Disentangling the Relationship Between Urinary Metal Exposure and Osteoporosis Risk Across a Broad Population: A Comprehensive Supervised and Unsupervised Analysis
Abstract
:1. Introduction
2. Methods
2.1. Data Source and Study Population
2.2. Exposure Variables: Urinary Metals
2.3. Outcome Variables: Osteoporosis
2.4. Statistical Analysis
2.5. Software and Statistical Significance
3. Results
3.1. Baseline Characteristics of the Study Population
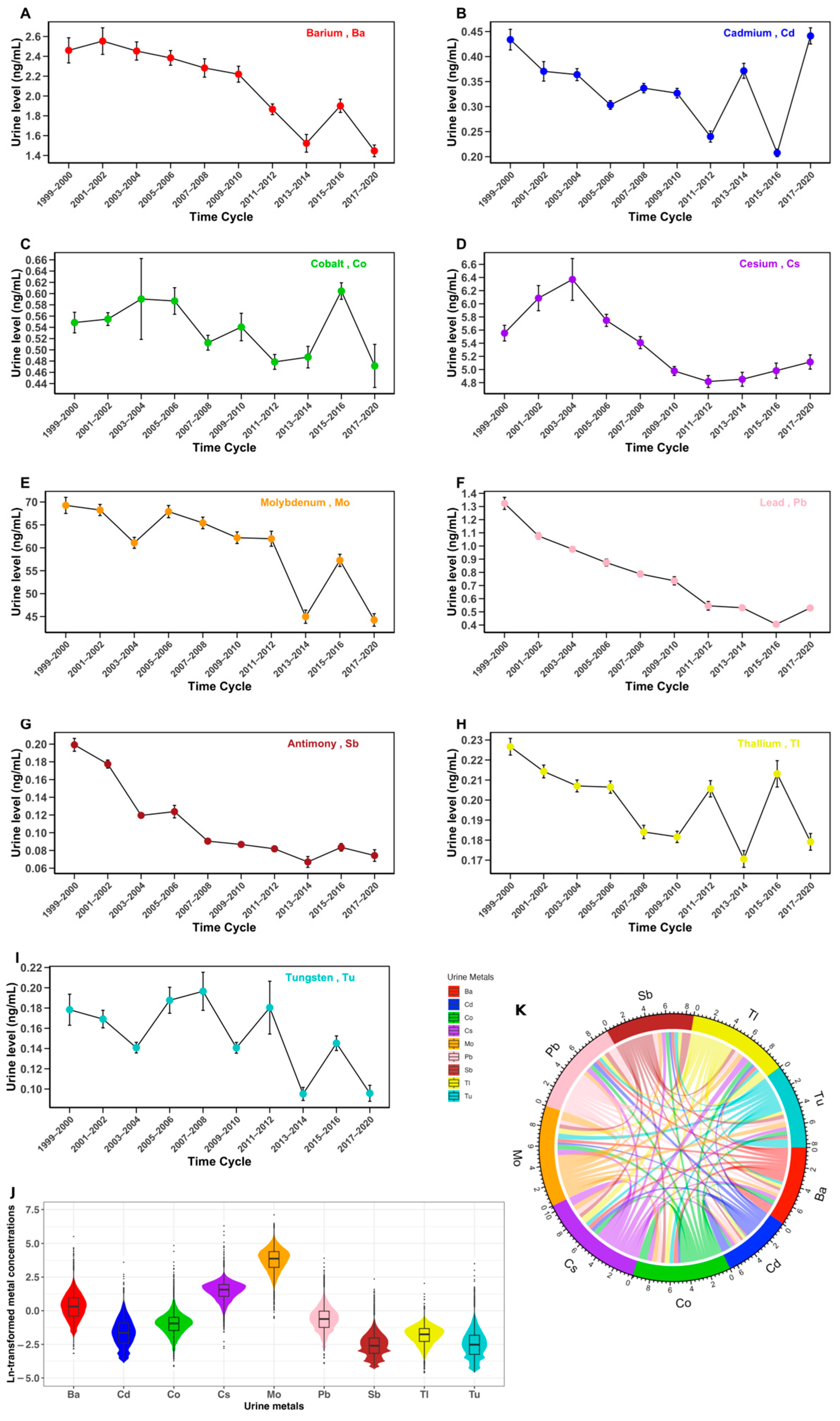
3.2. Urinary Metal Profiles
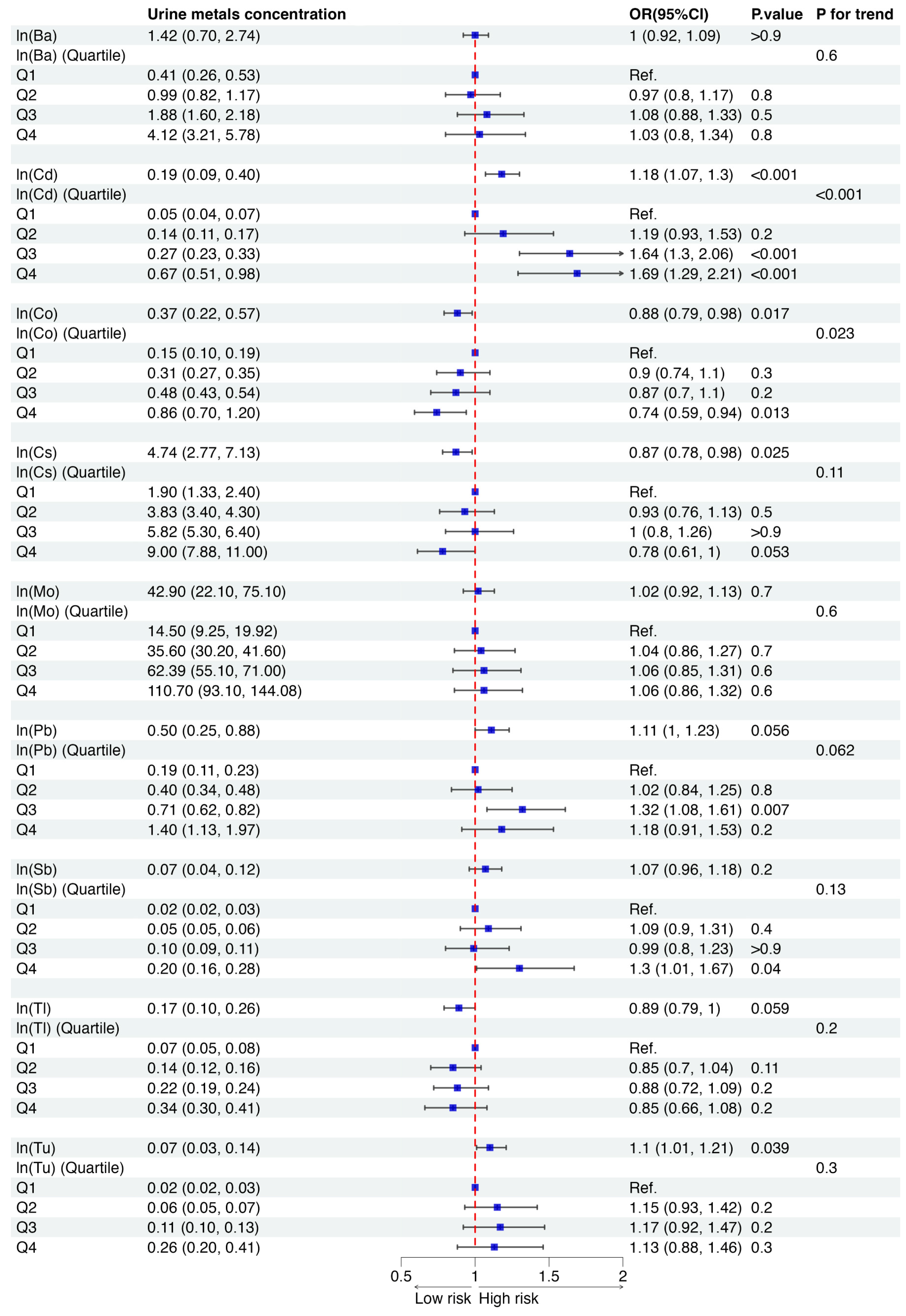
3.3. Association of Each Urinary Metal with Osteoporosis Risk
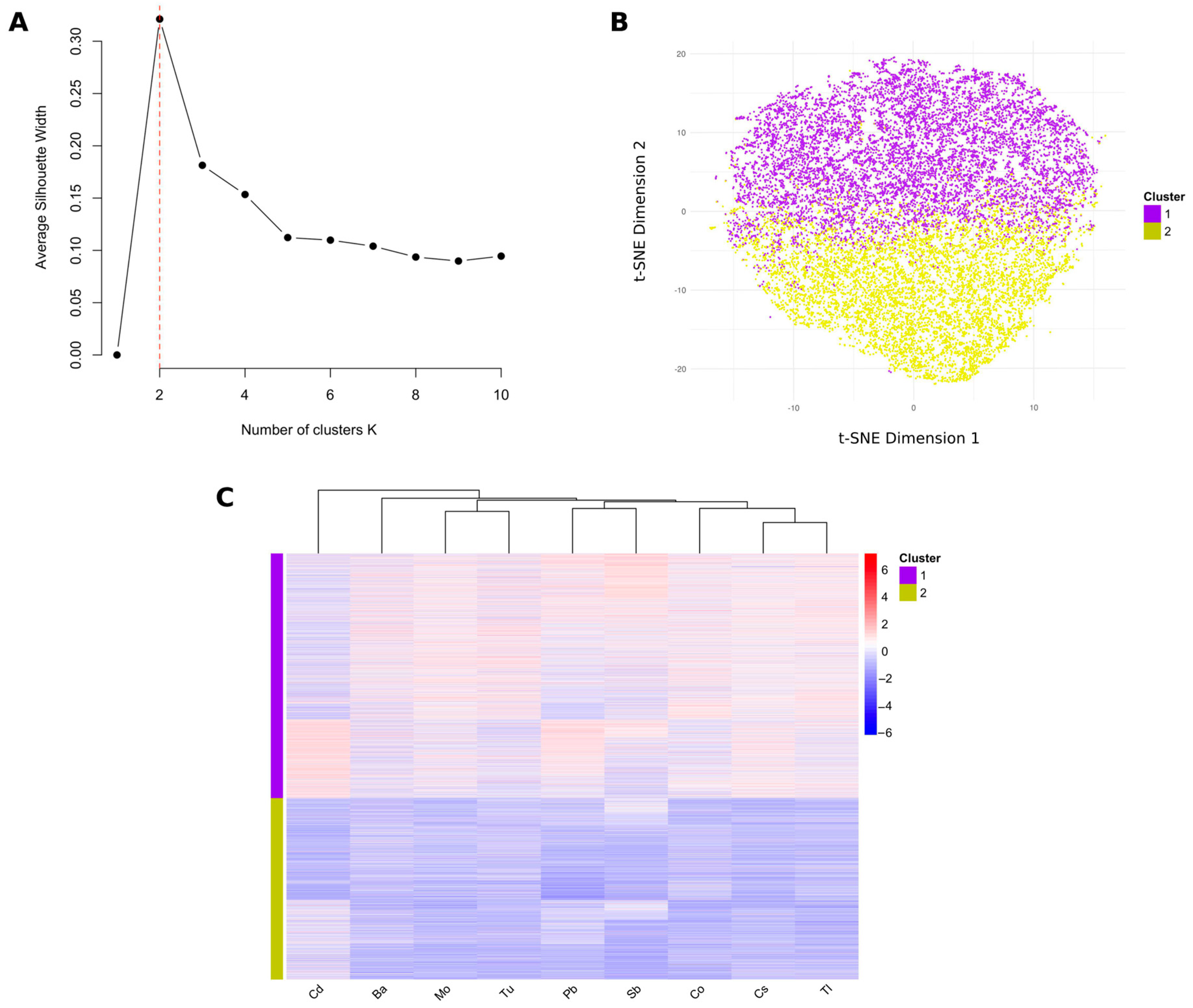
3.4. Identification of Different Mixed Metal Exposure Groups Using PAM Clustering
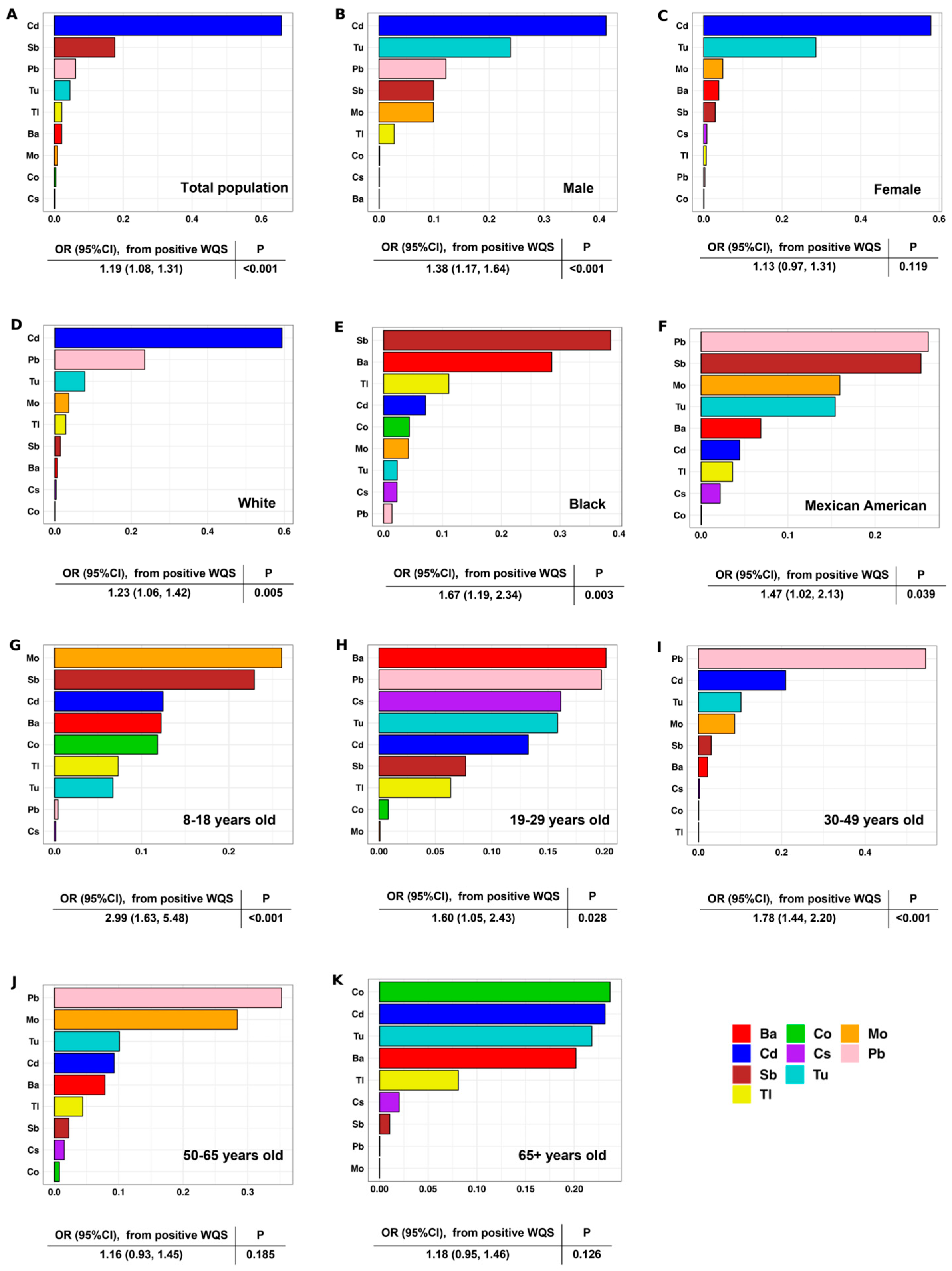
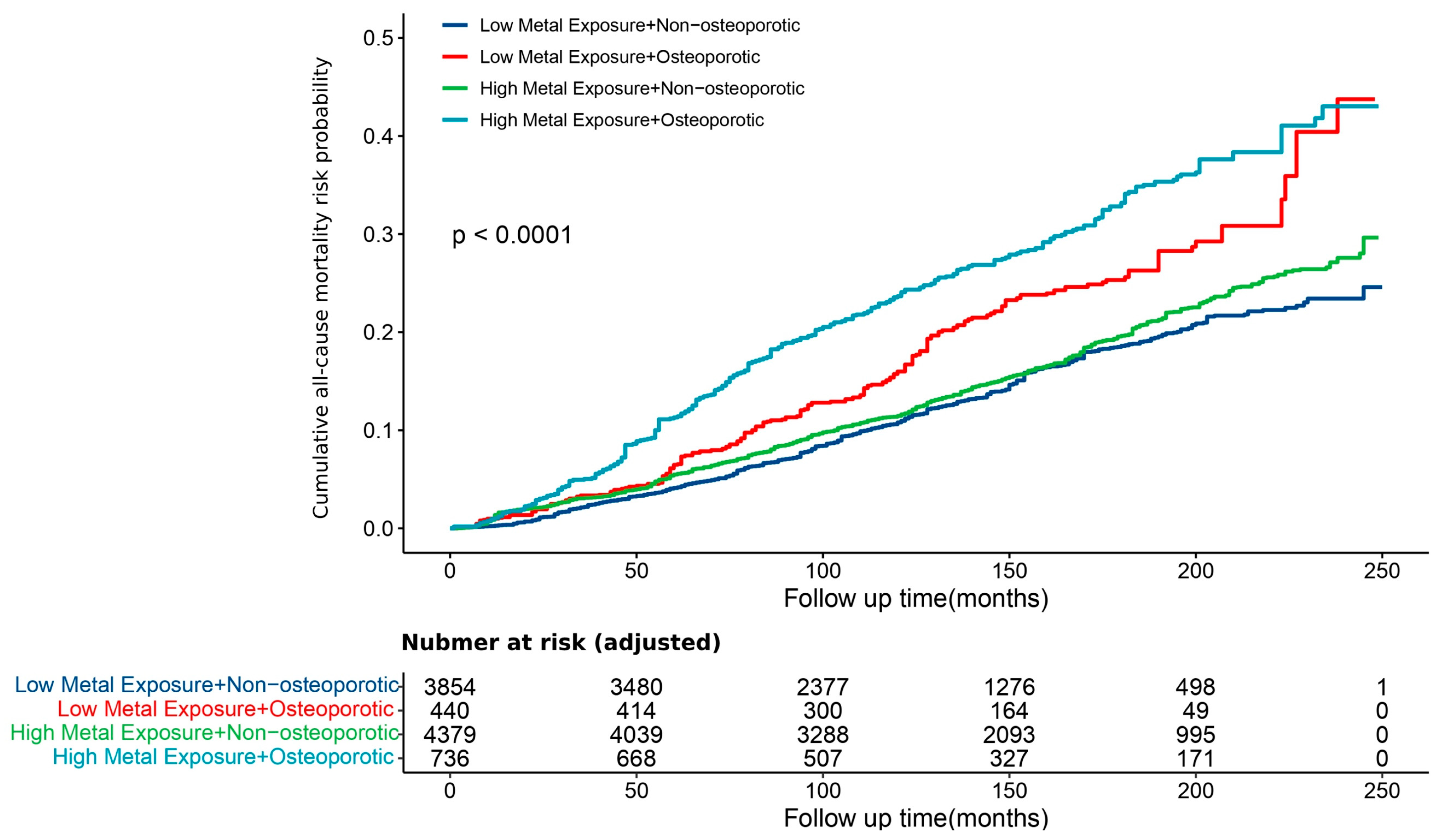
3.5. Association Between the Exposure Level of Urinary Mixed Metals and Osteoporosis Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez Rodriguez, E.; Debrach-Schneider, A.C.; Lamy, O. Osteoporosis. Rev. Med. Suisse 2022, 18, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Yuan, Y.; Liang, X.; Lei, J.; Zhu, X.; Zhang, Z.; Cai, L. Loss of longitudinal superiority marks the microarchitecture deterioration of osteoporotic cancellous bones. Biomech. Model. Mechanobiol. 2021, 20, 2013–2030. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Prada, D.; Crandall, C.J.; Kupsco, A.; Kioumourtzoglou, M.A.; Stewart, J.D.; Liao, D.; Yanosky, J.D.; Ramirez, A.; Wactawski-Wende, J.; Shen, Y.; et al. Air pollution and decreased bone mineral density among Women’s Health Initiative participants. EClinicalMedicine 2023, 57, 101864. [Google Scholar] [CrossRef]
- Scimeca, M.; Feola, M.; Romano, L.; Rao, C.; Gasbarra, E.; Bonanno, E.; Brandi, M.L.; Tarantino, U. Heavy metals accumulation affects bone microarchitecture in osteoporotic patients. Environ. Toxicol. 2017, 32, 1333–1342. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, X.; Wang, H.; Zhang, S.; Du, X.; Wei, H. Relationship of blood heavy metals and osteoporosis among the middle-aged and elderly adults: A secondary analysis from NHANES 2013 to 2014 and 2017 to 2018. Front. Public Health 2023, 11, 1045020. [Google Scholar] [CrossRef]
- Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Karlsson, M.K.; Lorentzon, M.; Ohlsson, C.; Mellstrom, D. Low-Level Cadmium Exposure Is Associated with Decreased Bone Mineral Density and Increased Risk of Incident Fractures in Elderly Men: The MrOS Sweden Study. J. Bone Miner. Res. 2016, 31, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Mohamed, N.; Ima-Nirwana, S.; Chin, K.Y. A Review of Knowledge, Belief and Practice Regarding Osteoporosis among Adolescents and Young Adults. Int. J. Environ. Res. Public Health 2018, 15, 1727. [Google Scholar] [CrossRef]
- Baradaran Mahdavi, S.; Zamani, S.; Riahi, R.; Taheri, E.; Vahdatpour, B.; Sharifianjazi, F.; Kelishadi, R. Exposure to Environmental Chemicals and Human Bone Health; A Systematic Review and Meta-Analysis. Expo. Health 2023, 16, 861–883. [Google Scholar] [CrossRef]
- Blomberg, A.; Mortensen, J.; Weihe, P.; Grandjean, P. Bone mass density following developmental exposures to perfluoroalkyl substances (PFAS): A longitudinal cohort study. Environ. Health 2022, 21, 113. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, H.H.; Kim, T.H.; Lee, B.R. Relationship between Heavy Metal Exposure and Bone Mineral Density in Korean Adult. J. Bone Metab. 2016, 23, 223–231. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Chapman, P.M. Heavy metal-music, not science. Environ. Sci. Technol. 2007, 41, 6C. [Google Scholar] [CrossRef]
- Hubner, R.; Astin, K.B.; Herbert, R.J. ‘Heavy metal’--time to move on from semantics to pragmatics? J. Environ. Monit. 2010, 12, 1511–1514. [Google Scholar] [CrossRef]
- Pourret, O.; Bollinger, J.C. “Heavy metal”-What to do now: To use or not to use? Sci. Total Environ. 2018, 610–611, 419–420. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef]
- Parida, L.; Patel, T.N. Systemic impact of heavy metals and their role in cancer development: A review. Environ. Monit. Assess. 2023, 195, 766. [Google Scholar] [CrossRef]
- Kemnic, T.R.; Coleman, M. Thallium Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Huang, X.Y.; Hu, D.W.; Zhao, F.J. Molybdenum: More than an essential element. J. Exp. Bot. 2022, 73, 1766–1774. [Google Scholar] [CrossRef]
- Novotny, J.A.; Peterson, C.A. Molybdenum. Adv. Nutr. 2018, 9, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, O.; Caceres, H.; Gimenez, L.; Soto, L.; Montenegro, M.; Rueda, J.A.A. Thallium poisoning: A case report. J. Yeungnam Med. Sci. 2023, 40, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, J.; Nishimoto, N. Thallium-poisoner’s poison: An overview and review of current knowledge on the toxicological effects and mechanisms. Curr. Res. Toxicol. 2024, 6, 100157. [Google Scholar] [CrossRef]
- Jalili, C.; Kazemi, M.; Taheri, E.; Mohammadi, H.; Boozari, B.; Hadi, A.; Moradi, S. Exposure to heavy metals and the risk of osteopenia or osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 1671–1682. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium toxicity: A role in bone cell function and teeth development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar] [CrossRef]
- Ciosek, Z.; Kot, K.; Kosik-Bogacka, D.; Lanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of thirty elements on bone metabolism. J. Trace Elem. Med. Biol. 2015, 32, 86–106. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Jt. Surg. Am. 2021, 103, 1451–1461. [Google Scholar] [CrossRef]
- Espinosa-Vellarino, F.L.; Garrido, I.; Ortega, A.; Casimiro, I.; Espinosa, F. Effects of Antimony on Reactive Oxygen and Nitrogen Species (ROS and RNS) and Antioxidant Mechanisms in Tomato Plants. Front. Plant Sci. 2020, 11, 674. [Google Scholar] [CrossRef]
- Elwej, A.; Ghorbel, I.; Chaabane, M.; Soudani, N.; Mnif, H.; Boudawara, T.; Zeghal, N.; Sefi, M. Zinc and selenium modulate barium-induced oxidative stress, cellular injury and membrane-bound ATPase in the cerebellum of adult rats and their offspring during late pregnancy and early postnatal periods. Arch. Physiol. Biochem. 2018, 124, 237–246. [Google Scholar] [CrossRef]
- Wasel, O.; Freeman, J.L. Comparative Assessment of Tungsten Toxicity in the Absence or Presence of Other Metals. Toxics 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Puerto-Parejo, L.M.; Aliaga, I.; Canal-Macias, M.L.; Leal-Hernandez, O.; Roncero-Martin, R.; Rico-Martin, S.; Moran, J.M. Evaluation of the Dietary Intake of Cadmium, Lead and Mercury and Its Relationship with Bone Health among Postmenopausal Women in Spain. Int. J. Environ. Res. Public Health 2017, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.J.; Shen, Z.H.; Duan, S.Y.; Wang, R.; He, P.; Zhang, Z.Y.; Dai, Y.Q.; Li, M.Y.; Chen, Y.; Li, X.Y.; et al. Associations of urinary metal concentrations with anemia: A cross-sectional study of Chinese community-dwelling elderly. Ecotoxiol. Environ. Safe 2024, 15, 115828. [Google Scholar] [CrossRef] [PubMed]
- Zha, B.; Liu, Y.; Xu, H. Associations of mixed urinary metals exposure with metabolic syndrome in the US adult population. Chemosphere 2023, 344, 140330. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Cui, X.; Liu, B.; Liu, C.; Xiao, Y.; Lu, W.; Guo, H.; He, M.; Zhang, X.; Yuan, J.; et al. Association of urinary metal profiles with altered glucose levels and diabetes risk: A population-based study in China. PLoS ONE 2015, 10, e0123742. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, P.; Wang, R.; Luo, C.; Zhang, Z.; Yu, S.; Wu, Q.; Li, Y.; Zhang, Y.; Zhang, H.; et al. Relationships between urinary metals concentrations and cognitive performance among U.S. older people in NHANES 2011–2014. Front. Public Health 2022, 10, 985127. [Google Scholar] [CrossRef]
- Calvetti, D.; Somersalo, E. Computationally Efficient Sampling Methods for Sparsity Promoting Hierarchical Bayesian Models. Siam-Asa J. Uncertain. 2024, 12, 524–548. [Google Scholar] [CrossRef]
- McCoy, D.; Schuler, A.; Hubbard, A.; van der Laan, M. SuperNOVA: Semi-Parametric Identification and Estimation of Interaction and Effect Modification in Mixed Exposures using Stochastic Interventions in R. J. Open Source Softw. 2023, 8, 5422. [Google Scholar] [CrossRef]
- Liu, J.; Mozaffarian, D. Trends in Diet Quality Among U.S. Adults From 1999 to 2020 by Race, Ethnicity, and Socioeconomic Disadvantage. Ann. Intern. Med. 2024, 177, 841–850. [Google Scholar] [CrossRef]
- Vieux, F.; Maillot, M.; Rehm, C.D.; Barrios, P.; Drewnowski, A. Trends in tap and bottled water consumption among children and adults in the United States: Analyses of NHANES 2011-16 data. Nutr. J. 2020, 19, 10. [Google Scholar] [CrossRef]
- Pirkle, J.L. Inorganic and Radiation Analytical Toxicology, Division of Laboratory Sciences. In Metals-Urine Procedure Manual (NHANES); National Center for Environmental Health: Washington, DC, USA, 2019. [Google Scholar]
- Yao, X.; Steven Xu, X.; Yang, Y.; Zhu, Z.; Zhu, Z.; Tao, F.; Yuan, M. Stratification of population in NHANES 2009-2014 based on exposure pattern of lead, cadmium, mercury, and arsenic and their association with cardiovascular, renal and respiratory outcomes. Environ. Int. 2021, 149, 106410. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Force, U.S.P.S.T.; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 2521–2531. [Google Scholar] [CrossRef]
- Ward, L.M.; Weber, D.R.; Munns, C.F.; Hogler, W.; Zemel, B.S. A Contemporary View of the Definition and Diagnosis of Osteoporosis in Children and Adolescents. J. Clin. Endocrinol. Metab. 2020, 105, e2088–e2097. [Google Scholar] [CrossRef]
- Division of the National Health and Nutrition Examination Surveys. The National Health and Nutrition Examination Survey (NHANES) Analytic and Reporting Guidelines; Division of the National Health and Nutrition Examination Surveys, 2018. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 10 August 2024).
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2015, 20, 100–120. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Rustom, S.; Carli, M.; Whitehead, T.P.; Ward, M.H.; Metayer, C. Assessment of Grouped Weighted Quantile Sum Regression for Modeling Chemical Mixtures and Cancer Risk. Int. J. Environ. Res. Public Health 2021, 18, 504. [Google Scholar] [CrossRef]
- Al Abid, F.B. A novel approach for PAM clustering method. Int. J. Comput. Appl. 2014, 86, 0975–8887. [Google Scholar]
- Lengyel, A.; Botta-Dukát, Z. Silhouette width using generalized mean-A flexible method for assessing clustering efficiency. Ecol. Evol. 2019, 9, 13231–13243. [Google Scholar] [CrossRef]
- Baek, S.; Park, S.H.; Won, E.; Park, Y.R.; Kim, H.J. Propensity score matching: A conceptual review for radiology researchers. Korean J. Radiol. 2015, 16, 286–296. [Google Scholar] [CrossRef]
- Van Larebeke, N.; Sioen, I.; Hond, E.D.; Nelen, V.; Van de Mieroop, E.; Nawrot, T.; Bruckers, L.; Schoeters, G.; Baeyens, W. Internal exposure to organochlorine pollutants and cadmium and self-reported health status: A prospective study. Int. J. Hyg. Environ. Health 2015, 218, 232–245. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, P.; Huang, R.; Liang, X.; Wang, P.; Tan, J.; Chen, Z.; Dun, Z.; Wang, J.; Jiang, Q.; et al. Cadmium Exposure and Osteoporosis: A Population-Based Study and Benchmark Dose Estimation in Southern China. J. Bone Miner. Res. 2017, 32, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Richardson, D.H.S. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut. Ser. B Chem. Phys. 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Kinraide, T.B. Improved scales for metal ion softness and toxicity. Environ. Toxicol. Chem. 2009, 28, 525–533. [Google Scholar] [CrossRef]
- Matsushita, M.T.; Xia, Z. Cadmium inhibits calcium activity in hippocampal CA1 neurons of freely moving mice. Toxicol. Sci. 2024, 200, 199–212. [Google Scholar] [CrossRef]
- Chung, S.M. Long-Term Sex-Specific Effects of Cadmium Exposure on Osteoporosis and Bone Density: A 10-Year Community-Based Cohort Study. J. Clin. Med. 2022, 11, 2899. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Mitigation of Cadmium Toxicity through Modulation of the Frontline Cellular Stress Response. Stresses 2022, 2, 355–372. [Google Scholar] [CrossRef]
- Brzoska, M.M.; Rogalska, J.; Kupraszewicz, E. The involvement of oxidative stress in the mechanisms of damaging cadmium action in bone tissue: A study in a rat model of moderate and relatively high human exposure. Toxicol. Appl. Pharmacol. 2011, 250, 327–335. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Lv, Y.J.; Wei, Q.Z.; Zhang, Y.C.; Huang, R.; Li, B.S.; Tan, J.B.; Wang, J.; Ling, H.T.; Wu, S.X.; Yang, X.F. Low-dose cadmium exposure acts on rat mesenchymal stem cells via RANKL/OPG and downregulate osteogenic differentiation genes. Environ. Pollut. 2019, 249, 620–628. [Google Scholar] [CrossRef]
- Khan, Z.; Elahi, A.; Bukhari, D.A.; Rehman, A. Cadmium sources, toxicity, resistance and removal by microorganisms-A potential strategy for cadmium eradication. J. Saudi Chem. Soc. 2022, 26, 101569. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Bruyere, O.; Bergmann, P.; Cavalier, E.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Lapauw, B.; Laurent, M.R.; De Schepper, J.; et al. How to manage osteoporosis before the age of 50. Maturitas 2020, 138, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Kemal, O.; Lu, M.; Lix, L.M.; Leslie, W.D.; Yang, S. Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005-2014. Bone 2020, 131, 115163. [Google Scholar] [CrossRef] [PubMed]
- Shahida, S.; Rehman, S.; Ilyas, N.; Khan, M.I.; Hameed, U.; Hafeez, M.; Iqbal, S.; Elboughdiri, N.; Ghernaout, D.; Salih, A.A.; et al. Determination of Blood Calcium and Lead Concentrations in Osteoporotic and Osteopenic Patients in Pakistan. ACS Omega 2021, 6, 28373–28378. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.L.; Pan, W.H.; Chung, Y.T.; Wu, T.N.; Tseng, Y.C.; Liou, S.H.; Wang, S.L. Association between urinary lead and bone health in a general population from Taiwan. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Manocha, A.; Srivastava, L.M.; Bhargava, S. Lead as a Risk Factor for Osteoporosis in Post-menopausal Women. Indian J. Clin. Biochem. 2017, 32, 261–265. [Google Scholar] [CrossRef]
- Fadhillah, R.; Ilyas, M.; Liem, J.F.; Khoe, L.C.; Mansyur, M. P-351 Correlation Between Serum 25(OH)D and Blood Lead Levels to Delta-Aminolevulinic Acid Dehydratase in Lead-Exposed Workers. Occup. Med. 2024, 74. [Google Scholar] [CrossRef]
- Almasmoum, H.; Refaat, B.; Ghaith, M.M.; Almaimani, R.A.; Idris, S.; Ahmad, J.; Abdelghany, A.H.; BaSalamah, M.A.; El-Boshy, M. Protective effect of Vitamin D3 against lead induced hepatotoxicity, oxidative stress, immunosuppressive and calcium homeostasis disorders in rat. Environ. Toxicol. Pharmacol. 2019, 72, 103246. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Mandalunis, P.M. A Review of Metal Exposure and Its Effects on Bone Health. J. Toxicol. 2018, 2018, 4854152. [Google Scholar] [CrossRef] [PubMed]
- Carmouche, J.J.; Puzas, J.E.; Zhang, X.; Tiyapatanaputi, P.; Cory-Slechta, D.A.; Gelein, R.; Zuscik, M.; Rosier, R.N.; Boyce, B.F.; O’Keefe, R.J.; et al. Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ. Health Perspect. 2005, 113, 749–755. [Google Scholar] [CrossRef]
- Pu, Y.; Sun, H.; Liu, J.; Amantai, D.; Yao, W.X.; Han, X.Z.; He, H.Y. Cobalt Chloride Promotes Osteogenesis of Rat Bone Marrow Mesenchymal Stem Cells and In Vivo. Indian J. Pharm. Sci. 2023, 85, 1–10. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Silina, E.V.; Stupin, V.A.; Zaitsev, O.N.; Sotnikova, T.I.; Tazina, S.I.; Zhang, F.; Guo, X.; Tinkov, A.A. The Role of Trace Elements and Minerals in Osteoporosis: A Review of Epidemiological and Laboratory Findings. Biomolecules 2023, 13, 1006. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, C.W.; Wu, D.W.; Lee, W.H.; Chen, Y.C.; Li, C.H.; Tsai, C.C.; Lin, W.Y.; Chen, S.C.; Hung, C.H.; et al. Association of Heavy Metals with Overall Mortality in a Taiwanese Population. Nutrients 2021, 13, 2070. [Google Scholar] [CrossRef] [PubMed]
- Praveen, A.D.; Aspelund, T.; Ferguson, S.J.; Sigurethsson, S.; Guethnason, V.; Palsson, H.; Matchar, D.; Helgason, B. Refracture and mortality risk in the elderly with osteoporotic fractures: The AGES-Reykjavik study. Osteoporos. Int. 2024, 35, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Thomas, T.; Paccou, J.; Bizouard, G.; Crochard, A.; Toth, E.; Lemaitre, M.; Maurel, F.; Perrin, L.; Tubach, F. Refracture and mortality following hospitalization for severe osteoporotic fractures: The Fractos Study. JBMR Plus 2021, 5, e10507. [Google Scholar] [CrossRef] [PubMed]
- Akerstrom, M.; Barregard, L.; Lundh, T.; Sallsten, G. Variability of urinary cadmium excretion in spot urine samples, first morning voids, and 24 h urine in a healthy non-smoking population: Implications for study design. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 171–179. [Google Scholar] [CrossRef]
- Danziger, J.; Dodge, L.E.; Hu, H.; Mukamal, K.J. Susceptibility to Environmental Heavy Metal Toxicity among Americans with Kidney Disease. Kidney360 2022, 3, 1191–1196. [Google Scholar] [CrossRef]

| Characteristic 2 | Median (25th, 75th) or N (%) 1 |
|---|---|
| Age, years | 43.0 (25.0, 56.0) |
| Sex, N (%) | |
| Female | 7554 (49.38%) |
| Male | 8369 (50.62%) |
| Race, N (%) | |
| White | 6567 (69.17%) |
| Black | 3739 (11.12%) |
| Mexican American | 3190 (7.97%) |
| Other Hispanic | 1202 (5.58%) |
| Other/multiracial | 1225 (6.16%) |
| Family poverty–income ratio (PIR) | 2.93 (1.48, 4.95) |
| Marital status, N (%) | |
| Married/Living with Partner | 6953 (54.46%) |
| Widowed/Divorced/Separated | 2446 (15.56%) |
| Never married | 6524 (29.98%) |
| Urinary Creatinine, mg/dL | 109.00 (61.00, 169.00) |
| Diabetes status, N (%) | |
| Non-diabetes | 9849 (62.37%) |
| Prediabetes | 4232 (27.66%) |
| Diabetes | 1842 (9.97%) |
| General obesity (based on BMI, kg/m2), N (%) | |
| Underweight (<18.5) | 1483 (6.29%) |
| Normal (18.5 to <25) | 5463 (32.83%) |
| Overweight (25 to <30) | 4705 (31.95%) |
| Obese (30 or greater) | 4272 (28.93%) |
| Central obesity, N (%) | 6595 (45.70%) |
| Barium (Ba), ng/mL | 1.42 (0.70, 2.74) |
| Cadmium (Cd), ng/mL | 0.19 (0.09, 0.40) |
| Cobalt (Co), ng/mL | 0.37 (0.22, 0.57) |
| Cesium (Cs), ng/mL | 4.74 (2.77, 7.13) |
| Molybdenum (Mo), ng/mL | 42.90 (22.10, 75.10) |
| Lead (Pb), ng/mL | 0.50 (0.25, 0.88) |
| Antimony (Sb), ng/mL | 0.07 (0.04, 0.12) |
| Thallium (Tl), ng/mL | 0.17 (0.10, 0.26) |
| Tungsten (Tu), ng/mL | 0.07 (0.03, 0.14) |
| Osteoporosis, N (%) | 1683 (12.67%) |
| Urinary Metals | Concentration Ratio | t | p-Value |
|---|---|---|---|
| Ba | 2.44 | 30.77 | <0.001 |
| Cd | 2.20 | 28.18 | <0.001 |
| Co | 2.54 | 22.15 | <0.001 |
| Cs | 2.34 | 42.86 | <0.001 |
| Mo | 2.90 | 81.29 | <0.001 |
| Pb | 2.84 | 48.02 | <0.001 |
| Sb | 2.71 | 35.88 | <0.001 |
| Tl | 2.31 | 76.02 | <0.001 |
| Tu | 3.16 | 21.46 | <0.001 |
| N * | OR | 95%CI | p Value | |
|---|---|---|---|---|
| Model 1 1 | 15,923 | 2.01 | (1.67, 2.41) | <0.001 |
| Model 2 2 | 15,923 | 1.80 | (1.48, 2.19) | <0.001 |
| Model 3 3 | 15,923 | 1.74 | (1.43, 2.12) | <0.001 |
| Sensitivity analysis—PSM 4 | 4850 | 1.99 | (1.59, 2.50) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, K. Disentangling the Relationship Between Urinary Metal Exposure and Osteoporosis Risk Across a Broad Population: A Comprehensive Supervised and Unsupervised Analysis. Toxics 2024, 12, 866. https://doi.org/10.3390/toxics12120866
Liu J, Wang K. Disentangling the Relationship Between Urinary Metal Exposure and Osteoporosis Risk Across a Broad Population: A Comprehensive Supervised and Unsupervised Analysis. Toxics. 2024; 12(12):866. https://doi.org/10.3390/toxics12120866
Chicago/Turabian StyleLiu, Jianing, and Kai Wang. 2024. "Disentangling the Relationship Between Urinary Metal Exposure and Osteoporosis Risk Across a Broad Population: A Comprehensive Supervised and Unsupervised Analysis" Toxics 12, no. 12: 866. https://doi.org/10.3390/toxics12120866
APA StyleLiu, J., & Wang, K. (2024). Disentangling the Relationship Between Urinary Metal Exposure and Osteoporosis Risk Across a Broad Population: A Comprehensive Supervised and Unsupervised Analysis. Toxics, 12(12), 866. https://doi.org/10.3390/toxics12120866






