-1 α, IL-1β, and IL-1RA.

Table 6.
Loadings of variables in each of the four principal components extracted by principal component analysis.
4. Discussion
We successfully used THP-1 cells as an experimental model for evaluating the innate immune system’s response to airborne PM10. This monocytic human leukemia cell line has been widely used to study the functions, mechanisms, signaling pathways, nutrient, and drug transport of monocytes/macrophages [37,38], and we propose it can be used as a proxy of the inflammatory responses induced by airborne PM10 obtained in places such as Mexico City. Previous descriptions of THP-1 confirm that they can recognize, internalize, and process PM, activating NOD-like receptors (NLR) that, in turn, induce the activation of transcription factors, such as Nuclear Factor kB (NF-κB), AP-1, and SP-1 [38], and the signal transducer and activator of transcription-1 (STAT1) [39]. This activation results in the up-regulation of pro-inflammatory factors, such as TNF-α and IL-6, and the protein NLR3, necessary for the assembly of the inflammasome and the proteolytic activation of the pro-inflammatory cytokines IL-1β and IL-18 [40]. All these characteristics make THP-1 a well-characterized cell line that can be used to biomonitor the inflammation-mediated responses to air pollutants and to correlate these responses with health outcomes.
This study allowed the collection of PM10 over two years, simultaneously obtaining particles from five different sectors of a large city and evaluating them with the THP-1 biological system. Cytokine secretion patterns by THP-1 cells were consistent with the environmental characteristics of each region of the city. Sites with the highest industrial and pollution levels and a high population density, such as the northern and central sectors [41], induced a predominant pro-inflammatory effect on cells, calculated by the net amount of secreted chemokines and pro-inflammatory cytokines and as the ratio of pro-inflammatory/anti-inflammatory cytokines. Particles captured in areas of the city with well-preserved green spaces and little industrial activity showed a low capacity for the induction of cytokine secretion. Cytokine secretion by stimulated THP-1 cells also varied according to the season when the particles were collected, probably due to changes in their composition, which has been reported in previous studies [25,33,42].
Air pollution composition in Mexico City has cycles associated with changes in humidity, temperature, and precipitation levels in each season and other factors such as the intensity of vehicular traffic, industrial activity, construction, and demolition activities, which modify the content of several chemicals and diverse biological-derived materials. In the dry-cold season, pollution levels increase, with PM being higher in winter, while ozone is more elevated in the rainy season [43,44]. Mexico City is located in a basin, and the winds entering from the north and west disperse the particulate components in a gradient from north to south [45]. According to these changes, chemokines and pro-inflammatory cytokines were secreted by THP-1 cells in low concentrations when cells were exposed to particles captured during the rainy season. On the contrary, higher values were observed when using particles from the cold-dry season, supporting the existence of different city microenvironments in which we may expect non-identical exposure to pollutants and dissimilar extent and severity of health effects [46].
In this study, no attempt to characterize the composition of particles was made because they have been extensively studied in the past [33,47,48,49]. Studies by Manzano-Leon et al. in 2013 and 2016 [27,33], coupled with Rosas-Pérez et al. [48], show that the chemical properties of particles are consistent and induce proinflammatory responses resulting from complex interactions between PM constituents. In this study, we validate regional differential effects on the secretion of several cytokines, confirming region-specific contributions of particle constituents in THP-1 cells. Previous studies in Mexico City have shown that particles obtained from the northern area, but not those obtained from the south part of the city, induced cell death and DNA damage in proliferating cells and may be related to the high content of metals [50]. Pro-inflammatory effects of PM10 became more noticeable in the central zone, which may be the result of a synergy between metals, PAHs, and endotoxins. On the contrary, in the southern zone, the lowest proinflammatory effect of particles was identified, in coincidence with low levels of metals and PAHs in particles collected in this area in previous studies. In addition, PM10 levels are always lower in this location, which is characterized by relatively well-preserved extensive green areas.
The adverse health effects of PM are a consequence of its physical characteristics (size, mass, and even shape) and the chemical components they contain, such as polycyclic aromatic hydrocarbons (PAHs), heavy metals (HM), and biogenic materials such as lipopolysaccharide (LPS), all of them with potential pro-inflammatory effects. The response of THP-1 cells to PAHs and dioxins is mediated by the aryl hydrocarbon receptor (AhR), a highly conserved intracellular transcription factor that interacts with NF-kB [51] through physical association and transcriptional modulation. AhR, when activated in the presence of high concentrations of PAHs, a situation that may be induced by PM10 from the northern and central zones in our study, induces increased TNFα secretion by a mechanism related to MAPK and ERK [52,53]. On the other hand, NF-kB activity negatively regulates the inflammatory response mediated by LPS in monocytes and macrophages, through its interaction with Stat1 [39]. Thus, it induces the negative regulation of NLRP3, acting as a physiological suppressor of NLRP3 inflammasome and caspase-1 activation, directly affecting IL-1β secretion [54]. PM10 with low PAHs and high LPS concentrations such as those collected in the southern region results in preferential NF-kB activation that inhibits the activation of the AhR pathway [55].
A proposed biomonitor, such as THP-1 monocytes that evaluate the pro-inflammatory effects of particles, must be correlated with clinical and epidemiological outcomes. Cytokine secretion by THP-1 in response to PM10 is direct evidence that airborne particles can contribute to increased inflammatory factors and cellular recruitment in the lung, which promotes physiology alterations, resulting in enhanced acute respiratory symptoms as chronic obstructive pulmonary disease and asthma, pulmonary and systemic oxidative stress, and inflammation [56,57]. Likewise, PM can activate other cellular mediators that produce pulmonary fibrosis. All components present in the particle form a final complex mixture that will produce or activate inflammatory processes, damage, or oxygen-containing reactive species (ROS) in the lung. All these changes harm the epithelium, increasing epithelial permeability. In addition, once airway macrophages have phagocytized PM10, the macrophages can divert some of these cytokines to the systemic circulation, explaining the long-distance effects of the cytokines in the cardiovascular system, modulating carcinogenesis [43,58,59] and inducing perinatal complications such as preterm labor or premature rupture of the membranes [60].
Recent epidemiological evidence of the COVID-19 pandemic in Mexico City suggests that air pollution may explain excess mortality in cities with high pollution levels [22,23]. Considering that inflammation is a primary contributor to morbidity and mortality, conditioned by the innate immune response to SARS-CoV-2, our results provide direct evidence that PM10 can induce additional pro-inflammatory responses that may explain the asymmetry in both COVID-19-related excess mortality and morbidity during different seasons of the year [61]. Identifying areas of the city with the highest capacity to induce inflammatory responses by the THP-1 bioindicator may aid in developing focalized government efforts to modify air pollution sources and consequently lower population exposure to air pollutants [62].
5. Conclusions
We validated the potential use of THP-1 as a biomonitor for the inflammatory effects of PM10. It was interesting to observe that there are differences in the secretion of cytokines according to the geographical region and season from which the particulate material comes. Using data reduction techniques, we also identified four possible ‘sentinel’ cytokines that can indicate the level of inflammatory balance in the cellular response, potentially reducing the analysis costs and allowing for regular monitoring of the biological effects of PM by season and location.
Our results support the hypothesis that particle composition may explain differences in inflammation and toxic responses induced by air pollution in different sectors and seasons in a megacity. This is relevant as it would help to carry out preventive measures in the population according to where they live and the season of the year [63], as well as in the treatment of people with different respiratory diseases exposed to particulate matter.
Author Contributions
Conceptualization: N.M.-C., M.S.O. and F.V.-O.; Methodology: N.M.-C., N.M.-L., M.d.C.G.d.L.M., R.Q.-B. and L.S.T.; Formal analysis: N.M.-C., D.E.S.-C. and F.V.-O.; Investigation: N.M.-C., N.M.-L., D.E.S.-C., M.d.C.G.d.L.M., R.Q.-B., L.S.T., A.R.O.-V., M.A.B., M.S.O. and F.V.-O.; Resources: A.R.O.-V., M.S.O. and F.V.-O.; Data curation: D.E.S.-C.; Writing—original draft preparation: N.M.-C., N.M.-L., M.d.C.G.d.L.M. and F.V.-O.; Writing—review and editing: N.M.-C., N.M.-L., D.E.S.-C., M.d.C.G.d.L.M., R.Q.-B., L.S.T., A.R.O.-V., M.A.B., M.S.O. and F.V.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the following sources: National Institute of Environmental Health Sciences R01ES016932, R01ES017022.
Institutional Review Board Statement
The ethics committees of Facultad de Medicina, UNAM (102-2009) and the University of Michigan Institutional Review Board (HUM00023514) approved the protocols for sample collection and analysis.
Informed Consent Statement
Does not apply.
Data Availability Statement
All relevant data are within the manuscript.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Almetwally, A.; Bin-Jumah, A. Ambient air pollution and its influence on human health and welfare: An overview. Environ. Sci. Pollut. Res. Int. 2020, 27, 24815–24830. [Google Scholar] [CrossRef]
- Valverde, M.; Granados, A.; Milić, M.; Ceppi, M.; Sollano, L.; Bonassi, S.; Rojas, E. Effect of Air Pollution on the Basal DNA Damage of Mother–Newborn Couples of México City. Toxics 2023, 11, 766. [Google Scholar] [CrossRef]
- Glinianaia, S.V.R.J.; Bell, R.; Pless-Mulloli, T.; Howel, D. Particulate air pollution and fetal health: A systematic review of the epidemiologic evidence. Epidemiology 2004, 15, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Maisonet, M.C.A.; Misra, D.; Jaakkola, J.J.K. A review of the literature on the effects of ambient air pollution on fetal growth. Environ. Res. 2004, 95, 106–115. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.; Osornio-Vargas, A.R.; Buxton, M.; Sanchez, B.; Rojas-Bracho, L.; Castillo-Castrejon, M.; Mordhukovich, I.; Brown, D.; Vadillo-Ortega, F. Air pollution, inflammation and preterm birth in Mexico City: Study design and methods. Sci. Total Environ. 2013, 448, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.W.M.; Hoggatt, K.J.; Ghosh, J.K.C. Ambient air pollution and preterm birth in the Environment and Pregnancy Outcomes Study at the University of California, Los Angeles. Am. J. Epidemiol. 2007, 166, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, R.; Ansotegui, I.; Bousquet, J.; Canonica, W.; D’Amato, G.; Rosario, N.; Pawankar, R.; Peden, D.; Bergmann, K.; Bielory, L.; et al. International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants: Impact of air pollution on patients with AR: Current knowledge and future strategies. World Allergy Organ. J. 2020, 13, 100106. [Google Scholar] [CrossRef]
- Nel, A.E.; Diaz-Sanchez, D.; Li, N. The role of particulate pollutants in pulmonary inflammation and asthma: Evidence for the involvement of organic chemicals and oxidative stress. Curr. Opin. Pulm. Med. 2001, 7, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Saldiva, P.H.; Clarke, R.W.; Coull, B.A.; Stearns, R.C.; Lawrence, J.; Murthy, G.G.; Diaz, E.; Koutrakis, P.; Suh, H.; Tsuda, A.; et al. Lung inflammation induced by concentrated ambient air particles is related to particle composition. Am. J. Respir. Crit. Care Med. 2002, 165, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Contiero, P.; Boffi, R.; Tagliabue, G.; Scaburri, A.; Tittarelli, A.; Bertoldi, M.; Borgini, A.; Favia, I.; Ruprecht, A.; Maiorino, A.; et al. A case-crossover study to investigate the effects of atmospheric particulate matter concentrations, season, and air temperature on accident and emergency presentations for cardiovascular events in Northern Italy. Int. J. Environ. Res. Public Health 2019, 16, 4627. [Google Scholar] [CrossRef]
- Delfino, R.; Sioutas, C.; Malik, S. Potential role of ultrafine particles in association between airborne particle mass and cardiovascular health. Environ. Health Perspect. 2005, 113, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Tager, I.; Lurmann, F.; Segal, M.; Quesenberry, C.; Lugg, M.M.; Shan, J.; Van Den Eeden, S.K. Air pollution and hospital admissions for ischemic heart disease: A prospective study and metanalysis. Environ. Health Perspect. 2002, 110, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.; Burnet, R.; Thurston, G.; Thun, M.; Calle, E.; Krewski, D. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Moreno, E.; Ponce de Leon, S.; Osornio-Vargas, A.; Garcia-Cuellar, C.; Martinez, L.; Rosas, I. Potential toxic effects associated to metals and endotoxin present in PM10: An ancillary study using multivariate analysis. Inhal. Toxicol. 2007, 19, 49–53. [Google Scholar] [CrossRef]
- Peters, A.; Fröhlich, M.; Doring, A.; Immervoll, T.; Wichmann, H.E.; Hutchinson, W.L.; Pepys, M.B.; Koenig, W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur. Heart J. 2001, 22, 1198–1204. [Google Scholar] [CrossRef]
- Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res. 2005, 592, 119–137. [Google Scholar] [CrossRef]
- Bello-Medina, P.; Rodríguez-Martínez, E.; Prado-Alcalá, R.; Rivas-Arancibia, S. Ozone pollution, oxidative stress, synaptic plasticity, and neurodegeneration. Neurologia 2022, 37, 277–286. [Google Scholar] [CrossRef]
- Ovrevik, J.; Refsnes, M.; Lag, M.; Holme, J.; Schwarze, P. Activation of pro-inflammatory responses in the airway mucosa cells by particulate matter. Oxid.—Non-Oxid.-Mediat. Triggering Mech. Biomol. 2015, 5, 1399–1440. [Google Scholar]
- Rouadi, P.; Idriss, S.; Naclerio, R.; Peden, D.; Ansotegui, I.; Canonica, G.; Gonzalez-Diaz, S.; Rosario Filho, N.; Ivancevich, J.; Hellings, P.; et al. Immunopathological features of air pollution and its impact on inflammatory airways diseases (IAD). World Allergy Organ. J. 2020, 13, 100467. [Google Scholar] [CrossRef]
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T. A work report on ultrafine particles (American Academy of Allergy, Asthma and Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J. Allergy Clin. Immunol. 2016, 138, 386–396. [Google Scholar] [CrossRef]
- Lowther, S.; Jones, K.; Wang, X.; Whyatt, J.; Wild, O.; Booker, D. Particulate matter measurement indoors: A review of metrics, sensors, needs, and applications. Environ. Sci. Technol. 2019, 53, 11644–11656. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Perez-Guevara, F.; Roy, P.; Elizalde-Martinez, I.; Shruti, V. Impacts of the COVID-19 lockdown on air quality and its association with human mortality trends in megalopolis Mexico City. Air Qual. Atmos. Health 2021, 14, 553–562. [Google Scholar] [CrossRef]
- Lopez-Feldman, A.; Heres, D.; Marquez-Padilla, F. Air pollution exposure and COVID-19: A look at mortality in Mexico City using individual-level data. Sci. Total Environ. 2021, 756, 143929. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lai, C.; Chu, C. Air pollution diffusion simulation and seasonal spatial risk analysis for industrial areas. Environ. Res. 2020, 194, 110693. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Rivas-Santiago, C.; Ibironke, O.; Carranza, C.; Meng, Q.; Osornio-Vargas, A.; Zhang, J.; Torres, M.; Chow, J.; Watson, J.; et al. Season and size of urban particulate matter differentially affect cytotoxicity and human immune responses to Mycobacterium tuberculosis. PLoS ONE 2019, 14, e0219122. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Su, S.; Wang, B.; Zhu, X.; Wang, X.; Zeng, E.; Xing, B.; Tao, S. Seasonal and spatial variations in the chemical components and the cellular effects of particulate matter collected in Northern China. Sci. Total Environ. 2018, 627, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Manzano-León, N.; Quintana, R.; Sánchez, B.; Serrano, J.; Vega, E.; Osornio, A. Variation in the composition and in vitro pro-inflammatory effect of urban particulate matter from different sites. J. Biochem. Mol. Toxicol. 2013, 27, 87–97. [Google Scholar] [CrossRef]
- Mirowsky, J.; Hickey, C.; Horton, L.; Blaustein, M.K.G. The effect of particle size, location, and season on urban and rural particulate matter toxicity. Inhal. Toxicol. 2013, 25, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Mamkhezri, J.; Bohara, A.K.; Islas Camargo, A. Air pollution and daily mortality in the Mexico City Metropolitan Area. Atmósfera 2020, 33, 249–267. [Google Scholar] [CrossRef]
- World Health Organization. WHO Ambient (Outdoor) Air Quality Database; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Astudillo-García, C.; Rodriguez-Villamizar, L.; Cortez-Lugo, M.; Cruz-De la Cruz, J.; Fernandez -Niño, J. Air pollution and suicide in Mexico City: A time series analysis, 2000–2016. Int. Environ. Res. Public Health 2019, 16, 2971. [Google Scholar] [CrossRef]
- SMA-GDF. Inventario de emisiones de la Zona Metropolitana del Valle de Mexico 2010. In Secretaría del Medio Ambiente; del Gobierno, D.F., Ed.; Secretaría del Medio Ambiente: Ciudad de México, Mexico, 2012. [Google Scholar]
- Manzano-Leon, N.; Serrano-Lomelín, J.; Sanchez, B.; Quintana-Belmares, R.; Vega, E.; Osornio-Vargas, A. TNF-alpha and IL-6 responses to particulate matter in vitro: Variation according to PM size, season, and polycyclic aromatic hydrocarbon and soil content. Environ. Health Perspect. 2016, 124, 406–412. [Google Scholar] [CrossRef]
- Zeb, B.; Alam, K.; Sorooshian, A.; Blaschke, T.; Ahmad, I.; Shahid, I. On the morphology and composition of particulate matter in an urban environment. Aerosol Air Qual. Res. 2018, 18, 1431–1447. [Google Scholar]
- INE Instituto Nacional de Ecología. Cuarto Almanaque de Datos y Tendencias de la Calidad del Aire en 20 Ciudades Mexicanas (2000–2009), 1st ed.; Gobierno Federal, SEMARNAT: Ciudad de México, Mexico, 2011. [Google Scholar]
- Palacio, F.; Apodaca, M.; Crisci, J. Análisis Multivariado para datos Biológicos, Teoría y su Aplicación Utilizando el Lenguaje R; Vázquez Mazzini Editores: Mexico City, Mexico, 2020. [Google Scholar]
- Bosshart, H.; Heizelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef]
- Chanput, W.; Mess, J.; Wichers, H. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Naka, T.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fuji-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 2009, 206, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Aguirre-Salado, A.; Vaquera-Huerta, H.; Aguirre-Salado, C.; Reyes-Mora, S.; Olvera-Cervantes, A. Developing a hierarchical model for the spatial analysis of PM10 pollution extremes in the Mexico City metropolitan area. Int. J. Environ. Res. Public Health 2017, 14, 734. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Dailey, L.; Soukup, J.; Grambow, S.; Devlin, R.; Huang, Y. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ. Health Perspect. 2005, 113, 1032–1038. [Google Scholar] [CrossRef]
- Corona-Vazquez, T.; Flores, R.; Rodríguez, V.; Cervantes-Arriaga, A. Air pollution, multiple sclerosis and its relevance to Mexico City. Arch. Med. Res. 2019, 50, 111–112. [Google Scholar] [CrossRef]
- Gutierrez, I.; Calderon Nepomuceno, D.; Gutierrez Cruz, D.; Aquina Verga, E. Correlación entre diferentes contaminantes atmosféricos de la Ciudad de México y el área metropolitana. Cienc. Ergo Sum Rev. Cient. Multidiscip. Prospect. 2020, 27, 1. [Google Scholar]
- Secretaría del Medio Ambiente, Gobierno del Distrito Federal. Informe Climatológico Ambiental del Valle de México; Secretaría del Medio Ambiente, Gobierno del Distrito Federal: Ciudad de México, Mexico, 2005. [Google Scholar]
- Januszek, R.; Staszczak, B.; Siudak, Z.; Bartus, J.; Plens, K.; Bartus, S.; Dudek, D. The relationship between increased air pollution expressed as PM10 concentration and the frequency of percutaneous coronary. Environ. Sci. Pollut. Res. 2020, 27, 21320–21330. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.; Rice, A.; Lindroos, P.; O’Brien, P.; Dreher, K.; Rosas, I.; Alfaro-Moreno, E.; Osornio-Vargas, A. Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from Mexico City. Am. J. Respir. Cell Mol. Biol. 1998, 19, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Rosas Perez, I.S.J.; Alfaro-Moreno, E.; Baumgardner, D.; Garcia-Cuellar, C.; Martin Del Campo, J.M.; Raga, G.B.; Castillejos, M.; Colin, R.D.; Osornio Vargas, A.R. Relations between PM10 composition and cell toxicity: A multivariate and graphical approach. Chemosphere 2007, 67, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Chirino, Y.; Sánchez-Perez, Y.; Osornio-Vargas, A.; Rosas, I.; García-Cuellar, C. Sampling and composition of airborne particulate matter (PM10) from two locations of Mexico City. Data Brief 2015, 4, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Moreno, E.; Martínez, L.; García-Cuellar, C.; Bonner, J.; Murray, J.; Rosas, I.; Rosales, S.; Osornio-Vargas, A. Biologic effects induced in vitro by PM10 from three zones of Mexico City. Environ. Health Perspect. 2002, 110, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ke, S.; Denison, M.S.; Rabson, A.B.; Gallo, M.A. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J. Biol Chem. 1999, 274, 510–515. [Google Scholar] [CrossRef]
- Fardel, O. Cytokines as molecular targets for aryl hydrocarbon receptor ligands: Implications for toxicity and xenobiotic detoxification. Expert Opin. Drug Metab. Toxicol. 2013, 9, 141–152. [Google Scholar] [CrossRef]
- Guarnieri, T.; Abruzzo, P.M.; Bolotta, A. More than a cell biosensor: Aryl hydrocarbon receptor at the intersection of physiology and inflammation. Am. J. Physiol. Cell Physiol. 2020, 318, C1078–C1082. [Google Scholar] [CrossRef]
- Huai, W.; Zhao, R.; Song, H.; Zhao, J.; Zhang, L.; Gao, C.; Han, L.; Zhao, W. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat. Commun. 2014, 5, 4738. [Google Scholar] [CrossRef]
- Ke, S.; Rabson, A.B.; Germino, J.F.; Gallo, M.A.; Tian, Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J. Biol. Chem. 2001, 276, 39638–39644. [Google Scholar] [CrossRef]
- Guarneros, M.; López-Rivera, C.; Gonsebatt, M.E.; Alcaraz-Zubeldia, M.; Hummel, H.; Schriever, V.A.; Valdez, B.; Hudson, R. Metal-containing particulate matter and associated reduced olfatory identification ability in children from an area of high atmospheric exposure in Mexico City. Chem. Senses 2020, 45, 59–67. [Google Scholar] [CrossRef]
- Maciel-Ruiz, J.A.; López-Rivera, C.; Robles-Morales, R.; Veloz-Martínez, M.G.; López-Arellano, R.; Rodríguez-Patiño, G.; Petrosyan, P.; Govezensky, T.; Salazar, A.M.; Ostrosky-Wegman, P.; et al. Prenatal exposure to particulate matter and ozone: Bulky DNA adducts, plasma isoprostanes, allele risk variants, and neonate susceptibility in the Mexico City Metropolitan Area. Environ. Mol. Mutagen. 2019, 60, 428–442. [Google Scholar] [CrossRef]
- Jeaniean, M.; Bind, M.; Roux, J. Ozone, NO2 and PM10 are associated with the occurrence of multiple sclerosis relapses. Evidence from seasonal multi-pollutant analyses. Environ. Res. 2018, 163, 43–52. [Google Scholar] [CrossRef]
- Roux, J.; Bard, D.; LePabic, E. Air pollution by particulate matter PM10 may trigger multiple sclerosis relapses. Environ. Res. 2017, 156, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Buxton, M.A.; Perng, W.; Tellez-Rojo, M.M.; Rodríguez-Carmona, Y.; Cantoral, A.; Sánchez, B.N.; Rivera-González, L.O.; Gronlund, C.J.; Shivappa, N.; Hébert, J.R.; et al. Particulate matter exposure, dietary inflammatory index and preterm birth in Mexico city, Mexico. Environ. Res. 2020, 189, 109852. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Sánchez, P.; Landeros-López, S.; Velazquez-Trejo, D.; Martínez-Kobeh, J.P.; Vadillo-Ortega, F. COVID-19 epidemiological indicators and exposition to airborne particle matter in Mexico City. Environ. Res. 2021; submitted. [Google Scholar]
- Antonio-Villa, N.E.; Bello-Chavolla, O.Y.; Fermín-Martínez, C.A.; Aburto, J.M.; Fernández-Chirino, L.; Ramírez-García, D.; Pisanty-Alatorre, J.; González-Díaz, A.; Vargas-Vázquez, A.; Barquera, S.; et al. Socio-demographic inequalities and excess non-COVID-19 mortality during the COVID-19 pandemic: A data-driven analysis of 1 069 174 death certificates in Mexico. Int. J. Epidemiol. 2022, 51, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, C.; Salvi, S.; Wong, G.W.K.; Chung, K.F. Personal strategies to minimize effects of air pollution on respiratory health: Advice for providers, patients and the public. Eur. Respir J. 2020, 55, 902056. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).