Olfactory Learning Behavior and Mortality of the Honey Bee Apis mellifera jemenitica in Response to Pyrethroid Insecticide (Deltamethrin)
Abstract
1. Introduction
2. Materials and Methods
2.1. Capture and Conservation of Bees
2.2. Insecticides and Application
2.3. Toxicity Bioassay
2.3.1. Topical Application
2.3.2. Oral Feeding
2.4. Probit Analysis
2.5. Olfactory Learning Trials and Memory Tests
2.6. Statistical Analysis
3. Results
3.1. Percent Mortality of Honey Bees
3.1.1. Topical Application of Deltamethrin
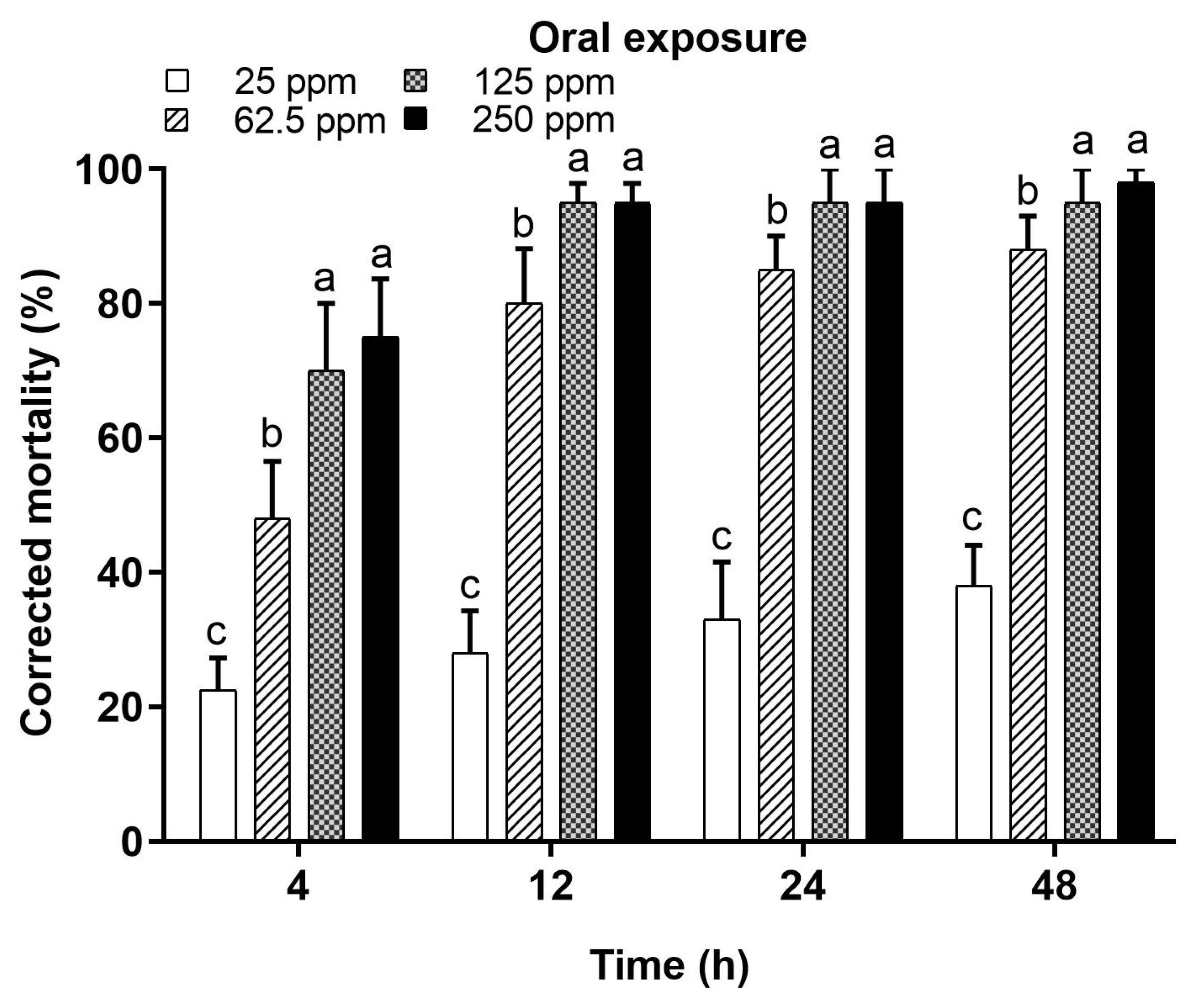
3.1.2. Oral Application of Deltamethrin
3.1.3. Food Consumption during Oral Feeding (Sensitivity Response)
3.1.4. Lethal Concentrations of Deltamethrin
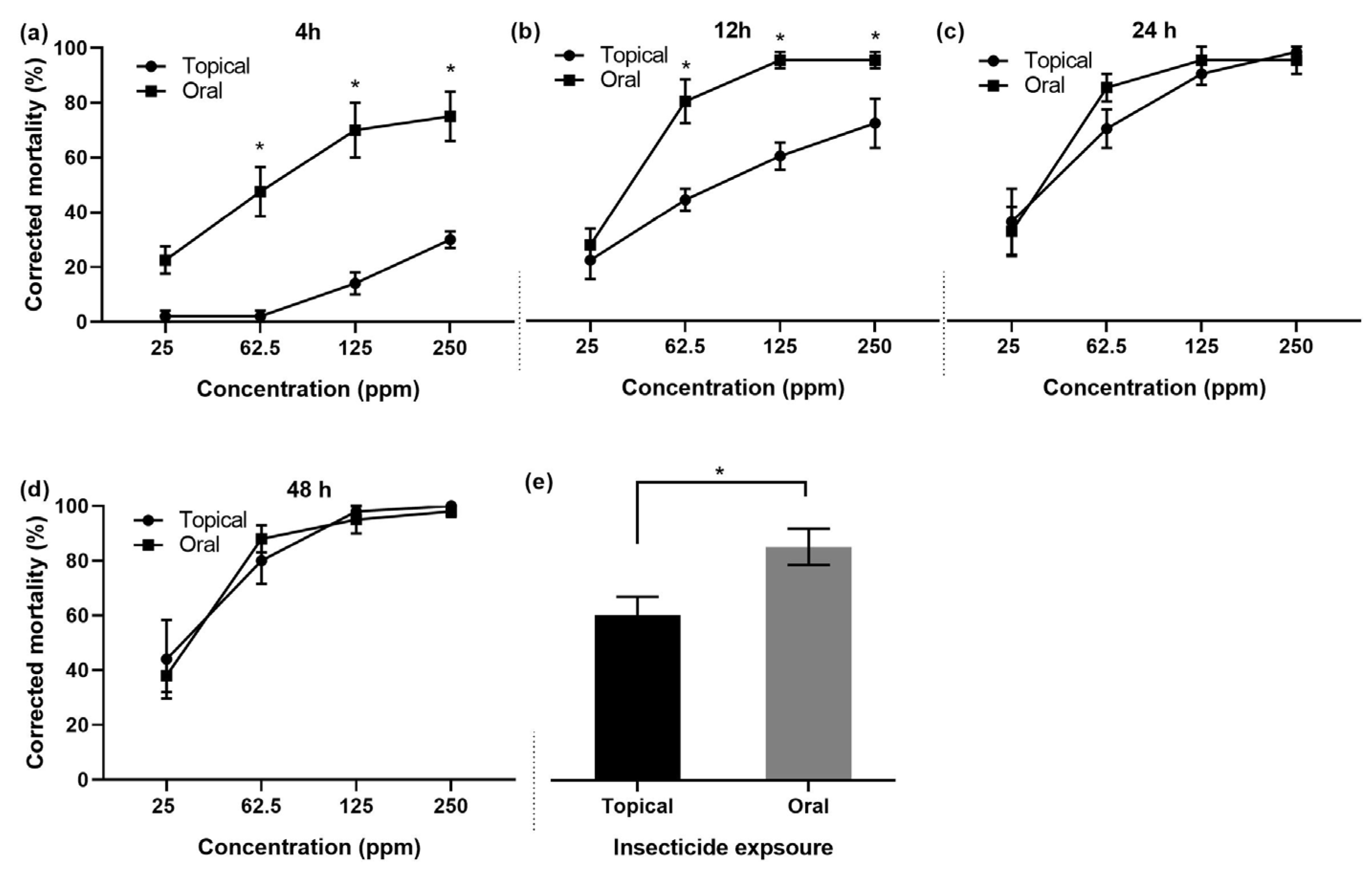
3.1.5. Mortality of Bees: Topical vs. Oral Exposure
3.2. Olfactory Behavior of Honey Bees
3.2.1. Associative Learning and Topical Exposure to Deltamethrin
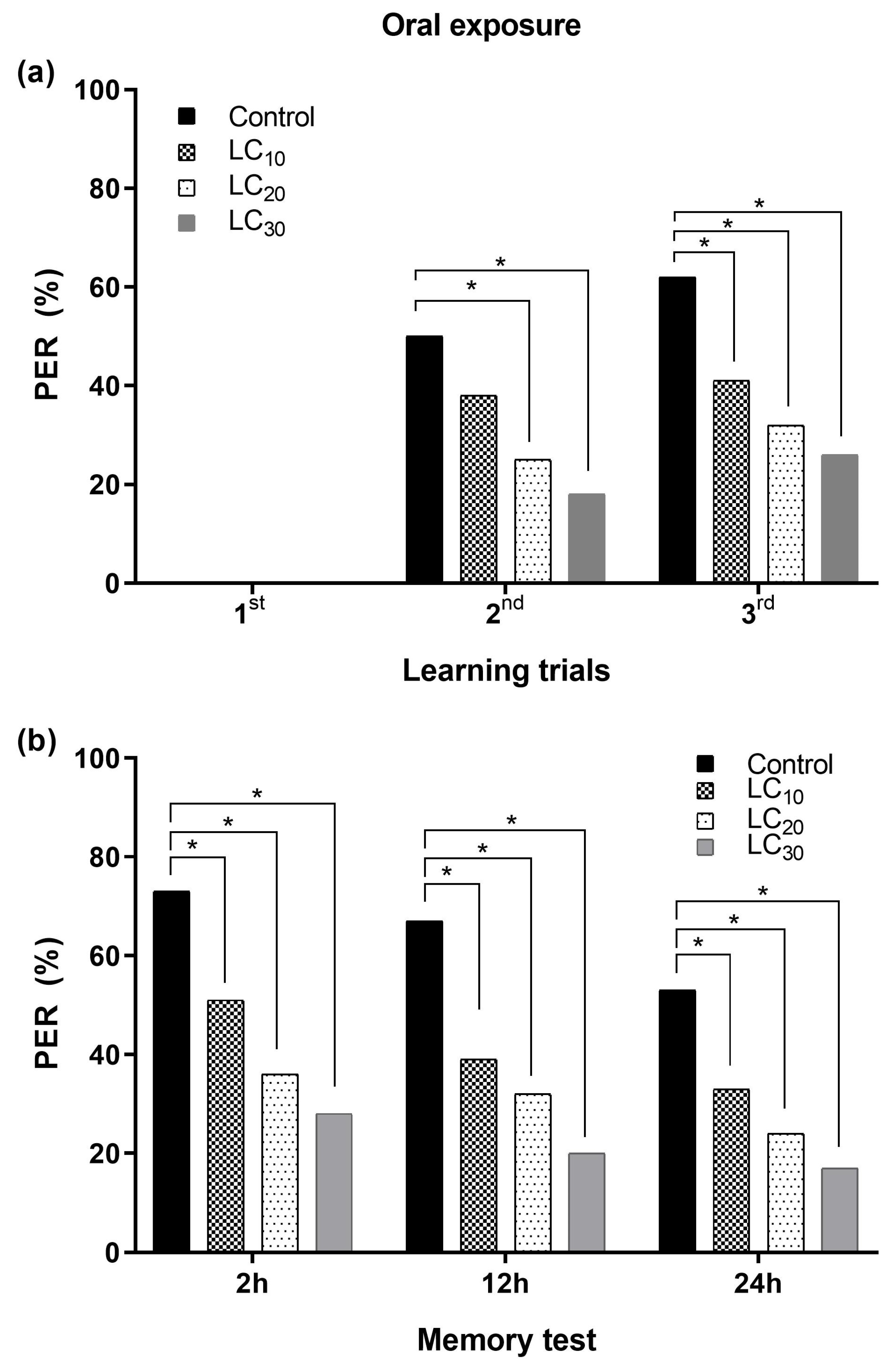
3.2.2. Associative Learning and Oral Exposure to Deltamethrin
4. Discussion
4.1. Topical Application and Mortality
4.2. Oral Application and Mortality
4.3. Comparison of Topical vs. Oral Exposure to Deltamethrin for Bee Mortality
4.4. Lethal Concentrations
4.5. Associative Learning of Honey Bees
4.5.1. Topical Route and Learning and Memory
4.5.2. Oral Route and Learning and Memory
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eilers, E.J.; Kremen, C.; Smith Greenleaf, S.; Garber, A.K.; Klein, A.-M. Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS ONE 2011, 6, e21363. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. Lond. B. Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembski, S.; Lindner, A.; Konaté, S.; Linsenmair, E.K. Bee pollination increases yield quantity and quality of cash crops in Burkina Faso, West Africa. Sci. Rep. 2017, 7, 17691. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Ghosh, A.; Roy, C.; Mazumder, I.; Marrazzo, P. Revolutionizing the use of honeybee products in healthcare: A focused review on using bee pollen as a potential adjunct material for biomaterial functionalization. J. Funct. Biomater. 2023, 14, 352. [Google Scholar] [CrossRef]
- Nowak, A.; Szczuka, D.; Górczyńska, A.; Motyl, I.; Kręgiel, D. Characterization of Apis mellifera gastrointestinal microbiota and lactic acid bacteria for honeybee protection—A review. Cells 2021, 10, 701. [Google Scholar] [CrossRef]
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and abiotic factors associated with colonies mortalities of managed honey bee (Apis mellifera). Diversity 2019, 11, 237. [Google Scholar] [CrossRef]
- Grover, A.; Kalia, P.; Sinha, R.; Garg, P. Colony collapse disorder: A peril to apiculture. J. Appl. Nat. Sci. 2022, 14, 729–739. [Google Scholar] [CrossRef]
- James, R.R.; Li, Z. Chapter 12—From silkworms to bees: Diseases of beneficial insects. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 425–459. [Google Scholar] [CrossRef]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors associated with honey bee colony losses: A Mini-review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef]
- Pudasaini, R. Behavioral changes due to sub-lethal doses of pesticides in bees. J. Entomol. 2020, 17, 84–92. [Google Scholar] [CrossRef]
- Milford, A.B.; Hatteland, B.A.; Ursin, L.Ø. The responsibility of farmers, public authorities and consumers for safeguarding bees against harmful pesticides. J. Agric. Environ. Ethics 2022, 35, 13. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes: Volume 1: Implications in Crop Science; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar] [CrossRef]
- Faraj, T.K. Determination of pesticide residues in most commonly consumed leafy vegetables in Riyadh Region (Al-Kharej Province). J. King Abdulaziz Univ. Meteorol. Environ. Arid Land Agric. Sci. 2019, 28, 63–73. [Google Scholar] [CrossRef]
- Belzunces, L.P.; Tchamitchian, S.; Brunet, J.-L. Neural effects of insecticides in the honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, Z.; Huang, Q.; Zeng, Z.J. The effects of sublethal doses of imidacloprid and deltamethrin on honeybee foraging time and the brain transcriptome. J. Appl. Entomol. 2022, 146, 1169–1177. [Google Scholar] [CrossRef]
- Gradish, A.E.; van der Steen, J.; Scott-Dupree, C.D.; Cabrera, A.R.; Cutler, G.C.; Goulson, D.; Klein, O.; Lehmann, D.M.; Lückmann, J.; O’Neill, B.; et al. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and Bumble bees (Hymenoptera: Apidae): Implications for risk assessments. Environ. Entomol. 2018, 48, 12–21. [Google Scholar] [CrossRef]
- Zhao, H.; Li, G.; Cui, X.; Wang, H.; Liu, Z.; Yang, Y.; Xu, B. Review on effects of some insecticides on honey bee health. Pestic. Biochem. Physiol. 2022, 188, 105219. [Google Scholar] [CrossRef]
- López, J.H.; Krainer, S.; Engert, A.; Schuehly, W.; Riessberger-Gallé, U.; Crailsheim, K. Sublethal pesticide doses negatively affect survival and the cellular responses in American foulbrood-infected honeybee larvae. Sci. Rep. 2017, 7, 40853. [Google Scholar] [CrossRef]
- Chmiel, J.A.; Daisley, B.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Understanding the effects of sublethal pesticide exposure on honey bees: A role for probiotics as mediators of environmental stress. Front. Ecol. Evol. 2020, 8, 22. [Google Scholar] [CrossRef]
- Iqbal, J.; Alqarni, A.S.; Raweh, H.S.A. Effect of sub-lethal doses of imidacloprid on learning and memory formation of indigenous Arabian bee (Apis mellifera jemenitica Ruttner) adult foragers. Neotrop. Entomol. 2019, 48, 373–380. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J.; Genecque, E.; Menach, K.L.; Budzinski, H.; Cluzeau, S.; Pham-Delègue, M.H. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch. Environ. Contam. Toxicol. 2005, 48, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.L.; Wang, Q.; Sun, J.-H.; Liu, F.; Wang, X.; Wu, Y.-Y.; Zhou, T. Effects of sublethal concentrations of bifenthrin and deltamethrin on fecundity, growth, and development of the honeybee Apis mellifera ligustica. Environ. Toxicol. Chem. 2010, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Chen, W.W.; Dong, S.H.; Liu, X.W.; Wang, Y.C.; Nieh, J.C. Imidacloprid alters foraging and decreases bee avoidance of predators. PLoS ONE 2014, 9, e102725. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; David, A.; Horwood, J.; Abdul-Sada, A.; Nicholls, E.; Hill, E.; Goulson, D. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 2015, 49, 12731–12740. [Google Scholar] [CrossRef]
- Francisco, S.-B.; Koichi, G. Impacts of Pesticides on Honey Bees. In Beekeeping and Bee Conservation; Emerson Dechechi, C., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Saggu, S.; Rehman, H.; Alzeiber, F.M.; Aziz, A. Current situation of pesticide consumption and poisoning in Saudi Arabia. J. Entomol. Zool. Stud. 2016, 4, 153–158. [Google Scholar]
- El-Saeid, M.H.; Khan, H.A. Determination of pyrethroid insecticides in crude and canned vegetable samples by supercritical fluid chromatography. Int. J. Food Prop. 2015, 18, 1119–1127. [Google Scholar] [CrossRef]
- EL-Saeid, M.H.; Selim, M. Multiresidue analysis of 86 pesticides using gas chromatography mass spectrometry: II-nonleafy vegetables. J. Chem. 2013, 2013, 727149. [Google Scholar] [CrossRef]
- Osman, K.A.; Al-Humaid, A.; Al-Rehiayani, S.; Al-Redhaiman, K. Estimated daily intake of pesticide residues exposure by vegetables grown in greenhouses in Al-Qassim region, Saudi Arabia. Food Control 2011, 22, 947–953. [Google Scholar] [CrossRef]
- EL-Saeid, M.; Shaht, M. Detection of pesticide residues and heavy metals in some fresh fruits and vegetables collected from Cairo. In Proceedings of the 1st Mansoura Conf. of Food and Dairy Tech., Cairo, Egypt, 17–19 October; pp. 183–203.
- Selim, M.; El-Saeid, M.; Al-Dossari, I. Multi-residues analysis of pesticides using gas chromatography mass spectrometry: I-leafy vegetables. Res. J. Environ. Sci. 2011, 5, 248–258. [Google Scholar] [CrossRef][Green Version]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, A.; Schmid, A.P.; Manzer, S.; Schulte, J.; Scheiner, R. Interaction of insecticides and fungicides in bees. Front. Insect Sci. 2022, 1, 808335. [Google Scholar] [CrossRef]
- Dieng, H.; Hassan, A.A.; Satho, T.; Miake, F.; Salmah, M.R.C.; AbuBakar, S. Insecticide susceptibility of the dengue vector Aedes aegypti (Diptera: Culicidae) in Makkah City, Saudi Arabia. Asian Pac. J. Trop. Dis. 2011, 1, 94–99. [Google Scholar] [CrossRef]
- Al-Sarar, A.S. Insecticide resistance of Culex pipiens (L.) populations (Diptera: Culicidae) from Riyadh city, Saudi Arabia: Status and overcome. Saudi J. Biol. Sci. 2010, 17, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Hafez, A.M. First comprehensive report of the resistance of Culex quinquefasciatus Say (Diptera: Culicidae) to commonly used insecticides in Riyadh, Saudi Arabia. Heliyon 2023, 9, e12709. [Google Scholar] [CrossRef] [PubMed]
- Al Nazawi, A.M.; Weetman, D. Age-dependence of susceptibility to single and repeated deltamethrin exposure in pyrethroid-resistant Aedes aegypti strains. Curr. Res. Parasitol. Vector-Borne Dis. 2023, 3, 100121. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.H.; Abbas, Z.K.; Ansari, A.A.; Khan, M.N.; Ansari, W.A. Pesticides and their effects on plants: A case study of deltamethrin. In Agrochemicals in Soil and Environment: Impacts and Remediation; Naeem, M., Bremont, J.F.J., Ansari, A.A., Gill, S.S., Eds.; Springer: Singapore, 2022; pp. 183–193. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J.; Cluzeau, S.; Charreton, M.; Pham-Delègue, M.-H. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 2004, 57, 410–419. [Google Scholar] [CrossRef]
- Dong, Z.-X.; Tang, Q.-H.; Li, W.-L.I.; Wang, Z.-W.; Li, X.-J.; Fu, C.-M.; Li, D.; Qian, K.; Tian, W.-L.I.; Guo, J. Honeybee (Apis mellifera) resistance to deltamethrin exposure by Modulating the gut microbiota and improving immunity. Environ. Pollut. 2022, 314, 120340. [Google Scholar] [CrossRef]
- Van dame, R.; Meled, M.; Colin, M.; Belzunces, L. Alteration of the homing-flight in the honey bee Apis mellifera L. Exposed to sublethal dose of deltamethrin. Environ. Toxicol. Chem. 1995, 14, 855–860. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, Z.; Huang, Q.; Zhang, X.W.; Ke, L.; Yan, W.; Zhang, L.Z.; Zeng, Z.-J. Deltamethrin impairs honeybees (Apis mellifera) dancing communication. Arch. Environ. Contam. Toxicol. 2020, 78, 117–123. [Google Scholar] [CrossRef]
- Thom, C.; Gilley, D.C.; Hooper, J.; Esch, H.E. The scent of the waggle dance. PLoS Biol. 2007, 5, e228. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.A.; Gott, A.; Scott, J.; Chavarria, N.L.; Wright, G.A. Honeybees fail to discriminate floral scents in a complex learning task after consuming a neonicotinoid pesticide. J. Exp. Biol. 2020, 223, jeb217174. [Google Scholar] [CrossRef] [PubMed]
- Dani, F.R.; Jones, G.R.; Corsi, S.; Beard, R.; Pradella, D.; Turillazzi, S. Nestmate recognition cues in the honey bee: Differential importance of cuticular alkanes and alkenes. Chem. Senses 2005, 30, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Bell, H.C.; Montgomery, C.N.; Benavides, J.E.; Nieh, J.C. Effects of Nosema ceranae (Dissociodihaplophasida: Nosematidae) and Flupyradifurone on olfactory learning in honey bees Apis mellifera (Hymenoptera: Apidae). J. Insect Sci. 2020, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.J.; Pettit, T.J.; Irga, P.; McArthur, C.; Hochuli, D.F. Acute exposure to urban air pollution impairs olfactory learning and memory in honeybees. Ecotoxicol. 2019, 28, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Piechowicz, B.; Początek, E.; Woś, I.; Zaręba, L.; Koziorowska, A.; Podbielska, M.; Grodzicki, P.; Szpyrka, E.; Sadło, S. Insecticide and fungicide effect on thermal and olfactory behavior of bees and their disappearance in bees’ tissues. Environ. Toxicol. Pharmacol. 2022, 95, 103975. [Google Scholar] [CrossRef] [PubMed]
- Aliouane, Y.; Hassani, A.; Gary, V.; Armengaud, C.; Lambin, M.; Gauthier, M. Subchronic exposure of honeybees to sublethal doses of pesticides: Effects on behavior. Environ. Toxicol. Chem. 2008, 28, 113–122. [Google Scholar] [CrossRef]
- Haynes, K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Nuru, A. Beekeeping in the Kingdom of Saudi Arabia opportunities and challenges. Bee World 2013, 90, 54–57. [Google Scholar] [CrossRef]
- Adgaba, N.; Al-Ghamdi, A.A.; Shenkute, A.G.; Ismaiel, S.M.; Al-Kahtani, S.N.; Tadess, Y.; Ansari, M.J.; Abebe, W.; Abdulaziz, M. Socio-economic analysis of beekeeping and determinants of box hive technology adoption in the kingdom of Saudi Arabia. J. Anim. Plant Sci. 2014, 24, 1876–1884. [Google Scholar]
- Alattal, Y.; Al Ghamdi, A.; Al Sharhi, M.; Fuchs, S. Morphometric characterisation of the native honeybee, Apis mellifera Linnaeus, 1758, of Saudi Arabia. Biol. Open 2014, 60, 226–235. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Hannan, M.A.; Owayss, A.A.; Engel, M.S. The indigenous honey bees of Saudi Arabia (Hymenoptera, Apidae, Apis mellifera jemenitica Ruttner): Their natural history and role in beekeeping. Zookeys 2011, 134, 83–98. [Google Scholar] [CrossRef]
- Iqbal, J.; Ali, H.; Owayss, A.A.; Raweh, H.S.A.; Engel, M.S.; Alqarni, A.S.; Smith, B.H. Olfactory associative behavioral differences in three honey bee Apis mellifera L. races under the arid zone ecosystem of central Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alqarni, A.S.; Owayss, A.A.; Hassan, A.M.; Smith, B.H. Osmotic concentration in three races of honey bee, Apis mellifera L. under environmental conditions of arid zone. Saudi J. Biol. Sci. 2017, 24, 1081–1085. [Google Scholar] [CrossRef]
- OECD. Guidance Document on Honey Bee (Apis mellifera L.) Homing Flight Tests, Using Single Oral Exposure to Sublethal Doses of Test Chemicals; OECD: Paris, France, 2021. [Google Scholar]
- Williams, G.R.; Alaux, C.; Costa, C.; Csáki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; McMahon, D.P.; Medrzycki, P.; et al. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Ladurner, E.; Bosch, J.; Kemp, W.P.; Maini, S. Assessing delayed and acute toxicity of five formulated fungicides to Osmia lignaria Say and Apis mellifera. Apidologie 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Del Sarto, M.C.L.; Oliveira, E.E.; Guedes, R.N.C.; Campos, L.A.O. Differential insecticide susceptibility of the Neotropical stingless bee Melipona quadrifasciata and the honey bee Apis mellifera. Apidologie 2014, 45, 626–636. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Nasr, H.M.; Rabea, E.I. Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie 2015, 46, 177–193. [Google Scholar] [CrossRef]
- OECD. Test No. 214: Honeybees, acute contact toxicity test. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 1998; Volume 2, pp. 2–4. [Google Scholar]
- Bisrat, D.; Ulziibayar, D.; Begna, T.; Jung, C. Differential effects of the detoxicant on the honey bee intoxicated with seven pesticides and its chemical characterization. J. Apic. 2020, 35, 91–98. [Google Scholar] [CrossRef]
- Litsey, E.; Chung, S.; Fine, J.D. The behavioral toxicity of insect growth disruptors on Apis mellifera queen care. Front. Ecol. Evol. 2021, 9, 729208. [Google Scholar] [CrossRef]
- Shah, R.; Maawali, A.S.A.A.; Raeesi, A.A. Comparative toxicity of two neonicotinoids and a pyrethroid to forager honeybees (Apis mellifera L., 1758) (Hymenoptera: Apidae) by different exposure methods. Turk. J. Entomol. 2020, 44, 111–121. [Google Scholar] [CrossRef]
- OECD. Test No. 245: Honey Bee (Apis mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding); OECD: Paris, France, 2017. [Google Scholar] [CrossRef]
- Anwar, M.I.; Sadiq, N.; Aljedani, D.M.; Iqbal, N.; Saeed, S.; Khan, H.A.A.; Naeem-Ullah, U.; Aslam, H.M.F.; Ghramh, H.A.; Khan, K.A. Toxicity of different insecticides against the dwarf honey bee, Apis florea Fabricius (Hymenoptera: Apidae). J. King Saud Univ. Sci. 2022, 34, 101712. [Google Scholar] [CrossRef]
- Dhooria, M.S. Ane’s Encyclopedic Dictionary of General & Applied Entomology; Springer: Dordrecht, Germany, 2008. [Google Scholar] [CrossRef]
- Finney, D. A statistical treatment of the sigmoid response curve. In Probit Analysis; Cambridge University Press: London, UK, 1971; Volume 633. [Google Scholar]
- Baker, E. Ldp Line. 2007. Available online: http://www.ehabsoft.com/ldpline/ (accessed on 23 December 2023).
- Smith, B.H.; Burden, C.M. A proboscis extension response protocol for investigating behavioral plasticity in insects: Application to basic, biomedical, and agricultural research. J. Vis. Exp. 2014, 91, e51057. [Google Scholar] [CrossRef]
- Iqbal, J.; Mueller, U. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. B. 2007, 274, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Marter, K.; Grauel, M.K.; Lewa, C.; Morgenstern, L.; Buckemüller, C.; Heufelder, K.; Ganz, M.; Eisenhardt, D. Duration of the unconditioned stimulus in appetitive conditioning of honeybees differentially impacts learning, long-term memory strength, and the underlying protein synthesis. Learn. Mem. 2014, 21, 676–685. [Google Scholar] [CrossRef]
- Avalos, A.; Pérez, E.; Vallejo, L.; Pérez, M.E.; Abramson, C.I.; Giray, T. Social signals and aversive learning in honey bee drones and workers. Biol. Open 2017, 6, 41–49. [Google Scholar] [CrossRef]
- Ullah, A.; Gajger, I.; Majoros, A.; Dar, S.A.; Khan, S.; Kalimullah; Shah, A.H.; Khabir, M.N.; Hussain, R.; Khan, H.U.; et al. Viral impacts on honey bee populations: A review. Saudi J. Biol. Sci. 2021, 28, 523–530. [Google Scholar] [CrossRef]
- Radwan, M.; Sand, R.E.; Hendawy, M. Acute toxicity of some insecticides on honeybee, Apis mellifera L. Zagazig J. Agric. Res. 2020, 47, 65–70. [Google Scholar] [CrossRef]
- Costa, E.M.; Araujo, E.L.; Maia, A.V.P.; Silva, F.E.L.; Bezerra, C.E.S.; Silva, J.G. Toxicity of insecticides used in the Brazilian melon crop to the honey bee Apis mellifera under laboratory conditions. Apidologie 2014, 45, 34–44. [Google Scholar] [CrossRef]
- Nabti, D.; Achou, M.; Soltani, N. DECIS 25 EC toxicity to Algerian honeybees. J. Entomol. Zool. Stud. 2015, 3, 285–288. [Google Scholar]
- Stanley, J.; Sah, K.; Jain, S.K.; Bhatt, J.C.; Sushil, S.N. Evaluation of pesticide toxicity at their field recommended doses to honeybees, Apis cerana and A. mellifera through laboratory, semi-field and field studies. Chemosphere 2015, 119, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Beadle, K. Understanding the Molecular and Biochemical Basis of Insecticide Selectivity against Solitary Bee Pollinators; University of Exeter: Exeter, UK, 2018. [Google Scholar]
- Aljedani, D.M. Effects of abamectin and deltamethrin to the foragers honeybee workers of Apis mellifera jemenatica (Hymenoptera: Apidae) under laboratory conditions. Saudi J. Biol. Sci. 2017, 24, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Romero, R.; Chaufaux, J.; Pham-Delègue, M.-H. Effects of Cry1Ab protoxin, deltamethrin and imidacloprid on the foraging activity and the learning performances of the honeybee Apis mellifera, a comparative approach. Apidologie 2005, 36, 601–611. [Google Scholar] [CrossRef]
- Devillers, J.; Pham-Delègue, M.-H. Honey Bees: Estimating the Environmental Impact of Chemicals; Taylor & Francis: New York, NY, USA, 2002. [Google Scholar]
- Akça, R.; Saruhan, I. The effects of some insecticides on honeybees (Apis mellifera). Isr. J. Ecol. Evol. 2022, 69, 37–43. [Google Scholar] [CrossRef]
- Medrzycki, P.; Giffard, H.; Aupinel, P.; Belzunces, L.P.; Chauzat, M.-P.; Classen, C.; Colin, M.E.; Dupont, T.; Girolami, V.; Johnson, R. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 2013, 52, 1–60. [Google Scholar] [CrossRef]
- Sanchez-Arroyo, H. Differential toxicity of pyrethroid and organophosphate insecticides to the honey bee, Apis mellifera and the yellow fever mosquito, Aedes aegypti. J. Fla. Mosq. Control Assoc. 2021, 68, 70–78. [Google Scholar] [CrossRef]
- Sanchez-Arroyo, H. Laboratory toxicity of mosquito adulticides to the Asian tiger mosquitoes, Aedes albopictus and the honey bees, Apis mellifera. J. Fla. Mosq. Control Assoc. 2019, 66, 40–46. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Belzunces, L.P.; Carvalho, G.A.; Brunet, J.L.; Badiou-Beneteau, A. Enzymatic biomarkers as tools to assess environmental quality: A case study of exposure of the honeybee Apis mellifera to insecticides. Environ. Toxicol. Chem. 2013, 32, 2117–2124. [Google Scholar] [CrossRef]
- Tomé, H.V.; Ramos, G.S.; Araújo, M.F.; Santana, W.C.; Santos, G.R.; Guedes, R.N.; Maciel, C.D.; Newland, P.L.; Oliveira, E.E. Agrochemical synergism imposes higher risk to Neotropical bees than to honeybees. R. Soc. Open Sci. 2017, 4, 160866. [Google Scholar] [CrossRef]
- WHO. Deltamethrin; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Vaziritabar, S.; Oshidari, S.; Aghamirkarimi, A. A survey of pesticide remainders in pollen loads collected by honey bees in the Alborz province apiaries in Iran. J. Biodivers. Environ. Sci. 2015, 7, 107–124. [Google Scholar]
- Manzoor, F.; Pervez, M. Pesticide impact on honeybees declines and emerging food security crisis. In Global Decline of Insects; Hamadttu Abdel Farag, E.-S., Ed.; IntechOpen: Rijeka, Croatia, 2021; pp. 371–512. [Google Scholar] [CrossRef]
- Mamood, A.N.; Waller, G.D. Recovery of learning responses by honeybees following a sublethal exposure to permethrin. Physiol. Entomol. 1990, 15, 55–60. [Google Scholar] [CrossRef]
- Frost, E.H.; Shutler, D.; Hillier, N.K. Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J. Exp. Biol. 2013, 216, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; He, X.-J.; Wang, Z.-L.; Barron, A.B.; Zhang, B.; Zeng, Z.-J.; Wu, X.-B. Short-term exposure to lambda-cyhalothrin negatively affects the survival and memory-related characteristics of worker bees Apis mellifera. Arch. Environ. Contam. Toxicol. 2018, 75, 59–65. [Google Scholar] [CrossRef]
- Thany, S.H.; Bourdin, C.M.; Graton, J.; Laurent, A.D.; Mathé-Allainmat, M.; Lebreton, J.; Questel, J.Y. Similar comparative low and high doses of deltamethrin and acetamiprid differently impair the retrieval of the proboscis extension reflex in the forager honey hee (Apis mellifera). Insects 2015, 6, 805–814. [Google Scholar] [CrossRef]
- Dworzańska, D.; Moores, G.; Zamojska, J.; Strażyński, P.; Węgorek, P. The influence of acetamiprid and deltamethrin on the mortality and behaviour of honeybees (Apis mellifera carnica Pollman) in oilseed rape cultivations. Apidologie 2020, 51, 1143–1154. [Google Scholar] [CrossRef]




| Exposure Mode | Time (h) | LC50 (ppm) | LC90 (ppm) |
|---|---|---|---|
| Topical | 12 | 86.28 | 759.46 |
| 24 | 36.16 | 125.65 | |
| 48 | 29.19 | 79.62 | |
| Oral | 12 | 35.77 | 193.37 |
| 24 | 32.53 | 115.11 | |
| 48 | 30.78 | 83.24 |
| Time (h) | Sublethal Concentration (ppm) | |||||
|---|---|---|---|---|---|---|
| Topical Exposure | Oral Exposure | |||||
| LC10 | LC20 | LC30 | LC10 | LC20 | LC30 | |
| 12 | 9.80 | 20.68 | 35.43 | 6.61 | 11.81 | 17.93 |
| 24 | 10.40 | 15.95 | 21.72 | 9.19 | 14.19 | 19.40 |
| 48 | 10.70 | 15.10 | 19.36 | 11.83 | 16.02 | 20.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuagla, M.I.B.; Iqbal, J.; Raweh, H.S.A.; Alqarni, A.S. Olfactory Learning Behavior and Mortality of the Honey Bee Apis mellifera jemenitica in Response to Pyrethroid Insecticide (Deltamethrin). Toxics 2024, 12, 25. https://doi.org/10.3390/toxics12010025
Abuagla MIB, Iqbal J, Raweh HSA, Alqarni AS. Olfactory Learning Behavior and Mortality of the Honey Bee Apis mellifera jemenitica in Response to Pyrethroid Insecticide (Deltamethrin). Toxics. 2024; 12(1):25. https://doi.org/10.3390/toxics12010025
Chicago/Turabian StyleAbuagla, Mohamedazim I. B., Javaid Iqbal, Hael S. A. Raweh, and Abdulaziz S. Alqarni. 2024. "Olfactory Learning Behavior and Mortality of the Honey Bee Apis mellifera jemenitica in Response to Pyrethroid Insecticide (Deltamethrin)" Toxics 12, no. 1: 25. https://doi.org/10.3390/toxics12010025
APA StyleAbuagla, M. I. B., Iqbal, J., Raweh, H. S. A., & Alqarni, A. S. (2024). Olfactory Learning Behavior and Mortality of the Honey Bee Apis mellifera jemenitica in Response to Pyrethroid Insecticide (Deltamethrin). Toxics, 12(1), 25. https://doi.org/10.3390/toxics12010025








