Determination of Perfluorooctanoic Acid (PFOA) in the Indoor Dust Matter of the Sicily (Italy) Area: Analysis and Exposure Evaluations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Materials, Equipment, and Software
2.3. Sample Extraction and Method Optimization
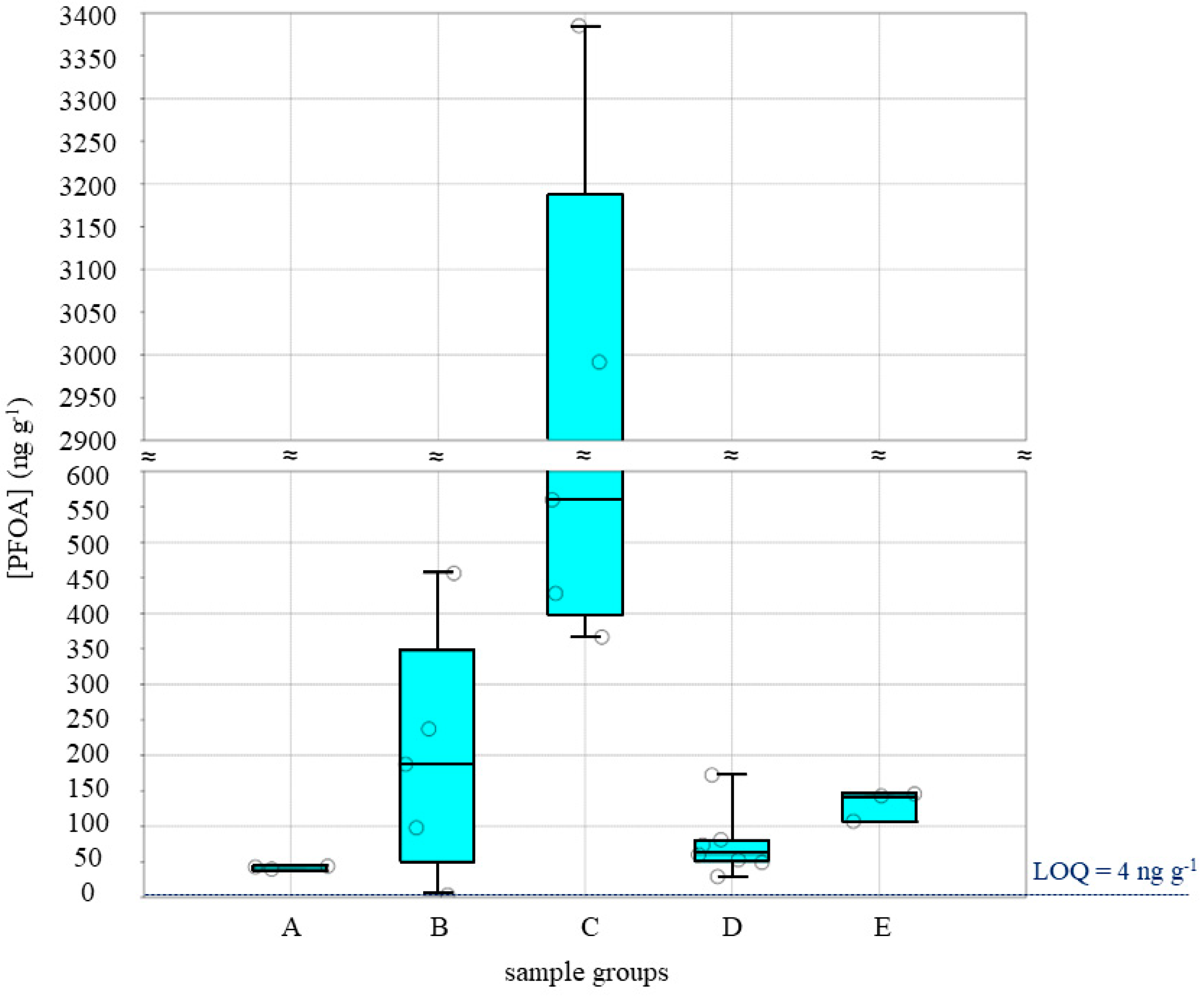
3. Results and Discussion
3.1. Sample Analysis and Evaluation
3.2. Evaluation of PFOA Exposure
- -
- PFOA dust is PFOA concentration in dust samples expressed as ng g−1;
- -
- Indoor hours are time (in hours) that people spent in indoor environments;
- -
- The 100 value is the correlation factor concerning PFOA levels from dust to blood samples.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yacoubi, L.; El Zrelli, R.B.; Hsu, H.H.; Lin, Y.-J.; Savoca, D.; Gopalan, J.; Nazal, M.; Bhuyan, M.S.; Arculeo, M.; Rabaoui, L.J. Bioaccumulation of Trace Elements and Hydrocarbons in Chondrichthyans of the Western Arabian Gulf: Environmental and Human Health Risk Assessment and Implications for Conservation. Sci. Total Environ. 2023, 901, 165990. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Barreca, S.; Lo Coco, R.; Punginelli, D.; Orecchio, S.; Maccotta, A. Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment. Environments 2023, 10, 99. [Google Scholar] [CrossRef]
- Binetti, R.; Calza, P.; Costantino, G.; Morgillo, S.; Papagiannaki, D. Perfluoroalkyl Substance Assessment in Turin Metropolitan Area and Correlation with Potential Sources of Pollution According to the Water Safety Plan Risk Management Approach. Separations 2019, 6, 17. [Google Scholar] [CrossRef]
- Orecchio, S.; Fiore, M.; Barreca, S.; Vara, G. Volatile Profiles of Emissions from Different Activities Analyzed Using Canister Samplers and Gas Chromatography-Mass Spectrometry (GC/MS) Analysis: A Case Study. Int. J. Environ. Res. Public Health 2017, 14, 195. [Google Scholar] [CrossRef]
- Bergamasco, A.; Culotta, L.; De Stefano, C.; Orecchio, S.; Sammartano, S.; Barreca, S. Composition, Distribution, and Sources of Polycyclic Aromatic Hydrocarbons in Sediments of the Gulf of Milazzo (Mediterranean Sea, Italy). Polycycl. Aromat. Compd. 2014, 34, 397–424. [Google Scholar] [CrossRef]
- Orecchio, S.; Indelicato, R.; Barreca, S. The Distribution of Phthalate Esters in Indoor Dust of Palermo (Italy). Environ. Geochem. Health 2013, 35, 613–624. [Google Scholar] [CrossRef]
- Kotthoff, M.; Müller, J.; Jürling, H.; Schlummer, M.; Fiedler, D. Perfluoroalkyl and Polyfluoroalkyl Substances in Consumer Products. Environ. Sci. Pollut. Res. 2015, 22, 14546–14559. [Google Scholar] [CrossRef]
- Barreca, S.; Orecchio, S.; Pace, A. Photochemical Sample Treatment for Extracts Clean up in PCB Analysis from Sediments. Talanta 2013, 103, 349–354. [Google Scholar] [CrossRef]
- Barreca, S.; Busetto, M.; Vitelli, M.; Colzani, L.; Clerici, L.; Dellavedova, P. Online Solid-Phase Extraction LC-MS/MS: A Rapid and Valid Method for the Determination of Perfluorinated Compounds at Sub ng·L−1 Level in Natural Water. J. Chem. 2018, 2018, 3780825. [Google Scholar] [CrossRef]
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; Von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.G.; Jones, K.C.; Sweetman, A.J. A First Global Production, Emission, And Environmental Inventory For Perfluorooctane Sulfonate. Environ. Sci. Technol. 2009, 43, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kotthoff, M.; Bücking, M. Four Chemical Trends Will Shape the Next Decade’s Directions in Perfluoroalkyl and Polyfluoroalkyl Substances Research. Front. Chem. 2018, 6, 103. [Google Scholar] [CrossRef]
- Savoca, D.; Pace, A. Bioaccumulation, Biodistribution, Toxicology and Biomonitoring of Organofluorine Compounds in Aquatic Organisms. Int. J. Mol. Sci. 2021, 22, 6276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, J.; Sumita; Shi, C.; Zhu, Q.; Li, C.; Pang, W. A Review of Recent Advances in Detection and Treatment Technology for Perfluorinated Compounds. Water 2022, 14, 3919. [Google Scholar] [CrossRef]
- Palma, D.; Papagiannaki, D.; Lai, M.; Binetti, R.; Sleiman, M.; Minella, M.; Richard, C. PFAS Degradation in Ultrapure and Groundwater Using Non-Thermal Plasma. Molecules 2021, 26, 924. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Scheringer, M.; Wang, Z. The High Persistence of PFAS Is Sufficient for Their Management as a Chemical Class. Environ. Sci. Process. Impacts 2020, 22, 2307–2312. [Google Scholar] [CrossRef]
- Barreca, S.; Busetto, M.; Colzani, L.; Clerici, L.; Marchesi, V.; Tremolada, L.; Daverio, D.; Dellavedova, P. Hyphenated High Performance Liquid Chromatography–Tandem Mass Spectrometry Techniques for the Determination of Perfluorinated Alkylated Substances in Lombardia Region in Italy, Profile Levels and Assessment: One Year of Monitoring Activities During 2018. Separations 2020, 7, 17. [Google Scholar] [CrossRef]
- Pontius, F. Pontius Regulation of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) in Drinking Water: A Comprehensive Review. Water 2019, 11, 2003. [Google Scholar] [CrossRef]
- Van Leeuwen, S.P.J.; Swart, C.P.; Van Der Veen, I.; De Boer, J. Significant Improvements in the Analysis of Perfluorinated Compounds in Water and Fish: Results from an Interlaboratory Method Evaluation Study. J. Chromatogr. A 2009, 1216, 401–409. [Google Scholar] [CrossRef]
- Savoca, D.; Melfi, R.; Palumbo Piccionello, A.; Barreca, S.; Buscemi, S.; Arizza, V.; Arculeo, M.; Pace, A. Presence and Biodistribution of Perfluorooctanoic Acid (PFOA) in Paracentrotus Lividus Highlight Its Potential Application for Environmental Biomonitoring. Sci. Rep. 2021, 11, 18763. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Pace, A.; Arizza, V.; Arculeo, M.; Melfi, R. Controlled Uptake of PFOA in Adult Specimens of Paracentrotus Lividus and Evaluation of Gene Expression in Their Gonads and Embryos. Environ. Sci. Pollut. Res. 2022, 30, 26094–26106. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, D.; Yan, S.; Cui, J.; Liang, Y.; Ren, S. Adverse Effects of Perfluorooctane Sulfonate on the Liver and Relevant Mechanisms. Toxics 2022, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Ducatman, A.; LaPier, J.; Fuoco, R.; DeWitt, J.C. Official Health Communications Are Failing PFAS-Contaminated Communities. Environ. Health 2022, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.; Ouyang, B. Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility? Int. J. Environ. Res. Public Health 2021, 18, 3794. [Google Scholar] [CrossRef] [PubMed]
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water PolicyText with EEA Relevance. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:en:PDF (accessed on 9 October 2023).
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic Acid (PFOA), an Emerging Drinking Water Contaminant: A Critical Review of Recent Literature. Environ. Res. 2012, 116, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Schultes, L.; Vestergren, R.; Volkova, K.; Westberg, E.; Jacobson, T.; Benskin, J.P. Per- and Polyfluoroalkyl Substances and Fluorine Mass Balance in Cosmetic Products from the Swedish Market: Implications for Environmental Emissions and Human Exposure. Environ. Sci. Process. Impacts 2018, 20, 1680–1690. [Google Scholar] [CrossRef]
- Stoiber, T.; Evans, S.; Naidenko, O.V. Disposal of Products and Materials Containing Per- and Polyfluoroalkyl Substances (PFAS): A Cyclical Problem. Chemosphere 2020, 260, 127659. [Google Scholar] [CrossRef]
- Valsecchi, S.; Conti, D.; Crebelli, R.; Polesello, S.; Rusconi, M.; Mazzoni, M.; Preziosi, E.; Carere, M.; Lucentini, L.; Ferretti, E.; et al. Deriving Environmental Quality Standards for Perfluorooctanoic Acid (PFOA) and Related Short Chain Perfluorinated Alkyl Acids. J. Hazard. Mater. 2017, 323, 84–98. [Google Scholar] [CrossRef]
- Björklund, J.A.; Thuresson, K.; De Wit, C.A. Perfluoroalkyl Compounds (PFCs) in Indoor Dust: Concentrations, Human Exposure Estimates, and Sources. Environ. Sci. Technol. 2009, 43, 2276–2281. [Google Scholar] [CrossRef]
- Orecchio, S.; Amorello, D. Platinum Levels in Urban Soils from Palermo (Italy); Analytical Method Using Voltammetry. Microchem. J. 2011, 99, 283–288. [Google Scholar] [CrossRef]
- Orecchio, S.; Amorello, D.; Indelicato, R.; Barreca, S.; Orecchio, S. A Short Review of Simple Analytical Methods for the Evaluation of PAHs and PAEs as Indoor Pollutants in House Dust Samples. Atmosphere 2022, 13, 1799. [Google Scholar] [CrossRef]
- Strynar, M.J.; Lindstrom, A.B. Perfluorinated Compounds in House Dust from Ohio and North Carolina, USA. Environ. Sci. Technol. 2008, 42, 3751–3756. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Wemken, N.; Drage, D.S.; Abdallah, M.A.-E.; Coggins, A.-M. Perfluoroalkyl Substances in Drinking Water, Indoor Air and Dust from Ireland: Implications for Human Exposure. Environ. Sci. Technol. 2019, 53, 13449–13457. [Google Scholar] [CrossRef]
- Gustafsson, Å.; Wang, B.; Gerde, P.; Bergman, Å.; Yeung, L.W.Y. Bioavailability of Inhaled or Ingested PFOA Adsorbed to House Dust. Environ. Sci. Pollut. Res. 2022, 29, 78698–78710. [Google Scholar] [CrossRef]
- Kato, K.; Calafat, A.M.; Needham, L.L. Polyfluoroalkyl Chemicals in House Dust. Environ. Res. 2009, 109, 518–523. [Google Scholar] [CrossRef]
- Kato, K.; Wong, L.-Y.; Jia, L.T.; Kuklenyik, Z.; Calafat, A.M. Trends in Exposure to Polyfluoroalkyl Chemicals in the U.S. Population: 1999–2008. Environ. Sci. Technol. 2011, 45, 8037–8045. [Google Scholar] [CrossRef]
| Codex Sample | Sampling Area | Sample Weight (g) |
|---|---|---|
| 1a | Milena country house | 0.14935 |
| 2a | Milena country house | 0.14907 |
| 3a | Milena country house | 0.14897 |
| 1b | Milena city house | 0.13941 |
| 2b | Milena city house | 0.12813 |
| 3b | Milena city house | 0.14631 |
| 4b | Milena city house | 0.13095 |
| 5b | Milena city house | 0.12751 |
| 1c | Palermo (University. STEBICEF) Laboratory I | 0.12495 |
| 2c | Palermo (University. STEBICEF) Laboratory I | 0.13510 |
| 3c | Palermo (University. STEBICEF) Laboratory I | 0.14309 |
| 4c | Palermo (University. STEBICEF) Laboratory I | 0.13178 |
| 5c | Palermo (University. STEBICEF) Laboratory I | 0.14626 |
| 1d | Palermo (Santi Romano. student University residence) | 0.09811 |
| 2d | Palermo (Santi Romano. student University residence) | 0.10196 |
| 3d | Palermo (Santi Romano. student University residence) | 0.10302 |
| 4d | Palermo (Santi Romano. student University residence) | 0.11484 |
| 5d | Palermo (Santi Romano. student University residence) | 0.09736 |
| 6d | Palermo (Santi Romano. student University residence) | 0.10929 |
| 7d | Palermo (Santi Romano. student University residence) | 0.10216 |
| 1e | Palermo (University. STEBICEF) Laboratory II | 0.15313 |
| 2e | Palermo (University. STEBICEF) Laboratory II | 0.17358 |
| 3e | Palermo (University. STEBICEF) Laboratory II | 0.16489 |
| City of Sampling | Codex Sample | PFOA ng g−1 |
|---|---|---|
| Milena country house | 1a | 44.01 |
| Milena country house | 2a | 42.92 |
| Milena country house | 3a | 39.82 |
| Milena city house | 1b | 237.21 |
| Milena city house | 2b | <LOQ |
| Milena city house | 3b | 98.05 |
| Milena city house | 4b | 456.45 |
| Milena city house | 5b | 187.53 |
| Palermo (University. STEBICEF) Laboratory I | 1c | 559.83 |
| Palermo (University. STEBICEF) Laboratory I | 2c | 428.02 |
| Palermo (University. STEBICEF) Laboratory I | 3c | 366.68 |
| Palermo (University. STEBICEF) Laboratory I | 4c | 3385.24 |
| Palermo (University. STEBICEF) Laboratory I | 5c | 2991.56 |
| Palermo (Santi Romano. student University residence) | 1d | 60.31 |
| Palermo (Santi Romano. student University residence) | 2d | 73.72 |
| Palermo (Santi Romano. student University residence) | 3d | 29.38 |
| Palermo (Santi Romano. student University residence) | 4d | 81.26 |
| Palermo (Santi Romano. student University residence) | 5d | 172.3 |
| Palermo (Santi Romano. student University residence) | 6d | 52.93 |
| Palermo (Santi Romano. student University residence) | 7d | 49.19 |
| Palermo (University. STEBICEF) Laboratory II | 1e | 145.75 |
| Palermo (University. STEBICEF) Laboratory II | 2e | 142.92 |
| Palermo (University. STEBICEF) Laboratory II | 3e | 107.22 |
| PFOA average in Palermo samples | 157.99 | |
| PFOA average in Milena samples | 576.42 | |
| Total average | 444.3 |
| Group a | Group b | Group c | Group d | Group e | |
|---|---|---|---|---|---|
| N samples | 3 | 5 | 5 | 7 | 3 |
| Min (ng g−1) | 39.8 | 2.5 | 367 | 29.4 | 107 |
| Max (ng g−1) | 44.0 | 456 | 3385 | 172 | 146 |
| Sum (ng g−1) | 127 | 982 | 7731 | 519 | 395 |
| Mean (ng g−1) | 42.3 | 196 | 1546 | 74.1 | 132 |
| Variance | 4.7257 | 29,160.01 | 2.271,413 | 2158.891 | 461.1766 |
| Group | IPEX Indoor Laboratory ng g−1 day | IPEX Indoor House ng g−1 day | IPEX Total Indoor ng g−1 day |
|---|---|---|---|
| Chemist Palermo from laboratory I | 124 | 7.4 | 131.4 |
| Chemist Palermo from laboratory II | 10.6 | 7.4 | 18 |
| Housewoman/Houseman from Milena rural house | --- | 7.6 | 7.6 |
| Housewoman/Houseman from Palermo | --- | 13.3 | 13.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreca, S.; Mancuso, M.M.M.; Sacristán, D.; Pace, A.; Savoca, D.; Orecchio, S. Determination of Perfluorooctanoic Acid (PFOA) in the Indoor Dust Matter of the Sicily (Italy) Area: Analysis and Exposure Evaluations. Toxics 2024, 12, 28. https://doi.org/10.3390/toxics12010028
Barreca S, Mancuso MMM, Sacristán D, Pace A, Savoca D, Orecchio S. Determination of Perfluorooctanoic Acid (PFOA) in the Indoor Dust Matter of the Sicily (Italy) Area: Analysis and Exposure Evaluations. Toxics. 2024; 12(1):28. https://doi.org/10.3390/toxics12010028
Chicago/Turabian StyleBarreca, Salvatore, Michele Marco Mizio Mancuso, Daniel Sacristán, Andrea Pace, Dario Savoca, and Santino Orecchio. 2024. "Determination of Perfluorooctanoic Acid (PFOA) in the Indoor Dust Matter of the Sicily (Italy) Area: Analysis and Exposure Evaluations" Toxics 12, no. 1: 28. https://doi.org/10.3390/toxics12010028
APA StyleBarreca, S., Mancuso, M. M. M., Sacristán, D., Pace, A., Savoca, D., & Orecchio, S. (2024). Determination of Perfluorooctanoic Acid (PFOA) in the Indoor Dust Matter of the Sicily (Italy) Area: Analysis and Exposure Evaluations. Toxics, 12(1), 28. https://doi.org/10.3390/toxics12010028










