The Roles of IL-18 in a Realistic Partial Body Irradiation with 5% Bone Marrow Sparing (PBI/BM5) Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mice and Animal Care
2.3. Irradiation
2.4. IL-18BP Treatment
2.5. Tissue Collection, Peripheral Blood Cell Counts and Clonogenicity Assay
2.6. Multi-Plex Cytokine Assay
2.7. IL-18 and IL-18BP ELISA Assays
2.8. Statistical Analysis
3. Results
3.1. Radiation Dose-Response Relationship (DRR) after PBI/BM5
3.2. IL-18 Knockout Mice Are Significantly More Radiation Resistant Compared to the Wild-Type Mice after 14.73 Gy PBI/BM5
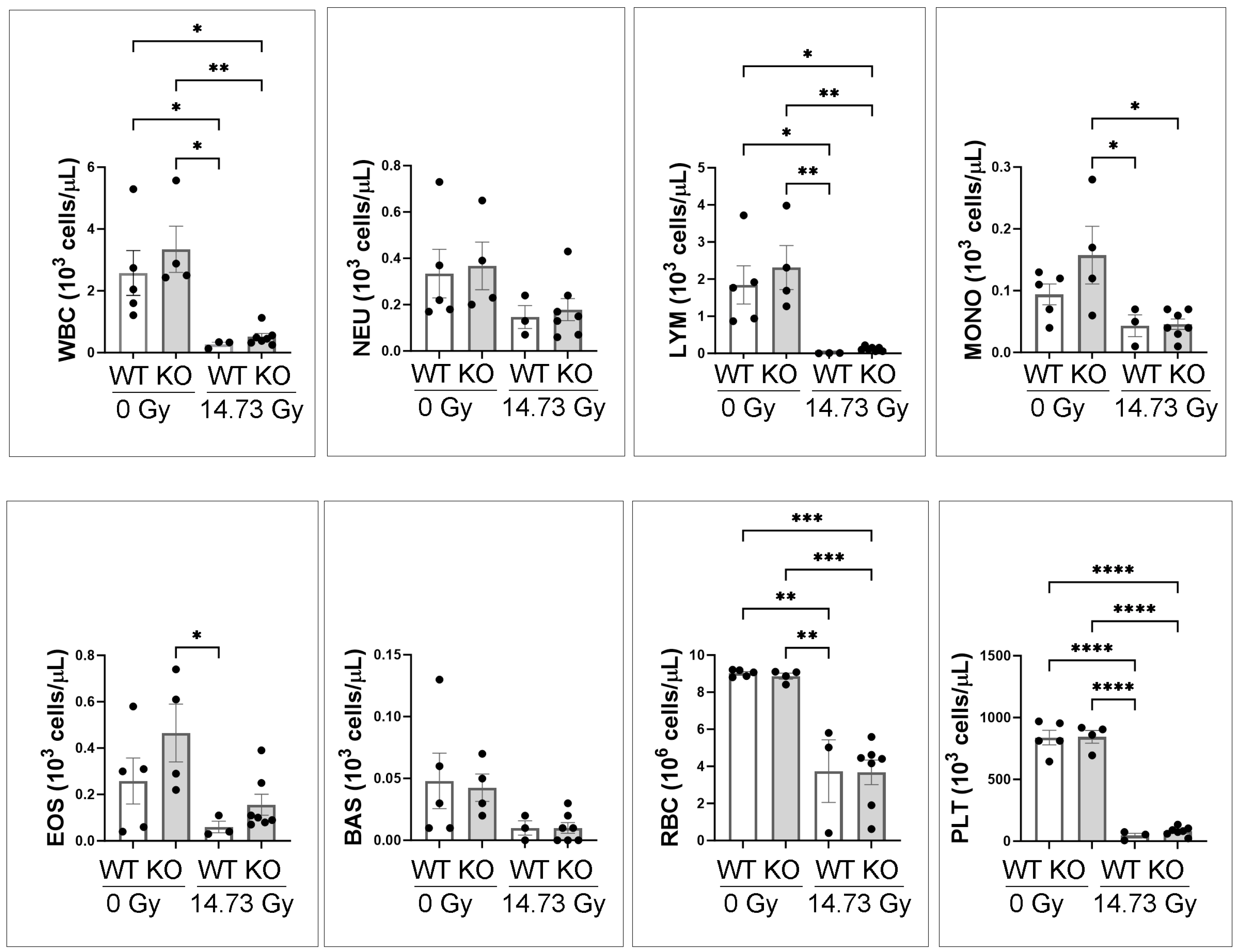
3.3. Complete Blood Counts (CBC) of 0 Gy and d14 14.73 Gy in WT and IL-18 KO Mice
3.4. Bone Marrow Colony Forming Assay of 0 Gy and d14 14.73 Gy in WT and IL-18 KO Mice
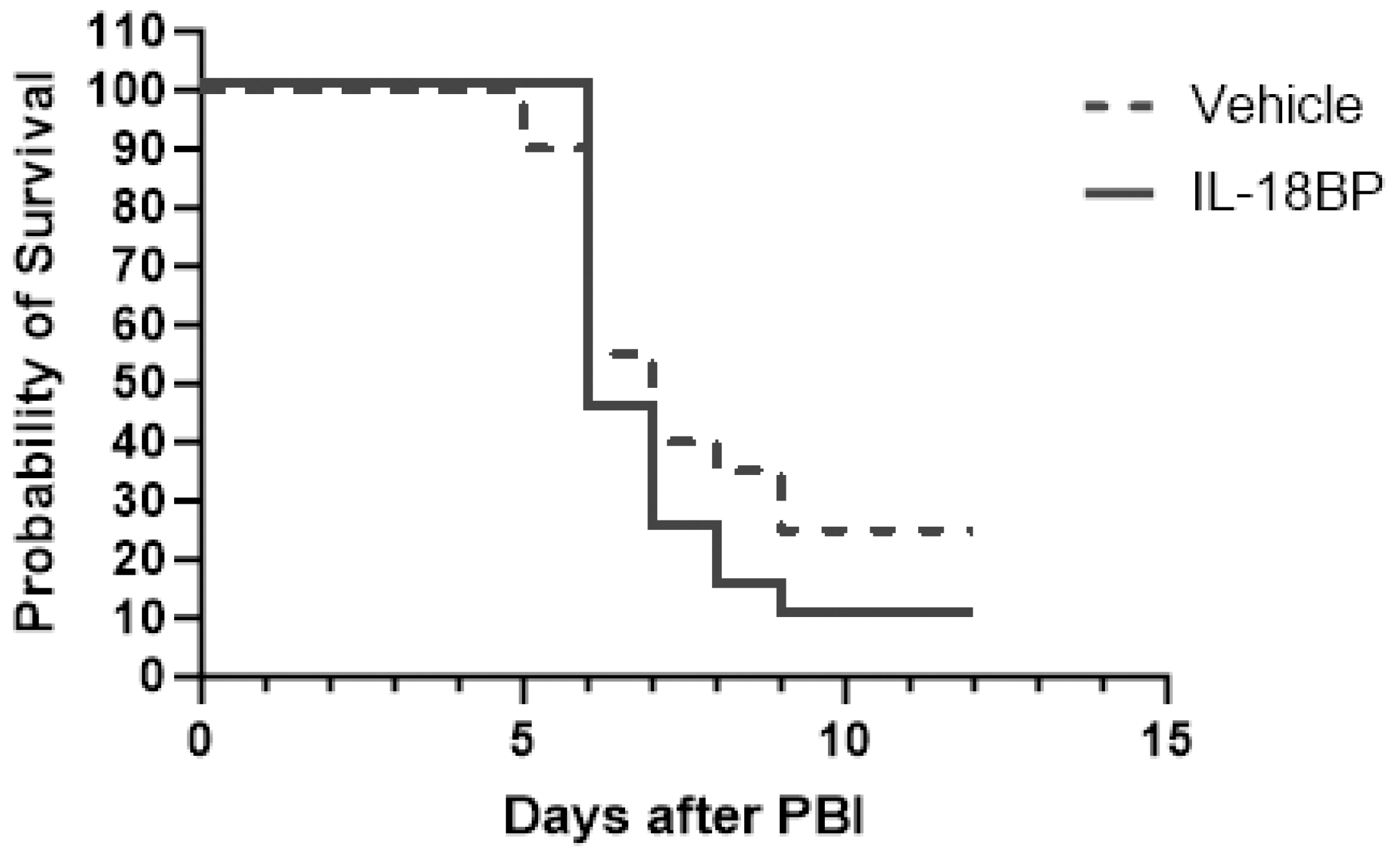
3.5. IL-18BP at 2.0 mg/kg Failed to Rescue C57BL6 Wild-Type Mice after 14.61 Gy PBI/BM5
3.6. Serum Cytokine Changes after PBI/BM5 Using a 45-Plex Assay
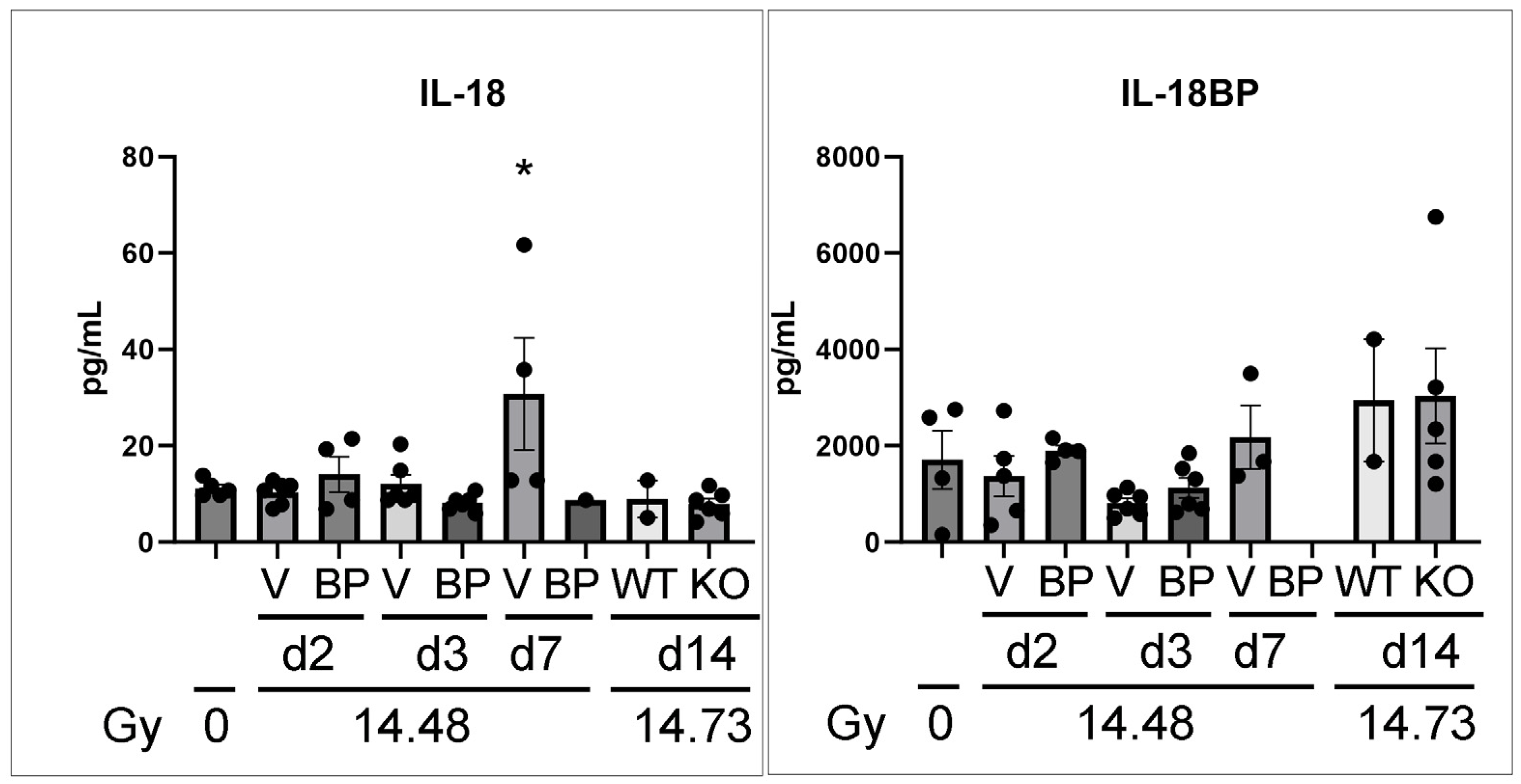
3.7. The Serum Levels of IL-18 and IL-18BP in C57BL6 Female Mice after PBI/BM5
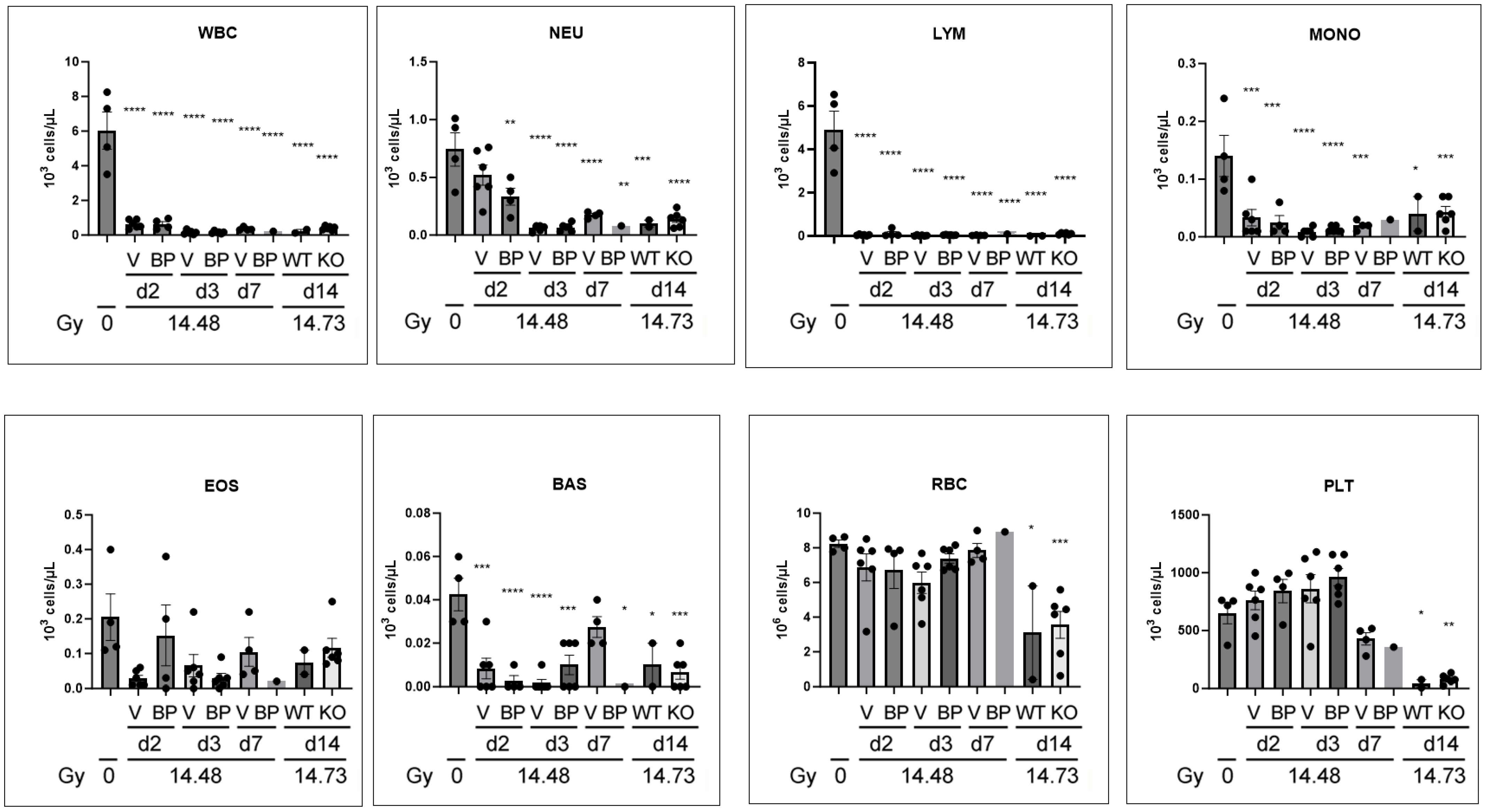
3.8. Complete Blood Count Changes after PBI/BM5
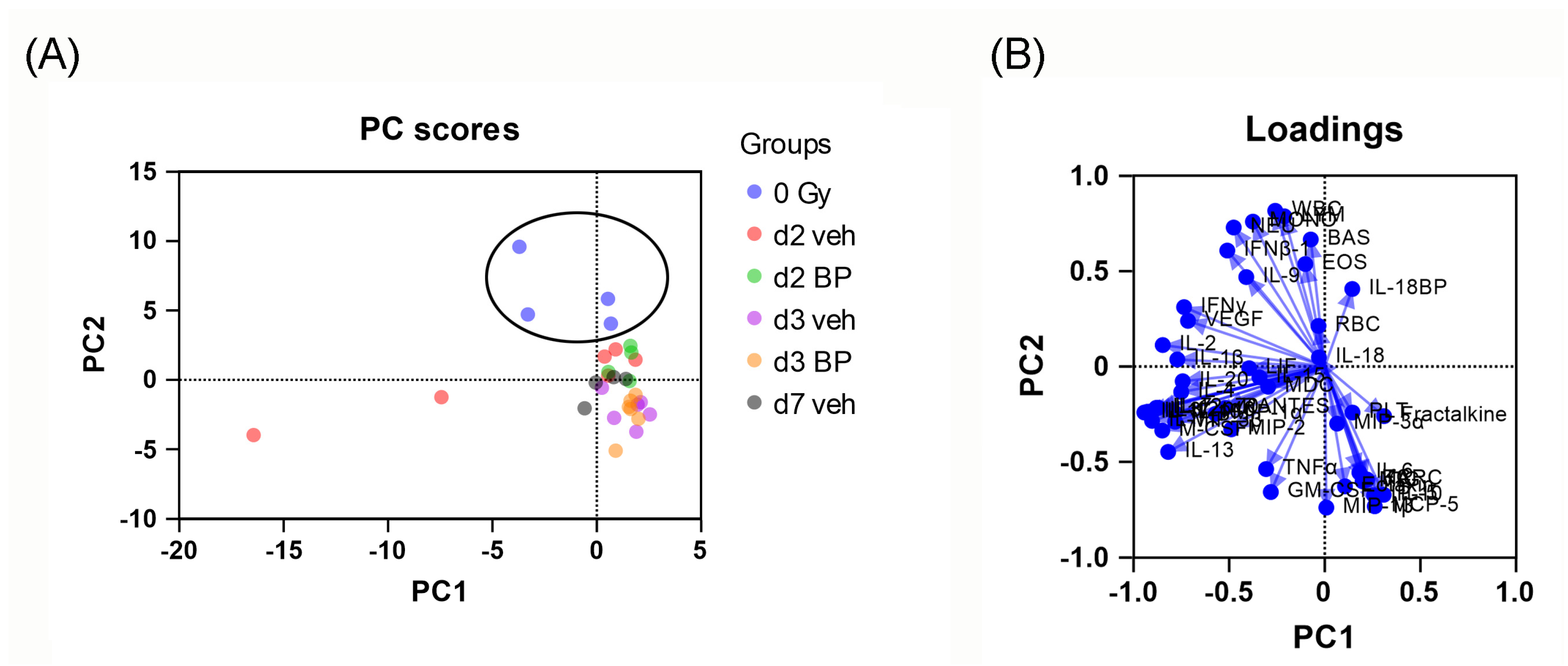
3.9. Principal Component Analysis (PCA) of Mouse Serum Cytokines and CBC Counts after Irradiation and IL-18BP Treatment
3.10. Individual Mouse Serum Cytokine Changes after 9.0 Gy TBI in CD2F1 Male Mice and 14.48 Gy PBI/BM5 in C57BL6 Male Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, S.; Dodt, A. Nuclear bomb and public health. J. Public. Health Policy 2023, 44, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Kearns, R.D.; Hickerson, W.L.; Carter, J.E. Radiation Injuries. Surg. Clin. N. Am. 2023, 103, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Orschell, C.M. The delayed effects of acute radiation exposure (DEARE): Characteristics, mechanisms, animal models, and promising medical countermeasures. Int. J. Radiat. Biol. 2023, 99, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- MacVittie, T.J.; Farese, A.M.; Kane, M.A. ARS, DEARE, and Multiple-organ Injury: A Strategic and Tactical Approach to Link Radiation Effects, Animal Models, Medical Countermeasures, and Biomarker Development to Predict Clinical Outcome. Health Phys. 2019, 116, 453. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M. The Role of Proinflammatory Cytokine Interleukin-18 in Radiation Injury. Health Phys. 2016, 111, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.T.; Li, X.H.; Fu, D.; Moroni, M.; Fisher, C.; Arnott, R.; Srinivasan, V.; Xiao, M. Circulating interleukin-18 as a biomarker of total-body radiation exposure in mice, minipigs, and nonhuman primates (NHP). PLoS ONE 2014, 9, e109249. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.T.; Li, X.; Fu, D.; Xiao, M. Circulating IL-18 Binding Protein (IL-18BP) and IL-18 as Dual Biomarkers of Total-Body Irradiation in Mice. Radiat. Res. 2016, 185, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, W.; Hull, L.; Wang, L.; Yu, T.; Xiao, M. IL-18 binding protein (IL-18BP) as a novel radiation countermeasure after radiation exposure in mice. Sci. Rep. 2020, 10, 18674. [Google Scholar] [CrossRef]
- Cui, W.; Hull, L.; Zizzo, A.; Wang, L.; Lin, B.; Zhai, M.; Xiao, M. Pharmacokinetic Study of rhIL-18BP and Its Effect on Radiation-Induced Cytokine Changes in Mouse Serum and Intestine. Toxics 2022, 11, 35. [Google Scholar] [CrossRef]
- Cui, W.; Hull, L.; Zizzo, A.; Wang, L.; Lin, B.; Zhai, M.; Xiao, M. The gut microbiome changes in wild type and IL-18 knockout mice after 9.0 Gy total body irradiation. Anim. Microbiome 2023, 5, 42. [Google Scholar] [CrossRef]
- Satyamitra, M.M.; Cassatt, D.R.; Marzella, L. A Trans-Agency Workshop on the Pathophysiology of Radiation-Induced Lung Injury. Radiat. Res. 2022, 197, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Cassatt, D.R.; Gorovets, A.; Karimi-Shah, B.; Roberts, R.; Price, P.W.; Satyamitra, M.M.; Todd, N.; Wang, S.-J.; Marzella, L. A Trans-Agency Workshop on the Pathophysiology of Radiation-Induced Lung Injury. Radiat. Res. 2022, 197, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Bouville, A.; Chumak, V.V.; Inskip, P.D.; Kryuchkov, V.; Luckyanov, N. The chornobyl accident: Estimation of radiation doses received by the Baltic and Ukrainian cleanup workers. Radiat. Res. 2006, 166, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Wuddie, K.; Tsioplaya, A.; Weaver, A.; Holmes-Hampton, G.P.; Ghosh, S.P. Development of a Multi-Organ Radiation Injury Model with Precise Dosimetry with Focus on GI-ARS. Radiat. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- MacVittie, T.J.; Bennett, A.; Booth, C.; Garofalo, M.; Tudor, G.; Ward, A.; Shea-Donohue, T.; Gelfond, D.; McFarland, E.; Jackson, W., III; et al. The prolonged gastrointestinal syndrome in rhesus macaques: The relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys. 2012, 103, 427–453. [Google Scholar] [CrossRef]
- Booth, C.; Tudor, G.; Tudor, J.; Katz, B.P.; MacVittie, T.J. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012, 103, 383–399. [Google Scholar] [CrossRef]
- Koch, A.; Gulani, J.; King, G.; Hieber, K.; Chappell, M.; Ossetrova, N. Establishment of Early Endpoints in Mouse Total-Body Irradiation Model. PLoS ONE 2016, 11, e0161079. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Maher, C.; Hick, J.L.; Hanfling, D.; Dainiak, N.; Chao, N.; Bader, J.L.; Coleman, C.N.; Weinstock, D.M. Radiation injury after a nuclear detonation: Medical consequences and the need for scarce resources allocation. Disaster Med. Public Health Prep. 2011, 5 (Suppl. S1), S32–S44. [Google Scholar] [CrossRef]
- Garty, G.; Xu, Y.; Elliston, C.; Marino, S.A.; Randers-Pehrson, G.; Brenner, D.J. Mice and the A-Bomb: Irradiation Systems for Realistic Exposure Scenarios. Radiat. Res. 2017, 187, 465–475. [Google Scholar] [CrossRef]
- Kumar, V.P.; Stone, S.; Biswas, S.; Sharma, N.; Ghosh, S.P. Gamma Tocotrienol Protects Mice From Targeted Thoracic Radiation Injury. Front. Pharmacol. 2020, 11, 587970. [Google Scholar] [CrossRef]
- Gosmann, C.; Frazer, I.H.; Mattarollo, S.R.; Blumenthal, A. IL-18, but not IL-12, induces production of IFN-gamma in the immunosuppressive environment of HPV16 E7 transgenic hyperplastic skin. J. Investig. Dermatol. 2014, 134, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kwon, J.Y.; Kim, S.Y.; Jung, K.; Cho, M.L. Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis. Sci. Rep. 2017, 7, 10133. [Google Scholar] [CrossRef] [PubMed]
- Mosovsky, K.; Silva, E.; Troyer, R.; Propst-Graham, K.; Dow, S. Interaction of Interferon gamma-induced reactive oxygen species with ceftazidime leads to synergistic killing of intracellular Burkholderia pseudomallei. Antimicrob. Agents Chemother. 2014, 58, 5954–5963. [Google Scholar] [CrossRef]
- MacVittie, T.J.; Farese, A.M.; Bennett, A.; Gelfond, D.; Shea-Donohue, T.; Tudor, G.; Booth, C.; McFarland, E.; Jackson, W., III. The acute gastrointestinal subsyndrome of the acute radiation syndrome: A rhesus macaque model. Health Phys. 2012, 103, 411–426. [Google Scholar] [CrossRef]




| CD2F1 Male Mice Total Body Irradiation 9.0 Gy | C57BL6 Female PBI/BM5 14.48 Gy | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokines | 0 Gy | d3 veh | p Value d3 veh vs. 0 Gy | d7 veh | p Value d7 veh vs. 0 Gy | Cytokines | 0 Gy | d2 veh | p Value d2 veh vs. 0 Gy | d3 veh | p Value d3 veh vs. 0 Gy | d7 veh | p Value d7 veh vs. 0 Gy | |
| 1 | MCP-1 | 13.31 ± 1.97 | 495.87 ± 194.8 | p < 0.05 | 138.79 ± 32.75 | n.s | MCP-1 | 40.61 ± 7.4 | 426.73 ± 136.24 | n.s | 332.87 ± 32.3 | n.s | 394.42 ± 79.67 | n.s |
| 2 | MDC | 325.67 ± 42.59 | 513.38 ± 72.71 | p < 0.05 | 187.58 ± 22.82 | n.s | MDC | 126.45 ± 21.34 | 352.43 ± 67.67 | n.s | 169.01 ± 18.27 | n.s | 142.07 ± 42.38 | n.s |
| 3 | MIP-3β | 178.38 ± 24.48 | 320.74 ± 11.59 | p < 0.01 | 283.41 ± 34.49 | p < 0.05 | MIP-3β | 87.01 ± 25.42 | 118.62 ± 41.07 | n.s | 104.38 ± 20.86 | n.s | 114.49 ± 24.46 | n.s |
| 4 | IL-17 | 3.12 ± 0.37 | 8.16 ± 1.56 | p < 0.01 | 3.33 ± 0.89 | n.s | IL-17 | 0.64 ± 0.18 | 2.66 ± 1.49 | n.s | 0.49 ± 0.12 | n.s | 0.52 ± 0.13 | n.s |
| 5 | MIG | 112.85 ± 42.23 | 690.56 ± 159.29 | p < 0.01 | 146.93 ± 29.25 | n.s | MIG | 90.95 ± 39.63 | 75.87 ± 13.04 | n.s | 298.66 ± 31.72 | n.s | 158.68 ± 85.08 | n.s |
| 6 | MIP-1α | 46.67 ± 7.3 | 95.7 ± 10.46 | p < 0.01 | 42.61 ± 4.72 | n.s | MIP-1α | 58.97 ± 28.03 | 80.51 ± 25.82 | n.s | 64.78 ± 13.45 | n.s | 41.07 ± 14.39 | n.s |
| 7 | TARC | 29.73 ± 3.86 | 132.74 ± 31.44 | p < 0.01 | 43.26 ± 6.11 | n.s | TARC | 21.1 ± 4.61 | 28.19 ± 5.8 | n.s | 94.19 ± 13.2 | n.s | 122.08 ± 38.53 | p < 0.01 |
| 8 | IL-5 | 4.75 ± 1.59 | 23.37 ± 3.52 | p < 0.001 | 18.5 ± 0.94 | p < 0.01 | IL-5 | 3.01 ± 1.01 | 15.5 ± 1.18 | n.s | 54.92 ± 6.1 | p < 0.001 | 4.96 ± 1.31 | n.s |
| 9 | IL-1α | 23.77 ± 9.04 | 113.51 ± 17.61 | p < 0.001 | 51.19 ± 7.54 | n.s | IL-1α | 74.28 ± 42.7 | 137.98 ± 61.95 | n.s | 80.02 ± 27.54 | n.s | 55.62 ± 17.77 | n.s |
| 10 | TIMP-1 | 1627.59 ± 330.01 | 4850.59 ± 707.44 | p < 0.001 | 1611.3 ± 190.79 | n.s | TIMP-1 | 1107.43 ± 79.64 | 1608.44 ± 260.94 | n.s | 2815.2 ± 384.94 | p < 0.05 | 3082.38 ± 123.71 | p < 0.05 |
| 11 | TNFα | 2.16 ± 0.83 | 12.25 ± 1.44 | p < 0.001 | 2.67 ± 1.56 | n.s | TNFα | 3.91 ± 2.09 | 5.03 ± 2.33 | n.s | 5.92 ± 0.74 | n.s | 7.02 ± 2.23 | n.s |
| 12 | MCP-5 | 196.13 ± 14.01 | 1112.96 ± 140.57 | p < 0.0001 | 672.18 ± 64 | p < 0.01 | MCP-5 | 41.17 ± 6.72 | 189.11 ± 20.09 | n.s | 418.19 ± 27.08 | p < 0.001 | 420.98 ± 91.89 | p < 0.001 |
| 13 | G-CSF | 378.82 ± 213.87 | 12031.13 ± 1317.17 | p < 0.0001 | 7820.85 ± 1429.67 | p < 0.001 | G-CSF | 164.86 ± 57.01 | 850.11 ± 144.38 | n.s | 9529.55 ± 2765.24 | n.s | 7176.39 ± 2771.2 | n.s |
| 14 | IFNγ | 1.07 ± 0.26 | 6.88 ± 1.04 | p < 0.0001 | 1.45 ± 0.17 | n.s | IFNγ | 2.26 ± 1.42 | 1.45 ± 0.69 | n.s | 0.71 ± 0.29 | n.s | 0.46 ± 0 | n.s |
| 15 | IP-10 | 66.32 ± 6.74 | 190.45 ± 19.34 | p < 0.0001 | 104.05 ± 10 | n.s | IP-10 | 17.96 ± 3.15 | 33.77 ± 5.44 | n.s | 61.96 ± 14.56 | n.s | 61.81 ± 7.07 | n.s |
| 16 | MIP-1β | 61.82 ± 10.59 | 161.69 ± 4.78 | p < 0.0001 | 64.12 ± 10.51 | n.s | MIP-1β | 15.85 ± 13.28 | 56.55 ± 12.59 | n.s | 78.45 ± 9.75 | p < 0.01 | 48.83 ± 17.74 | n.s |
| 17 | IL-16 | 1957.48 ± 396.85 | 935.04 ± 162.28 | n.s | 429.42 ± 86.09 | p < 0.01 | IL-16 | 1658.44 ± 304.57 | 1270.86 ± 213.62 | n.s | 721.28 ± 89.77 | p < 0.05 | 1264.56 ± 308.69 | n.s |
| 18 | MIP-3α | 80.5 ± 23.53 | 206.4 ± 44.29 | n.s | 343.82 ± 55.68 | p < 0.01 | MIP-3α | 106.8 ± 8.48 | 96.87 ± 13.84 | n.s | 165.22 ± 35.98 | n.s | 177.17 ± 71.92 | n.s |
| 19 | EPO | 246.74 ± 32.09 | 340.36 ± 30.09 | n.s | 791.16 ± 110.13 | p < 0.001 | EPO | 199.18 ± 59.94 | 224.84 ± 50.66 | n.s | 400.07 ± 39.37 | n.s | 272.76 ± 23.97 | n.s |
| 20 | 6Ckine/Exodus 2 | a.d | a.d | n.s | a.d | n.s | 6Ckine/Exodus 2 | 1285.35 ± 143.2 | 1054.42 ± 117.38 | n.s | 1264.96 ± 106.12 | n.s | 1236.74 ± 117.84 | n.s |
| 21 | Eotaxin | 826.05 ± 160.13 | 1472.6 ± 361.65 | n.s | 1357.4 ± 222.35 | n.s | Eotaxin | 163.36 ± 20.95 | 499.88 ± 114.34 | n.s | 485.57 ± 74.16 | n.s | 646.88 ± 67.9 | p < 0.01 |
| 22 | Fractalkine | 201.36 ± 19.64 | 370.76 ± 119.56 | n.s | 250 ± 43.94 | n.s | Fractalkine | 78.89 ± 4.51 | 90.77 ± 6.81 | n.s | 88.81 ± 10.77 | n.s | 161.91 ± 19.03 | p < 0.001 |
| 23 | GM-CSF | 3.96 ± 1.67 | 6.76 ± 3.06 | n.s | 2.24 ± 1.09 | n.s | GM-CSF | 0.46 ± 0 | 12.5 ± 7.21 | n.s | 22.64 ± 3.6 | n.s | 0.46 ± 0 | n.s |
| 24 | IFNβ-1 | 138.23 ± 59.63 | 75.54 ± 9.65 | n.s | 48.13 ± 1.62 | n.s | IFNβ-1 | 370.04 ± 132.54 | 165.51 ± 41.41 | p < 0.05 | 97.21 ± 12.76 | p < 0.01 | 131.16 ± 19.04 | p < 0.05 |
| 25 | IL-10 | 5.96 ± 1.9 | 4.3 ± 1.28 | n.s | 2.63 ± 0.84 | n.s | IL-10 | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 26 | IL-11 | 25.75 ± 3.47 | 39.79 ± 11.55 | n.s | 28.57 ± 19.57 | n.s | IL-11 | 18.23 ± 10.94 | 37.94 ± 22.91 | n.s | 18.18 ± 5.24 | n.s | 23.12 ± 4.29 | n.s |
| 27 | IL-12p40 | 1.52 ± 1.06 | 2.88 ± 1.18 | n.s | 2.3 ± 1.26 | n.s | IL-12p40 | 5.51 ± 3.69 | 20.05 ± 7.57 | n.s | 3.23 ± 1.38 | n.s | 7.34 ± 1.38 | n.s |
| 28 | IL-12p70 | 2.47 ± 2.02 | 2.38 ± 1.09 | n.s | 0.82 ± 0.37 | n.s | IL-12p70 | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 29 | IL-13 | 43.27 ± 5.81 | 48.95 ± 10.74 | n.s | 48.22 ± 6.14 | n.s | IL-13 | 9.11 ± 4.45 | 23.73 ± 9.97 | n.s | 13.45 ± 1.68 | n.s | 18.74 ± 2.26 | n.s |
| 30 | IL-15 | 3.84 ± 1.58 | 27.39 ± 13.8 | n.s | 2.54 ± 0.27 | n.s | IL-15 | 37.19 ± 14.89 | 74.65 ± 35.5 | n.s | 29.61 ± 4.68 | n.s | 30.46 ± 7.03 | n.s |
| 31 | IL-1β | 3.79 ± 0.45 | 3.81 ± 0.53 | n.s | 2.85 ± 0.69 | n.s | IL-1β | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 32 | IL-2 | 3.25 ± 0.8 | 6.16 ± 2.31 | n.s | 2.04 ± 0.95 | n.s | IL-2 | 20.52 ± 14.99 | 18.81 ± 11.58 | n.s | 3.98 ± 1.88 | n.s | 11.78 ± 4.75 | n.s |
| 33 | IL-20 | 78.37 ± 18.03 | 81.86 ± 11.61 | n.s | 67.13 ± 11.64 | n.s | IL-20 | 235.53 ± 107.47 | 273.68 ± 102.07 | n.s | 186.93 ± 47.32 | n.s | 112.52 ± 42.41 | n.s |
| 34 | IL-3 | n.d | n.d | n.s | n.d | n.s | IL-3 | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 35 | IL-4 | n.d | n.d | n.s | n.d | n.s | IL-4 | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 36 | IL-6 | 6.42 ± 3.28 | 26.44 ± 12.7 | n.s | 18.32 ± 7.34 | n.s | IL-6 | 7.73 ± 3.22 | 20.36 ± 2.89 | n.s | 40.59 ± 9.53 | n.s | 17.73 ± 5.25 | n.s |
| 37 | IL-7 | 0.51 ± 0.03 | 1.1 ± 0.64 | n.s | 0.45 ± 0 | n.s | IL-7 | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 38 | IL-9 | 6.27 ± 1.33 | 11.23 ± 3.97 | n.s | 5.48 ± 2.08 | n.s | IL-9 | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 39 | KC | 12.45 ± 4.9 | 812.75 ± 408.45 | n.s | 241.88 ± 62.98 | n.s | KC | 49.89 ± 20.16 | 106.07 ± 19.46 | n.s | 580.73 ± 75.11 | p < 0.01 | 394.36 ± 125.92 | n.s |
| 40 | LIF | 0.73 ± 0.28 | 0.82 ± 0.31 | n.s | 0.45 ± 0 | n.s | LIF | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 41 | M-CSF | 7.16 ± 1.83 | 11.87 ± 1.41 | n.s | 7.77 ± 1.55 | n.s | M-CSF | 3.9 ± 1.89 | 12.24 ± 7.2 | n.s | 5.93 ± 1.05 | n.s | 6.58 ± 1.45 | n.s |
| 42 | MIP-2 | 141.3 ± 2.91 | 147.92 ± 2.69 | n.s | 138.06 ± 1.44 | n.s | MIP-2 | 168.26 ± 6.1 | 172.87 ± 12.05 | n.s | 169.05 ± 5.35 | n.s | 192.61 ± 26.72 | n.s |
| 43 | RANTES | 19.32 ± 2.81 | 21.6 ± 7.15 | n.s | 22.69 ± 4.77 | n.s | RANTES | 29.24 ± 16.45 | 58.84 ± 17.25 | n.s | 21.6 ± 6.23 | n.s | 89.02 ± 30.87 | n.s |
| 44 | VEGF | n.d | 0.86 ± 0.32 | n.s | n.d | n.s | VEGF | n.d | n.d | n.s | n.d | n.s | n.d | n.s |
| 45 | LIX | 1306.46 ± 543.03 | 1151.18 ± 601.73 | n.s | 604.37 ± 257.14 | n.s | 1211.56 ± 434.32 | n.s | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Hull, L.; Zizzo, A.; Wang, L.; Lin, B.; Zhai, M.; Kumar, V.P.; Xiao, M. The Roles of IL-18 in a Realistic Partial Body Irradiation with 5% Bone Marrow Sparing (PBI/BM5) Model. Toxics 2024, 12, 5. https://doi.org/10.3390/toxics12010005
Cui W, Hull L, Zizzo A, Wang L, Lin B, Zhai M, Kumar VP, Xiao M. The Roles of IL-18 in a Realistic Partial Body Irradiation with 5% Bone Marrow Sparing (PBI/BM5) Model. Toxics. 2024; 12(1):5. https://doi.org/10.3390/toxics12010005
Chicago/Turabian StyleCui, Wanchang, Lisa Hull, Alex Zizzo, Li Wang, Bin Lin, Min Zhai, Vidya P. Kumar, and Mang Xiao. 2024. "The Roles of IL-18 in a Realistic Partial Body Irradiation with 5% Bone Marrow Sparing (PBI/BM5) Model" Toxics 12, no. 1: 5. https://doi.org/10.3390/toxics12010005
APA StyleCui, W., Hull, L., Zizzo, A., Wang, L., Lin, B., Zhai, M., Kumar, V. P., & Xiao, M. (2024). The Roles of IL-18 in a Realistic Partial Body Irradiation with 5% Bone Marrow Sparing (PBI/BM5) Model. Toxics, 12(1), 5. https://doi.org/10.3390/toxics12010005






