Respiratory Toxicology of Graphene-Based Nanomaterials: A Review
Abstract
1. Introduction
2. Database Search for Articles on GBNs and Respiratory Systems
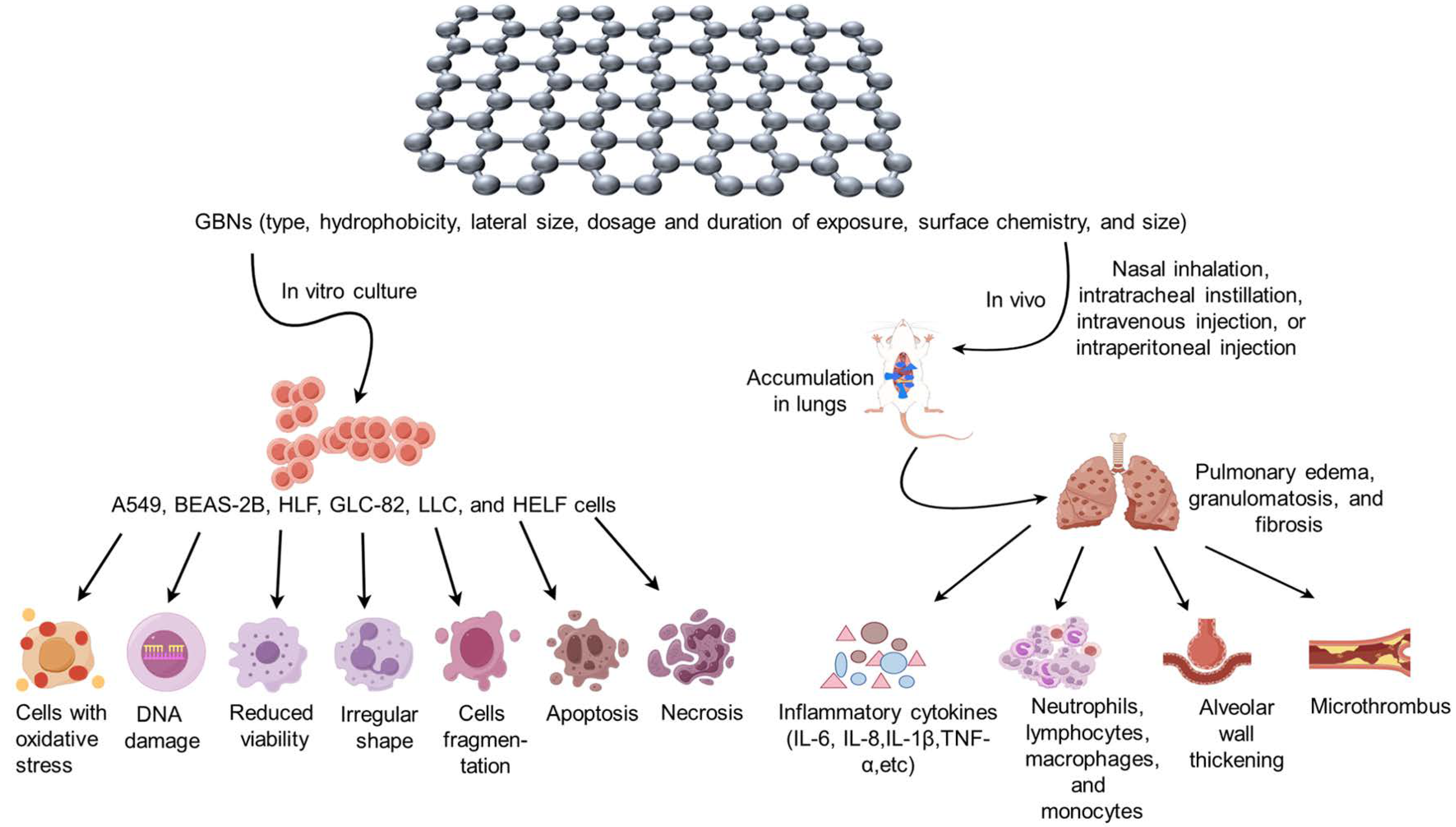
3. Exposure and Metabolism of GBNs
4. Respiratory Toxicity of GBNs
4.1. Graphene and GNS
4.2. GO
4.3. rGO
4.4. Functionalized GBNs
4.4.1. GBN/Inorganic Nanocomposites
4.4.2. GBN/Polymer Composites
| Exposure Model | Type | Lateral Size | Animals/Cells | Exposure Route | Exposure Concentration/Dose | Exposure Duration/Frequency | Time Points Post-Exposure | Toxicity | Adverse Effects | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| In vitro | ||||||||||
| Graphene and GNS | <2 μm | BEAS-2B, A549 cell | -- | 0.05~1000 μg /mL | -- | 6, 24, 48, and 72 h and 7 days | Yes | Reduce cell viability, apoptosis | [18,19,20] | |
| GO | 200~500 nm | HLF cell | -- | 1~100 μg/mL | -- | 2, 4, 12, and 24 h | Yes | ROS production, cellular morphological irregularity, fragmentation or apoptosis | [30] | |
| GO | 1~2 μm | FE1 cell | -- | 5~200 μg/mL | -- | 24 h | No | -- | [31] | |
| rGO | 1~2 μm | FE1 cell | -- | 5~200 μg/mL | -- | 24 h | No | -- | [31] | |
| rGO | 50–700 nm | A549 cell | -- | 1~20 μg/mL | -- | 48 and 72 h | No | -- | [48] | |
| rGO | approximately 900 nm | A549 cell | -- | ≥5 μg/mL | -- | 6 and 24 h | Yes | Cell viability inhibition, apoptosis | [3,49,50] | |
| Gr/NiO NCs, FePt/GO | 4~15 nm | HELF, A549, H460, H1975 and LLC cell | -- | 0~100 μg/mL | -- | 24 h | Yes | No change in the viability of HELF cells, decrease other cancer cell viability and cell death rate | [53,54] | |
| GO/TiO2/DOX | -- | A549 cell | -- | 50~1500 mg | -- | 24, 48, and 96 h | Yes | Suppress the proliferative capacity | [55] | |
| amino-modified GNS | 10 μm | TT1, 16HBE14o-cell | -- | 0~100 μg/mL | -- | 16 and 24 h | No | Reduce inflammation and genotoxicity | [56,57,58] | |
| PEG-GO | 50~150 nm | HLF cell | -- | 1~100 μg/mL | -- | 24 h | Yes | Lower toxicity compared to unmodified GO | [30] | |
| PEI-GO | 200~500 nm | HLF cell | -- | 1~100 μg/mL | -- | 24 h | Yes | DNA damage of HLF cells | [30] | |
| DOX-HA-Q-GS | -- | A549 cell | -- | 1 µg/mL | -- | 48 h | Yes | Apoptosis | [61] | |
| CS-GO, NGO-SS-HA-Gef, PTX-GO | 50~200 nm | A549, NCI-H460 cell | -- | 16~64 µg/mL, 1~400 µg/mL, 10~150 µg/mL | -- | 12, 24, 36, 48, and 72 h | Yes | Inhibitory effect of on the growth and proliferation of cell, apoptosis | [62,63,64] | |
| PEG-rGO, PrGO, PGE/Ag /HRG | 791.37 nm | A549 cell | -- | 1~1000 µg/mL | -- | 24 and 48 h | Yes | Decrease in cell viability, ROS production, decrease percentage of cells in the G0/G1 phase and increase accumulation of cells in the subG1 phase, apoptosis | [3,65,66] | |
| In vivo | ||||||||||
| Graphene | 10~130 nm | Rat | nasal inhalation | 0.68~3.86 mg/m3 | 6 h/day for 5 days | 1, 3, 7, and 28 days | Yes | Slight increase in the number of neutrophils, lymphocytes, and monocytes, the count of white blood cells, and lymphocytes | [22] | |
| Graphene | 1.33~3.26 μm | Mice | intratracheal instillation | 50 μg | Single administration | 1, 7, and 42 days | No | -- | [21] | |
| GNS | 123 nm | Rat | nasal inhalation | 0.12~1.88 mg/m3 | 6 h/day for 5 days | 1, 28, and 90 days | No | -- | [13] | |
| GNS | 2~7 μm | Rat | nasal inhalation | 0.2~3.2 mg/m3 | 6 h/day for 20 days | 1 and 29 days | No | -- | [23] | |
| GNS | 2 μm | Rat | intratracheal instillation | 0.3, 1, 3 mg | Single administration | 1, 7, and 28 days | Yes | Increase the number of neutrophils, acute multifocal alveolitis | [24] | |
| GNS | <4 μm | Mice | intratracheal instillation | 50 μg | Single administration | 1 and 7 days | Yes | Inflammatory cytokines, inflammatory edema, and granulomas | [25] | |
| GNS | <2 μm, 5 μm, 20 μm | Mice | intratracheal instillation | 40 μg | Single administration | 4 h, 1, 7, 28, and 60 days | Yes | Increase the number of neutrophils, increase activity levels of MCSF, MPO, and LDH | [27] | |
| GNS | Approximately 500 nm | Mice | intratracheal instillation | 25~100 μg/mice | Single administration | 90 days | Yes | Alterations in the lung gene profile and the expression level of Th1/Th2 cytokines | [28] | |
| GO | 0.5~5 μm | Rat | intranasal inhalation | 0.60~10 mg/m3 | 6 h/day for 5 days | 1, 3, and 21 days | No | -- | [32] | |
| GO | 0.1~10 μm | Mice, rat | intratracheal instillation, tail intravenous injection, intraperitoneal injection | 0.05~500 mg/kg | Once a day for 7 days | 2, 5, 10, 15, 30, and 180 min; 1, 7, 14, 28, 30, and 56 days | Yes | Neutrophils infiltration, increased pulmonary capillary permeability, microthrombosis, alveolar wall thickening, epithelioid granulomas, pulmonary edema, and peribronchiolar collagen deposition | [16,33,34,35,36,37,38,39] | |
| GO | -- | Mice | intraperitoneal injection | 0.04~4 mg/kg | 32 days | 8 days | Yes | Deterioration of airway hyperresponsiveness, collagen deposition, elevated IL-4, and decreased IFN-γ | [46] | |
| GO | -- | Mice | intratracheal instillation | 80 μg | Single administration | 31 days | Yes | Promoted airway hyperresponsiveness, airway remodeling, subepithelial fibrosis reducing eosinophilic inflammation in favor of the emergence of monocytes/macrophages, reduced OVA-induced AMCase enzymatic activity in the BALF | [47] | |
| GO | -- | Mice | intratracheal instillation | 40 μg/day | Once a day for 2 days | 2 days | Yes | Facilitated transient neutrophil accumulation in BALF, reduced OVA-induced AMCase enzymatic activity in the BALF | [47] | |
| rGO | 1~2 μm | Mice | intratracheal instillation | 18~162 μg/mice | Single administration | 1, 3, 28, and 90 days | Yes | Neutrophils inflammatory response, DNA damage | [9] | |
| COOH-/N-H-/N = H -modified GNS | 2 μm | Rat | intratracheal instillation | 1 mg/rat | Single administration | 1, 7, and 28 days | Yes | LDH and total protein elevation, neutrophilic inflammation | [24] | |
| PSS/PEG-modified GO | 500 nm; 314 nm | Mice | tail intravenous injection | 0~16 mg/kg | Single administration | 1, 7, 14, 28, 30, 90, and 180 days | Yes | Chronic inflammation with macrophage infiltration | [59,60] | |
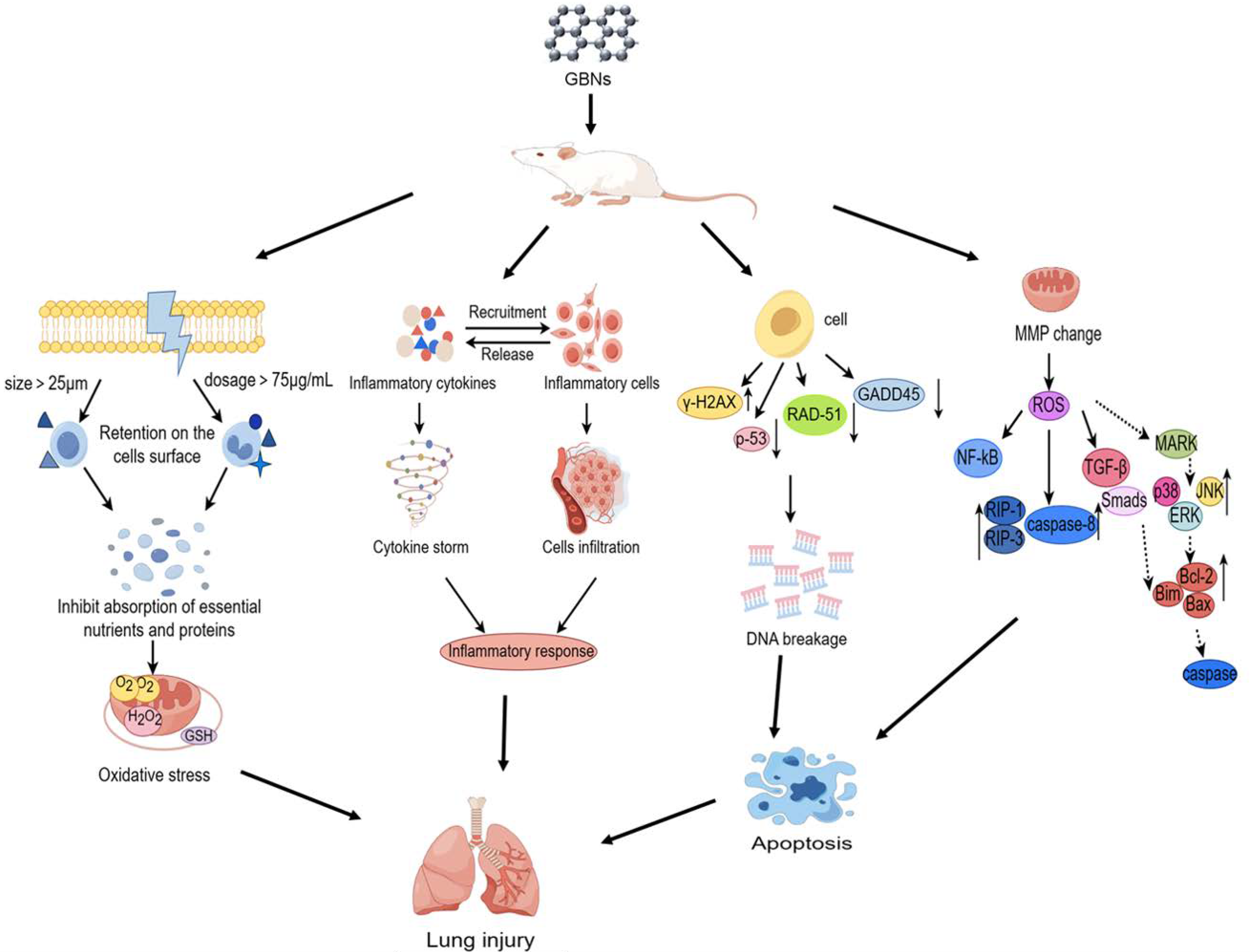
5. Mechanisms of GBN-Induced Respiratory Toxicity
5.1. Oxidative Stress
5.2. Inflammatory Response
5.3. Genotoxicity and Epigenetics
5.4. Apoptosis
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulzar, A.; Yang, P.; He, F.; Xu, J.; Yang, D.; Xu, L.; Jan, M.O. Bioapplications of graphene constructed functional nanomaterials. Chem.-Biol. Interact. 2017, 262, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.; Rivers-Auty, J.; Lemarchand, E.; Vranic, S.; Wang, E.; Buggio, M.; Rothwell, N.J.; Allan, S.M.; Kostarelos, K.; Brough, D. Small, Thin Graphene Oxide Is Anti-inflammatory Activating Nuclear Factor Erythroid 2-Related Factor 2 via Metabolic Reprogramming. ACS Nano 2018, 12, 11949–11962. [Google Scholar] [CrossRef]
- Reshma, S.C.; Syama, S.; Mohanan, P.V. Nano-biointeractions of PEGylated and bare reduced graphene oxide on lung alveolar epithelial cells: A comparative in vitro study. Colloids Surf. B Biointerfaces 2016, 140, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Gamo, M.; Honda, K. A review of toxicity studies on graphene-based nanomaterials in laboratory animals. Regul. Toxicol. Pharmacol. RTP 2017, 85, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ménard-Moyon, C.; Delogu, L.G.; Bianco, A. Graphene and the immune system: Challenges and potentiality. Adv. Drug Deliv. Rev. 2016, 105, 163–175. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, X.; Lynch, I.; Cui, F. Comparative evaluation of the mechanisms of toxicity of graphene oxide and graphene oxide quantum dots to blue-green algae Microcystis aeruginosa in the aquatic environment. J. Hazard. Mater. 2022, 425, 127898. [Google Scholar] [CrossRef]
- Liu, W.; Sun, C.; Liao, C.; Cui, L.; Li, H.; Qu, G.; Yu, W.; Song, N.; Cui, Y.; Wang, Z.; et al. Graphene Enhances Cellular Proliferation through Activating the Epidermal Growth Factor Receptor. J. Agric. Food Chem. 2016, 64, 5909–5918. [Google Scholar] [CrossRef]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of Nanomaterials: Exposure, Pathways, Assessment, and Recent Advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef]
- Bengtson, S.; Knudsen, K.B.; Kyjovska, Z.O.; Berthing, T.; Skaug, V.; Levin, M.; Koponen, I.K.; Shivayogimath, A.; Booth, T.J.; Alonso, B.; et al. Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide. PLoS ONE 2017, 12, e0178355. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, J.H.; Kim, J.H.; Kim, B.; Bello, D.; Kim, J.K.; Lee, G.H.; Sohn, E.K.; Lee, K.; Ahn, K.; et al. Exposure monitoring of graphene nanoplatelets manufacturing workplaces. Inhal. Toxicol. 2016, 28, 281–291. [Google Scholar] [CrossRef]
- Boccuni, F.; Ferrante, R.; Tombolini, F.; Lega, D.; Antonini, A.; Alvino, A.; Pingue, P.; Beltram, F.; Sorba, L.; Piazza, V.; et al. Workers’ Exposure to Nano-Objects with Different Dimensionalities in R&D Laboratories: Measurement Strategy and Field Studies. Int. J. Mol. Sci. 2018, 19, 349. [Google Scholar] [CrossRef] [PubMed]
- Heitbrink, W.A.; Lo, L.M.; Dunn, K.H. Exposure controls for nanomaterials at three manufacturing sites. J. Occup. Environ. Hyg. 2015, 12, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shin, J.H.; Lee, J.S.; Hwang, J.H.; Lee, J.H.; Baek, J.E.; Kim, T.G.; Kim, B.W.; Kim, J.S.; Lee, G.H.; et al. 28-Day inhalation toxicity of graphene nanoplatelets in Sprague-Dawley rats. Nanotoxicology 2016, 10, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Loret, T.; de Luna, L.A.V.; Lucherelli, M.A.; Fordham, A.; Lozano, N.; Bianco, A.; Kostarelos, K.; Bussy, C. Lung Persistence, Biodegradation, and Elimination of Graphene-Based Materials are Predominantly Size-Dependent and Mediated by Alveolar Phagocytes. Small 2023, 19, e2301201. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Bahadur, P.; Tiwari, S. Dispersed graphene materials of biomedical interest and their toxicological consequences. Adv. Colloid Interface Sci. 2020, 275, 102051. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.F.; Fojtů, M.; Fisher, A.C.; Pumera, M. Graphene Oxide Nanoplatelets Potentiate Anticancer Effect of Cisplatin in Human Lung Cancer Cells. Langmuir ACS J. Surf. Colloids 2019, 35, 3176–3182. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, G.H.; Han, B.S.; Lee, B.S.; Lee, S.; Cho, M.H.; Kim, J.H.; Kim, D.W. Toxic response of graphene nanoplatelets in vivo and in vitro. Arch. Toxicol. 2015, 89, 1557–1568. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Azari, M.R.; Rasoulzadeh, Y.; Mohammadian, Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol. Ind. Health 2019, 35, 79–87. [Google Scholar] [CrossRef]
- Frontiñan-Rubio, J.; González, V.J.; Vázquez, E.; Durán-Prado, M. Rapid and efficient testing of the toxicity of graphene-related materials in primary human lung cells. Sci. Rep. 2022, 12, 7664. [Google Scholar] [CrossRef]
- Schinwald, A.; Murphy, F.; Askounis, A.; Koutsos, V.; Sefiane, K.; Donaldson, K.; Campbell, C.J. Minimal oxidation and inflammogenicity of pristine graphene with residence in the lung. Nanotoxicology 2014, 8, 824–832. [Google Scholar] [CrossRef]
- Shin, J.H.; Han, S.G.; Kim, J.K.; Kim, B.W.; Hwang, J.H.; Lee, J.S.; Lee, J.H.; Baek, J.E.; Kim, T.G.; Kim, K.S.; et al. 5-Day repeated inhalation and 28-day post-exposure study of graphene. Nanotoxicology 2015, 9, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Creutzenberg, O.; Oliveira, H.; Farcal, L.; Schaudien, D.; Mendes, A.; Menezes, A.C.; Tischler, T.; Burla, S.; Ziemann, C. PLATOX: Integrated In Vitro/In Vivo Approach for Screening of Adverse Lung Effects of Graphene-Related 2D Nanomaterials. Nanomaterials 2022, 12, 1254. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeong, A.Y.; Bae, J.; Seok, J.H.; Yang, J.Y.; Roh, H.S.; Jeong, J.; Han, Y.; Jeong, J.; Cho, W.S. The role of surface functionalization on the pulmonary inflammogenicity and translocation into mediastinal lymph nodes of graphene nanoplatelets in rats. Arch. Toxicol. 2017, 91, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Schinwald, A.; Murphy, F.A.; Jones, A.; MacNee, W.; Donaldson, K. Graphene-based nanoplatelets: A new risk to the respiratory system as a consequence of their unusual aerodynamic properties. ACS Nano 2012, 6, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Hu, M.; Pan, B.; Xie, Y.; Petersen, E.J. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part. Fibre Toxicol. 2016, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.R.; Mercer, R.R.; Stefaniak, A.B.; Seehra, M.S.; Geddam, U.K.; Chaudhuri, I.S.; Kyrlidis, A.; Kodali, V.K.; Sager, T.; Kenyon, A.; et al. Evaluation of pulmonary and systemic toxicity following lung exposure to graphite nanoplates: A member of the graphene-based nanomaterial family. Part. Fibre Toxicol. 2016, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lee, S.J.; Lee, K.; Choi, Y.C.; Lee, B.S.; Lee, G.H.; Kim, D.W. Pulmonary persistence of graphene nanoplatelets may disturb physiological and immunological homeostasis. J. Appl. Toxicol. JAT 2017, 37, 296–309. [Google Scholar] [CrossRef]
- Chng, E.L.; Pumera, M. The toxicity of graphene oxides: Dependence on the oxidative methods used. Chemistry 2013, 19, 8227–8235. [Google Scholar] [CrossRef]
- Wang, A.; Pu, K.; Dong, B.; Liu, Y.; Zhang, L.; Zhang, Z.; Duan, W.; Zhu, Y. Role of surface charge and oxidative stress in cytotoxicity and genotoxicity of graphene oxide towards human lung fibroblast cells. J. Appl. Toxicol. JAT 2013, 33, 1156–1164. [Google Scholar] [CrossRef]
- Bengtson, S.; Kling, K.; Madsen, A.M.; Noergaard, A.W.; Jacobsen, N.R.; Clausen, P.A.; Alonso, B.; Pesquera, A.; Zurutuza, A.; Ramos, R.; et al. No cytotoxicity or genotoxicity of graphene and graphene oxide in murine lung epithelial FE1 cells in vitro. Environ. Mol. Mutagen. 2016, 57, 469–482. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jo, M.S.; Kim, J.K.; Shin, J.H.; Baek, J.E.; Park, H.S.; An, H.J.; Lee, J.S.; Kim, B.W.; Kim, H.P.; et al. Short-term inhalation study of graphene oxide nanoplates. Nanotoxicology 2018, 12, 224–238. [Google Scholar] [CrossRef]
- Liu, J.H.; Yang, S.T.; Wang, H.; Chang, Y.; Cao, A.; Liu, Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine 2012, 7, 1801–1812. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, S.; Zhang, H.; Qiu, M.; Dai, Y.; Wang, S.; Wang, Y.; Ou, J. Graphene oxide induces dose-dependent lung injury in rats by regulating autophagy. Exp. Ther. Med. 2021, 21, 462. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Tu, L.; Chen, D.; Luo, Z.; Liu, D.; Miao, Z.; Feng, G.; Qing, L.; Wang, S. Sub-Acute Toxicity Study of Graphene Oxide in the Sprague-Dawley Rat. Int. J. Environ. Res. Public Health 2016, 13, 1149. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef]
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769. [Google Scholar] [CrossRef]
- El-Yamany, N.A.; Mohamed, F.F.; Salaheldin, T.A.; Tohamy, A.A.; Abd El-Mohsen, W.N.; Amin, A.S. Graphene oxide nanosheets induced genotoxicity and pulmonary injury in mice. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2017, 69, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, M.K.; Nayak, M.K.; Kumari, S.; Shrivastava, S.; Grácio, J.J.; Dash, D. Thrombus inducing property of atomically thin graphene oxide sheets. ACS Nano 2011, 5, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Huang, Q.; Zhang, Y.; Peng, C.; Zhang, Y.; He, Y.; Shi, J.; Li, W.; Hu, J.; et al. Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice. NPG Asia Mater 2013, 5, e44. [Google Scholar]
- Taheriazam, A.; Abad, G.G.Y.; Hajimazdarany, S.; Imani, M.H.; Ziaolhagh, S.; Zandieh, M.A.; Bayanzadeh, S.D.; Mirzaei, S.; Hamblin, M.R.; Entezari, M.; et al. Graphene oxide nanoarchitectures in cancer biology: Nano-modulators of autophagy and apoptosis. J. Control. Release Off. J. Control. Release Soc. 2023, 354, 503–522. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Newman, L.; Jasim, D.; Mukherjee, S.P.; Wang, J.; Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A.; Fadeel, B.; Kostarelos, K.; et al. Size-Dependent Pulmonary Impact of Thin Graphene Oxide Sheets in Mice: Toward Safe-by-Design. Adv. Sci. 2020, 7, 1903200. [Google Scholar] [CrossRef]
- Wang, X.; Duch, M.C.; Mansukhani, N.; Ji, Z.; Liao, Y.P.; Wang, M.; Zhang, H.; Sun, B.; Chang, C.H.; Li, R.; et al. Use of a pro-fibrogenic mechanism-based predictive toxicological approach for tiered testing and decision analysis of carbonaceous nanomaterials. ACS Nano 2015, 9, 3032–3043. [Google Scholar] [CrossRef]
- Loret, T.; de Luna, L.A.V.; Fordham, A.; Arshad, A.; Barr, K.; Lozano, N.; Kostarelos, K.; Bussy, C. Innate but Not Adaptive Immunity Regulates Lung Recovery from Chronic Exposure to Graphene Oxide Nanosheets. Adv. Sci. 2022, 9, e2104559. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial Role of Lateral Size for Graphene Oxide in Activating Macrophages and Stimulating Pro-inflammatory Responses in Cells and Animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef]
- Shang, S.; Li, J.; Zhao, Y.; Xi, Z.; Lu, Z.; Li, B.; Yang, X.; Li, R. Oxidized graphene-aggravated allergic asthma is antagonized by antioxidant vitamin E in Balb/c mice. Environ. Sci. Pollut. Res. Int. 2017, 24, 1784–1793. [Google Scholar] [CrossRef]
- Shurin, M.R.; Yanamala, N.; Kisin, E.R.; Tkach, A.V.; Shurin, G.V.; Murray, A.R.; Leonard, H.D.; Reynolds, J.S.; Gutkin, D.W.; Star, A.; et al. Graphene oxide attenuates Th2-type immune responses, but augments airway remodeling and hyperresponsiveness in a murine model of asthma. ACS Nano 2014, 8, 5585–5599. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, W.; Huang, X.; Sun, Y.; Tian, S.; Cai, P. Reduced graphene oxide triggered epithelial-mesenchymal transition in A549 cells. Sci. Rep. 2018, 8, 15188. [Google Scholar] [CrossRef]
- Di Ianni, E.; Møller, P.; Vogel, U.B.; Jacobsen, N.R. Pro-inflammatory response and genotoxicity caused by clay and graphene nanomaterials in A549 and THP-1 cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 872, 503405. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Pranjol, M.Z.I.; Hayat, H.; Rahat, A.A.M.; Abdullah, T.M.; Whatmore, J.L.; Zhang, S. In vitro toxic effects of reduced graphene oxide nanosheets on lung cancer cells. Nanotechnology 2017, 28, 504001. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Saeed, Z.; Ahmad, A.; Pervaiz, M.; Younas, U.; Mahmood Khan, R.R.; Luque, R.; Rajendran, S. Green synthesis of graphene-based metal nanocomposite for electro and photocatalytic activity; recent advancement and future prospective. Chemosphere 2023, 311, 136982. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Kim, J.H. Differential Immunomodulatory Effect of Graphene Oxide and Vanillin-Functionalized Graphene Oxide Nanoparticles in Human Acute Monocytic Leukemia Cell Line (THP-1). Int. J. Mol. Sci. 2019, 20, 247. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Maruthupandy, M.; Quero, F.; Li, W.J. Graphene/nickel oxide nanocomposites against isolated ESBL producing bacteria and A549 cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 829–843. [Google Scholar] [CrossRef]
- Ma, S.; Miao, H.; Luo, Y.; Sun, Y.; Tian, X.; Wang, F.; You, C.; Peng, S.; Tang, G.; Yang, C.; et al. FePt/GO Nanosheets Suppress Proliferation, Enhance Radiosensitization and Induce Autophagy of Human Non-Small Cell Lung Cancer Cells. Int. J. Biol. Sci. 2019, 15, 999–1009. [Google Scholar] [CrossRef]
- Samadi, S.; Moradkhani, M.; Beheshti, H.; Irani, M.; Aliabadi, M. Fabrication of chitosan/poly(lactic acid)/graphene oxide/TiO2 composite nanofibrous scaffolds for sustained delivery of doxorubicin and treatment of lung cancer. Int. J. Biol. Macromol. 2018, 110, 416–424. [Google Scholar] [CrossRef]
- Burgum, M.J.; Clift, M.J.D.; Evans, S.J.; Hondow, N.; Tarat, A.; Jenkins, G.J.; Doak, S.H. Few-layer graphene induces both primary and secondary genotoxicity in epithelial barrier models in vitro. J. Nanobiotechnol. 2021, 19, 24. [Google Scholar] [CrossRef]
- Burgum, M.J.; Clift, M.J.D.; Evans, S.J.; Hondow, N.; Miller, M.; Lopez, S.B.; Williams, A.; Tarat, A.; Jenkins, G.J.; Doak, S.H. In Vitro Primary-Indirect Genotoxicity in Bronchial Epithelial Cells Promoted by Industrially Relevant Few-Layer Graphene. Small 2021, 17, e2002551. [Google Scholar] [CrossRef]
- Chatterjee, N.; Yang, J.; Choi, J. Differential genotoxic and epigenotoxic effects of graphene family nanomaterials (GFNs) in human bronchial epithelial cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 1–10. [Google Scholar] [CrossRef]
- Wen, K.P.; Chen, Y.C.; Chuang, C.H.; Chang, H.Y.; Lee, C.Y.; Tai, N.H. Accumulation and toxicity of intravenously-injected functionalized graphene oxide in mice. J. Appl. Toxicol. JAT 2015, 35, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.Y.; Yang, J.Z.; Zhang, Y.J.; Li, W.X.; Fan, C.H.; Huang, Q. Influence of polyethylene glycol coating on biodistribution and toxicity of nanoscale graphene oxide in mice after intravenous injection. Int. J. Nanomed. 2014, 9, 4697–4707. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, X.; Li, H.; Lin, Y.; Du, D. Hyaluronic Acid-Modified Multifunctional Q-Graphene for Targeted Killing of Drug-Resistant Lung Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 4048–4055. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Lian, S.; Zheng, J.; Li, B.; Li, T.; Jia, L. Redox-responsive hyaluronic acid-functionalized graphene oxide nanosheets for targeted delivery of water-insoluble cancer drugs. Int. J. Nanomed. 2018, 13, 7457–7472. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Li, Y.; Wang, M.; Shi, P.; Huang, X. Covalent functionalization of graphene oxide with biocompatible poly(ethylene glycol) for delivery of paclitaxel. ACS Appl. Mater. Interfaces 2014, 6, 17268–17276. [Google Scholar] [CrossRef]
- Arya, N.; Arora, A.; Vasu, K.S.; Sood, A.K.; Katti, D.S. Combination of single walled carbon nanotubes/graphene oxide with paclitaxel: A reactive oxygen species mediated synergism for treatment of lung cancer. Nanoscale 2013, 5, 2818–2829. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Chen, Y.; Fang, Z. In-vitro photothermal therapy using plant extract polyphenols functionalized graphene sheets for treatment of lung cancer. J. Photochem. Photobiol. B Biol. 2020, 204, 111587. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Al-Marri, A.H.; Al-Warthan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.; Nayak, V.L.; Kamal, A.; Adil, S.F. Apoptosis inducing ability of silver decorated highly reduced graphene oxide nanocomposites in A549 lung cancer. Int. J. Nanomed. 2016, 11, 873–883. [Google Scholar] [CrossRef]
- Poulsen, S.S.; Bengtson, S.; Williams, A.; Jacobsen, N.R.; Troelsen, J.T.; Halappanavar, S.; Vogel, U. A transcriptomic overview of lung and liver changes one day after pulmonary exposure to graphene and graphene oxide. Toxicol. Appl. Pharmacol. 2021, 410, 115343. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Rogers, L.K.; Cismowski, M.J. Oxidative Stress in the Lung—The Essential Paradox. Curr. Opin. Toxicol. 2018, 7, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Kumar, V.; Dhiman, N.; Chauhan, L.K.; Pasricha, R.; Pandey, A.K. Physico-chemical properties based differential toxicity of graphene oxide/reduced graphene oxide in human lung cells mediated through oxidative stress. Sci. Rep. 2016, 6, 39548. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alaizeri, Z.M.; Alhadlaq, H.A. Evaluation of the Cytotoxicity and Oxidative Stress Response of CeO2-RGO Nanocomposites in Human Lung Epithelial A549 Cells. Nanomaterials 2019, 9, 1709. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.; Koyakutty, M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Tan, X.; Peng, R.; Liu, Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small 2013, 9, 1492–1503. [Google Scholar] [CrossRef]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukhani, N.D.; Ji, Z.; Wang, X.; Liao, Y.P.; Jiang, W.; Sun, B.; Hersam, M.C.; et al. Surface Oxidation of Graphene Oxide Determines Membrane Damage, Lipid Peroxidation, and Cytotoxicity in Macrophages in a Pulmonary Toxicity Model. ACS Nano 2018, 12, 1390–1402. [Google Scholar] [CrossRef]
- Duch, M.C.; Budinger, G.R.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.E.; Campochiaro, L.A.; Gonzalez, A.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett. 2011, 11, 5201–5207. [Google Scholar] [CrossRef]
- Ma-Hock, L.; Strauss, V.; Treumann, S.; Küttler, K.; Wohlleben, W.; Hofmann, T.; Gröters, S.; Wiench, K.; van Ravenzwaay, B.; Landsiedel, R. Comparative inhalation toxicity of multi-wall carbon nanotubes, graphene, graphite nanoplatelets and low surface carbon black. Part. Fibre Toxicol. 2013, 10, 23. [Google Scholar] [CrossRef]
- Zelcer, N.; Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Lebre, F.; Boland, J.B.; Gouveia, P.; Gorman, A.L.; Lundahl, M.L.E.; Lynch, R.I.; O’Brien, F.J.; Coleman, J.; Lavelle, E.C. Pristine graphene induces innate immune training. Nanoscale 2020, 12, 11192–11200. [Google Scholar] [CrossRef]
- Panier, S.; Wang, S.; Schumacher, B. Genome Instability and DNA Repair in Somatic and Reproductive Aging. Annu. Rev. Pathol. 2024, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Wu, J.; Wang, Y.; Yang, X.; Yang, R.; Wang, B.; Yang, J.; Zhang, N. Graphene oxide can induce in vitro and in vivo mutagenesis. Sci. Rep. 2013, 3, 3469. [Google Scholar] [CrossRef]
- Peng, S.; Sun, Y.; Luo, Y.; Ma, S.; Sun, W.; Tang, G.; Li, S.; Zhang, N.; Ren, J.; Xiao, Y.; et al. MFP-FePt-GO Nanocomposites Promote Radiosensitivity of Non-Small Cell Lung Cancer Via Activating Mitochondrial-Mediated Apoptosis and Impairing DNA Damage Repair. Int. J. Biol. Sci. 2020, 16, 2145–2158. [Google Scholar] [CrossRef]
- Pérez, R.F.; Soto Fernández, A.Y.; Bousquets Muñoz, P.; Sierra, M.I.; Tejedor, J.R.; Morales-Sánchez, P.; Valdés, A.F.; Santamaría, R.; Blanco, C.; Torrecillas, R.; et al. No genome-wide DNA methylation changes found associated with medium-term reduced graphene oxide exposure in human lung epithelial cells. Epigenetics 2020, 15, 283–293. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Zhao, Y.; Bai, Y.; Chen, P.; Xia, T.; Wang, D. Response of microRNAs to in vitro treatment with graphene oxide. ACS Nano 2014, 8, 2100–2110. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, L.; Wang, L.L.; Zheng, P.D.; Zhang, F.Q.; Mao, Z.Y.; Zhang, H.J.; Liu, H.G. Programmed Cell Death in Asthma: Apoptosis, Autophagy, Pyroptosis, Ferroptosis, and Necroptosis. J. Inflamm. Res. 2023, 16, 2727–2754. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Fu, Y.; Wei, T.; Le Guyader, L.; Gao, G.; Liu, R.S.; Chang, Y.Z.; Chen, C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 2012, 33, 402–411. [Google Scholar] [CrossRef]
- Wu, A.Q.; Tian, X. Effects of Graphene Oxide on Cell Cycle and Apoptosis of Human Bronchial Epithelial Cells in Vitro. Asian J. Ecotoxicol. 2022, 17, 265–271. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, C.; Chen, J.; Li, P.; Wu, Y.; Zhang, G.; Sang, B.; Li, R.; Shi, Y.; Cui, X.; Zhou, T. Respiratory Toxicology of Graphene-Based Nanomaterials: A Review. Toxics 2024, 12, 82. https://doi.org/10.3390/toxics12010082
Kong C, Chen J, Li P, Wu Y, Zhang G, Sang B, Li R, Shi Y, Cui X, Zhou T. Respiratory Toxicology of Graphene-Based Nanomaterials: A Review. Toxics. 2024; 12(1):82. https://doi.org/10.3390/toxics12010082
Chicago/Turabian StyleKong, Chunxue, Junwen Chen, Ping Li, Yukang Wu, Guowei Zhang, Bimin Sang, Rui Li, Yuqin Shi, Xiuqing Cui, and Ting Zhou. 2024. "Respiratory Toxicology of Graphene-Based Nanomaterials: A Review" Toxics 12, no. 1: 82. https://doi.org/10.3390/toxics12010082
APA StyleKong, C., Chen, J., Li, P., Wu, Y., Zhang, G., Sang, B., Li, R., Shi, Y., Cui, X., & Zhou, T. (2024). Respiratory Toxicology of Graphene-Based Nanomaterials: A Review. Toxics, 12(1), 82. https://doi.org/10.3390/toxics12010082






