The Cytotoxic Effects of Fine Particulate Matter (PM2.5) from Different Sources at the Air–Liquid Interface Exposure on A549 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. PM2.5 Collection and Preparation
2.2. Analysis of PM2.5 Chemical Components
2.3. Cell Culture and Gas–Liquid Interface Exposure
2.4. Cell Vitality Detection
2.5. ROS Detection
2.6. Detection of Inflammatory Factors
2.7. Detection of Cell Apoptosis Rate
2.8. DNA Damage Detection
2.9. Data Analysis
3. Results and Discussion
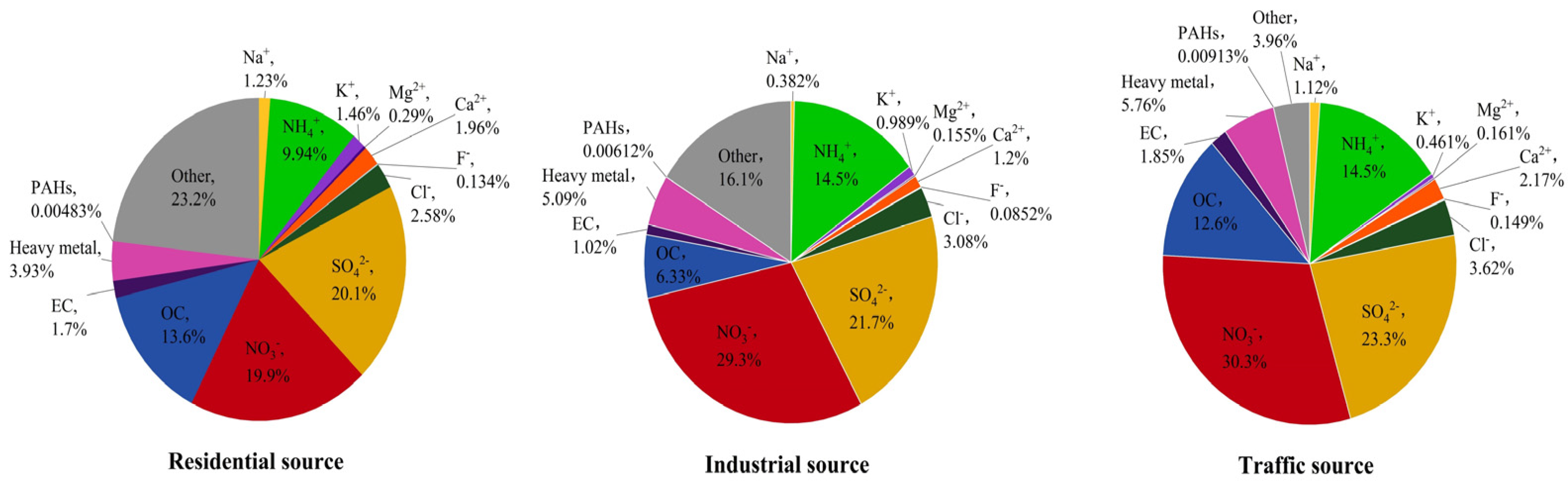
3.1. Analysis of PM2.5 Mass Concentration and Chemical Composition
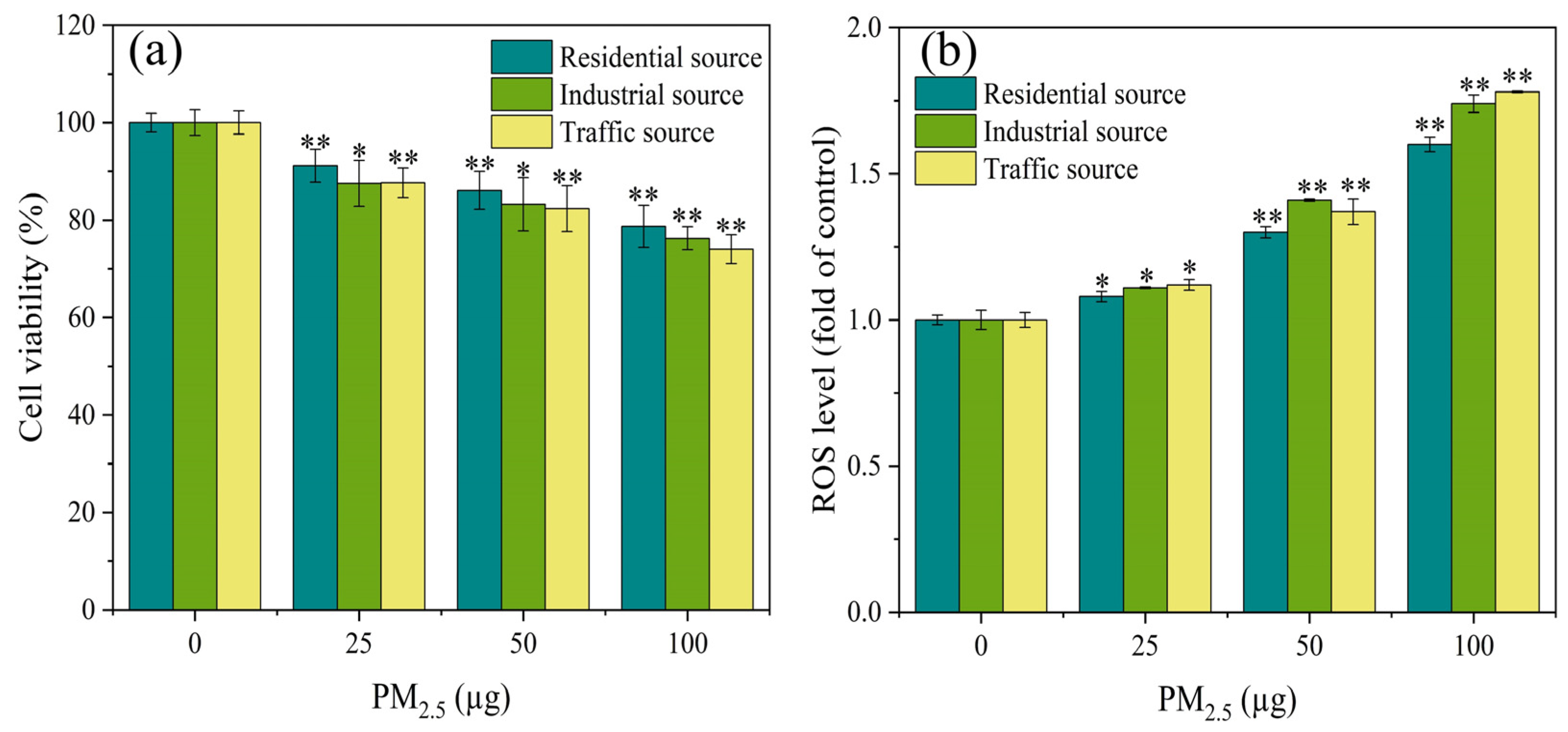
3.2. Cell Vitality
3.3. ROS Generation
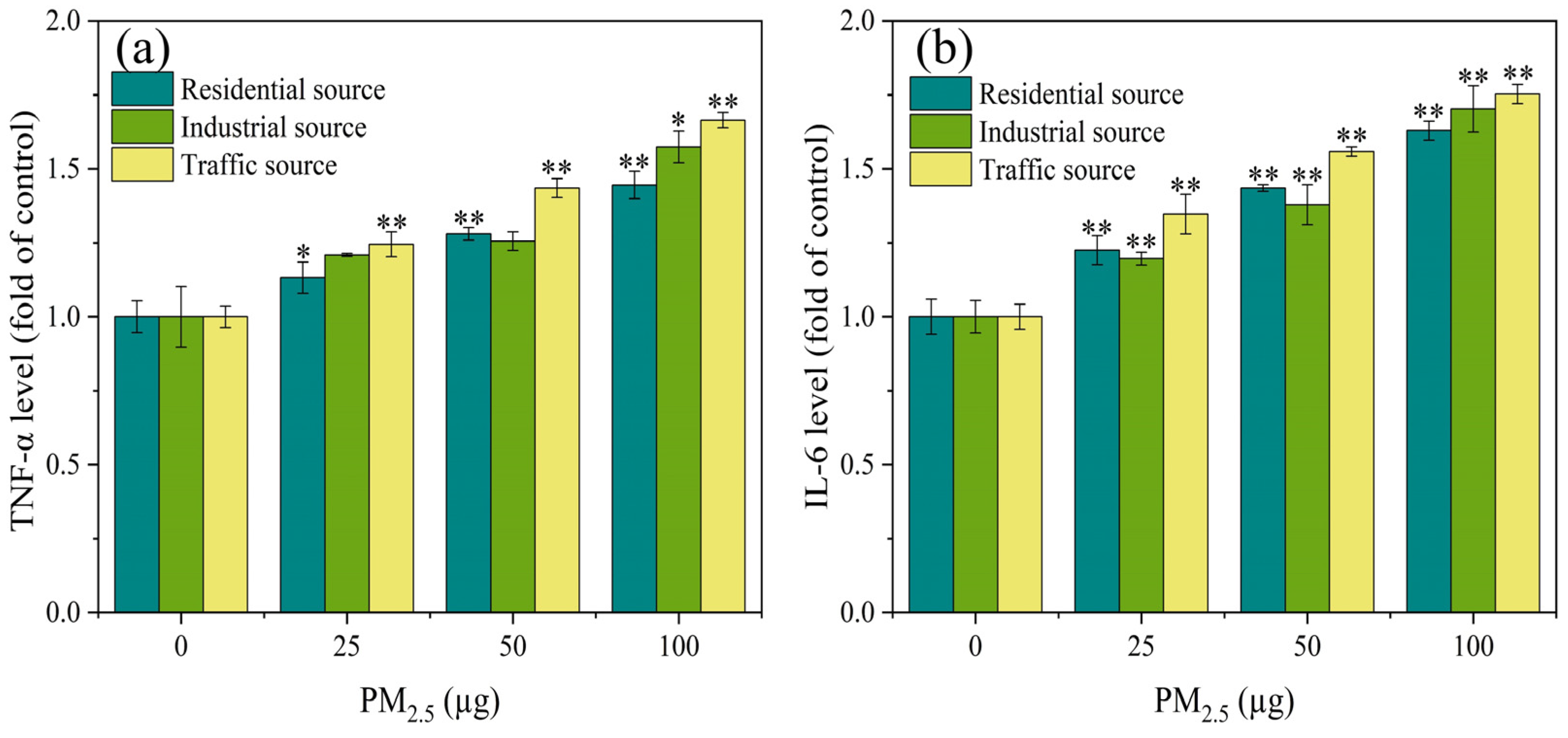
3.4. Expression Level of Inflammatory Factors
3.5. Cell Apoptosis Analysis
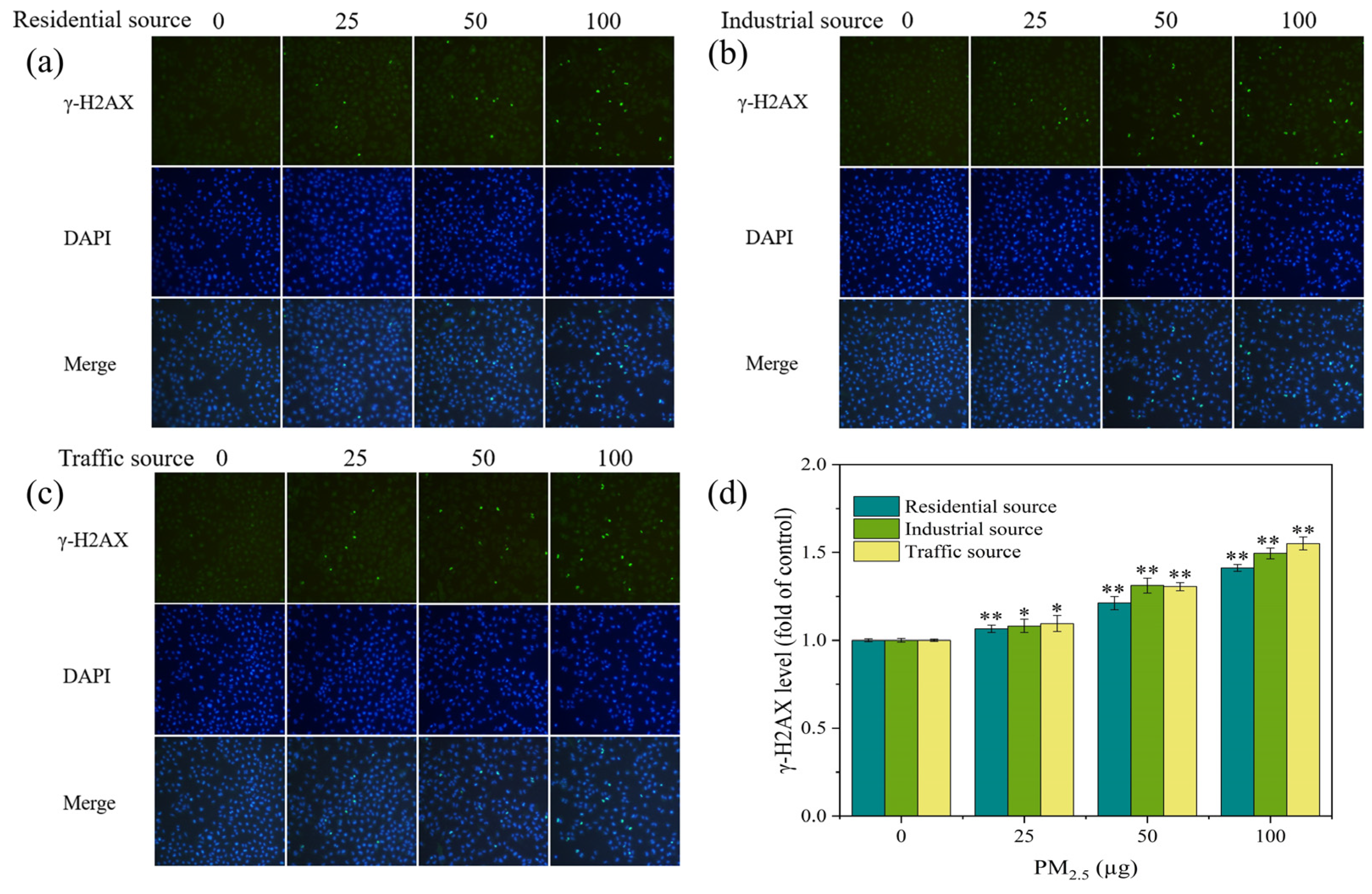
3.6. DNA Damage
3.7. Correlation Analysis between Biological Toxicity Indicators and Chemical Components of PM2.5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.-L.; Cao, F. Fine particulate matter (PM2.5) in China at a city level. Sci. Rep. 2015, 5, 14884. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, J.; Hao, Z.; Gong, C.; Tang, L.; Xu, Y.; Lu, D.; Li, Z.; Zhao, M. Suppression of progesterone synthesis in human trophoblast cells by fine particulate matter primarily derived from industry. Environ. Pollut. 2017, 231, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor Particulate Matter Exposure and Lung Cancer: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Liu, Z.; Chen, M.; Cui, Y.; Cao, M.; Liu, X. Chemical Characteristics and Cytotoxicity to GC-2spd(ts) Cells of PM(2.5) in Nanjing Jiangbei New Area from 2015 to 2019. Toxics 2023, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huo, T.; Zhang, X.; Ma, J.; Wang, Y.; Dong, F.; Deng, J. Oxidative stress and cell cycle arrest induced by short-term exposure to dustfall PM(2.5) in A549 cells. Environ. Sci. Pollut. Res. Int. 2018, 25, 22408–22419. [Google Scholar] [CrossRef] [PubMed]
- Lewtas, J. Air pollution combustion emissions: Characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat. Res./Rev. Mutat. Res. 2007, 636, 95–133. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Cho, C.-C.; Hsieh, W.-Y.; Tsai, C.-H.; Chen, C.-Y.; Chang, H.-F.; Lin, C.-S. In Vitro and In Vivo Experimental Studies of PM2.5 on Disease Progression. Int. J. Environ. Res. Public Health 2018, 15, 1380. [Google Scholar] [CrossRef]
- Park, M.; Joo, H.S.; Lee, K.; Jang, M.; Kim, S.D.; Kim, I.; Borlaza, L.J.S.; Lim, H.; Shin, H.; Chung, K.H.; et al. Differential toxicities of fine particulate matters from various sources. Sci. Rep. 2018, 8, 17007. [Google Scholar] [CrossRef]
- Rönkkö, T.J.; Jalava, P.I.; Happo, M.S.; Kasurinen, S.; Sippula, O.; Leskinen, A.; Koponen, H.; Kuuspalo, K.; Ruusunen, J.; Väisänen, O.; et al. Emissions and atmospheric processes influence the chemical composition and toxicological properties of urban air particulate matter in Nanjing, China. Sci. Total Environ. 2018, 639, 1290–1310. [Google Scholar] [CrossRef]
- MohseniBandpi, A.; Eslami, A.; Shahsavani, A.; Khodagholi, F.; Alinejad, A. Physicochemical characterization of ambient PM(2.5) in Tehran air and its potential cytotoxicity in human lung epithelial cells (A549). Sci. Total. Env. 2017, 593–594, 182–190. [Google Scholar] [CrossRef]
- Xin, L.; Wang, J.; Sun, J.; Zhang, C.; Tong, X.; Wan, J.; Feng, J.; Tian, H.; Zhang, Z. Cellular effects of PM2.5 from Suzhou, China: Relationship to chemical composition and endotoxin content. Environ. Sci. Pollut. Res. 2020, 28, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, G.; Erturk Ari, P.; Emerce, E.; Ari, A.; Odabasi, M.; Schins, R.; Burgaz, S.; Gaga, E.O. Investigation of spatial and temporal variation of particulate matter in vitro genotoxicity and cytotoxicity in relation to the elemental composition. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 842, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Landkocz, Y.; Ledoux, F.; Andre, V.; Cazier, F.; Genevray, P.; Dewaele, D.; Martin, P.J.; Lepers, C.; Verdin, A.; Courcot, L.; et al. Fine and ultrafine atmospheric particulate matter at a multi-influenced urban site: Physicochemical characterization, mutagenicity and cytotoxicity. Environ. Pollut. 2017, 221, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, X.S.; Zhao, Z.; Chen, Q.; Wu, D.; Sun, X.; Wu, L.; Jin, L. Summer-winter differences of PM(2.5) toxicity to human alveolar epithelial cells (A549) and the roles of transition metals. Ecotoxicol. Environ. Saf. 2018, 165, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Pang, Y.; Luo, X.S.; Chen, Q.; Wu, L.; Tang, M.; Hong, Y.; Chen, J.; Jin, L. The cytotoxicity and genotoxicity of PM(2.5) during a snowfall event in different functional areas of a megacity. Sci. Total Environ. 2020, 741, 140267. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Li, R.; Chen, W.; Chung, C.K.A.; Cai, Z. The cellular effects of PM(2.5) collected in Chinese Taiyuan and Guangzhou and their associations with polycyclic aromatic hydrocarbons (PAHs), nitro-PAHs and hydroxy-PAHs. Ecotoxicol. Environ. Saf. 2020, 191, 110225. [Google Scholar] [CrossRef]
- Kim, W.; Jeong, S.-C.; Shin, C.-Y.; Song, M.-K.; Cho, Y.; Lim, J.-H.; Gye, M.C.; Ryu, J.-C. A study of cytotoxicity and genotoxicity of particulate matter (PM2.5) in human lung epithelial cells (A549). Mol. Cell. Toxicol. 2018, 14, 163–172. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, F.; Rui, W.; Long, F.; Wang, L.; Feng, Z.; Chen, D.; Ding, W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. Vitr. 2013, 27, 1762–1770. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.Y.; Cao, J.Y.; Tao, F.B.; Zhu, X.X.; Yao, C.J.; Chen, D.J.; Che, Z.; Zhao, Q.H.; Wen, L.P. Oxidative stress, apoptosis, and cell cycle arrest are induced in primary fetal alveolar type II epithelial cells exposed to fine particulate matter from cooking oil fumes. Environ. Sci. Pollut. Res. Int. 2015, 22, 9728–9741. [Google Scholar] [CrossRef]
- Veerappan, I.; Sankareswaran, S.K.; Palanisamy, R. Morin Protects Human Respiratory Cells from PM(2.5) Induced Genotoxicity by Mitigating ROS and Reverting Altered miRNA Expression. Int. J. Environ. Res. Public Health 2019, 16, 2389. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant In Vitro Models for Investigating Air Pollutant-Induced Pulmonary Toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Offer, S.; Hartner, E.; Di Bucchianico, S.; Bisig, C.; Bauer, S.; Pantzke, J.; Zimmermann, E.J.; Cao, X.; Binder, S.; Kuhn, E.; et al. Effect of Atmospheric Aging on Soot Particle Toxicity in Lung Cell Models at the Air–Liquid Interface: Differential Toxicological Impacts of Biogenic and Anthropogenic Secondary Organic Aerosols (SOAs). Environ. Health Perspect. 2022, 130, 27003. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.H.; He, Q.; Carmieli, R.; Li, C.; Rudich, Y.; Pardo, M. Connecting the Oxidative Potential of Secondary Organic Aerosols with Reactive Oxygen Species in Exposed Lung Cells. Environ. Sci. Technol. 2019, 53, 13949–13958. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Ho, S.S.H.; Ho, K.F.; Huang, Y.; Sun, J.; Wang, Q.; Zhou, Y.; Zhao, Z.; Cao, J. Atmospheric levels and cytotoxicity of polycyclic aromatic hydrocarbons and oxygenated-PAHs in PM2.5 in the Beijing-Tianjin-Hebei region. Environ. Pollut. 2017, 231, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Z.; Luo, X.-S.; Fang, G.; Zhang, D.; Pang, Y.; Huang, W.; Mehmood, T.; Tang, M. Insight into urban PM2.5 chemical composition and environmentally persistent free radicals attributed human lung epithelial cytotoxicity. Ecotoxicol. Environ. Saf. 2022, 234, 113356. [Google Scholar] [CrossRef]
- Pang, Y.; Huang, W.; Luo, X.S.; Chen, Q.; Zhao, Z.; Tang, M.; Hong, Y.; Chen, J.; Li, H. In-vitro human lung cell injuries induced by urban PM(2.5) during a severe air pollution episode: Variations associated with particle components. Ecotoxicol. Environ. Saf. 2020, 206, 111406. [Google Scholar] [CrossRef]
- Yang, R.; Ge, P.; Liu, X.; Chen, W.; Yan, Z.; Chen, M. Chemical Composition and Transgenerational Effects on Caenorhabditis elegans of Seasonal Fine Particulate Matter. Toxics 2023, 11, 116. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, X.S.; Chen, Y.; Zhao, Z.; Hong, Y.; Pang, Y.; Huang, W.; Wang, Y.; Jin, L. Seasonally varied cytotoxicity of organic components in PM(2.5) from urban and industrial areas of a Chinese megacity. Chemosphere 2019, 230, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ge, P.; Lu, Z.; Yang, R.; Liu, Z.; Zhao, F.; Chen, M. Reproductive toxicity and underlying mechanisms of fine particulate matter (PM2.5) on Caenorhabditis elegans in different seasons. Ecotoxicol. Environ. Saf. 2022, 248, 114281. [Google Scholar] [CrossRef] [PubMed]
- Rahmatinia, T.; Kermani, M.; Farzadkia, M.; Jonidi Jafari, A.; Delbandi, A.A.; Rashidi, N.; Fanaei, F. The effect of PM(2.5)-related hazards on biomarkers of bronchial epithelial cells (A549) inflammation in Karaj and Fardis cities. Environ. Sci. Pollut. Res. Int. 2022, 29, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhou, T.; Shen, Y.; Rong, Y.; Zhang, Z.; Liu, Y.; Xiao, L.; Zhou, Y.; Li, W.; Chen, W. Different biological effects of PM(2.5) from coal combustion, gasoline exhaust and urban ambient air relate to the PAH/metal compositions. Environ. Toxicol. Pharmacol. 2019, 69, 120–128. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Lin, Z.; Wang, Y.; He, H.; Liu, T.; Kamp, D.W. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ. Toxicol. 2016, 31, 923–936. [Google Scholar] [CrossRef]
- Pardo, M.; Czech, H.; Offer, S.; Sklorz, M.; Di Bucchianico, S.; Hartner, E.; Pantzke, J.; Kuhn, E.; Paul, A.; Ziehm, T.; et al. Atmospheric aging increases the cytotoxicity of bare soot particles in BEAS-2B lung cells. Aerosol Sci. Technol. 2023, 57, 367–383. [Google Scholar] [CrossRef]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.-C.; Boland, S.; Baeza Squiban, A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef]
- Danielsen, P.H.; Møller, P.; Jensen, K.A.; Sharma, A.K.; Wallin, H.; Bossi, R.; Autrup, H.; Mølhave, L.; Ravanat, J.-L.; Briedé, J.J.; et al. Oxidative Stress, DNA Damage, and Inflammation Induced by Ambient Air and Wood Smoke Particulate Matter in Human A549 and THP-1 Cell Lines. Chem. Res. Toxicol. 2011, 24, 168–184. [Google Scholar] [CrossRef]
- Barzgar, F.; Sadeghi-Mohammadi, S.; Aftabi, Y.; Zarredar, H.; Shakerkhatibi, M.; Sarbakhsh, P.; Gholampour, A. Oxidative stress indices induced by industrial and urban PM(2.5)-bound metals in A549 cells. Sci. Total Environ. 2023, 877, 162726. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Env. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.Y.; Li, W.K.; Li, J.S.; Hong, Q.H.; Khodahemmati, S.; Gao, J.F.; Zhou, Z.X. Effects of DNA Damage and Oxidative Stress in Human Bronchial Epithelial Cells Exposed to PM(2.5) from Beijing, China, in Winter. Int. J. Environ. Res. Public Health 2020, 17, 4874. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, S.; Gianotti, V.; Bonetta, S.; Gosetti, F.; Oddone, M.; Gennaro, M.C.; Carraro, E. DNA damage in A549 cells exposed to different extracts of PM(2.5) from industrial, urban and highway sites. Chemosphere 2009, 77, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, M.; Wang, G.; Du, L.; Li, H.; Wang, Y. Chemical characteristics and cytotoxic correlation analysis of PM2.5 in Jinan. Air Qual. Atmos. Health 2022, 15, 1465–1475. [Google Scholar] [CrossRef]
- Guan, L.; Rui, W.; Bai, R.; Zhang, W.; Zhang, F.; Ding, W. Effects of Size-Fractionated Particulate Matter on Cellular Oxidant Radical Generation in Human Bronchial Epithelial BEAS-2B Cells. Int. J. Env. Res. Public Health 2016, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Al Hanai, A.H.; Antkiewicz, D.S.; Hemming, J.D.C.; Shafer, M.M.; Lai, A.M.; Arhami, M.; Hosseini, V.; Schauer, J.J. Seasonal variations in the oxidative stress and inflammatory potential of PM2.5 in Tehran using an alveolar macrophage model; The role of chemical composition and sources. Environ. Int. 2019, 123, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-κB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, F.; Qu, F.; Ding, W. Water-insoluble fraction of airborne particulate matter (PM10) induces oxidative stress in human lung epithelial A549 cells. Environ. Toxicol. 2014, 29, 226–233. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Q.; Yang, X.; Li, G.; Zhang, J.; Zhou, X.; Jiang, W. Cytotoxicity of the soluble and insoluble fractions of atmospheric fine particulate matter. J. Environ. Sci. 2020, 91, 105–116. [Google Scholar] [CrossRef]
- Liu, X.; Ge, P.; Lu, Z.; Cao, M.; Chen, W.; Yan, Z.; Chen, M.; Wang, J. Ecotoxicity induced by total, water soluble and insoluble components of atmospheric fine particulate matter exposure in Caenorhabditis elegans. Chemosphere 2023, 316, 137672. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.; Carpentier, J.; Simonin, O.; Gosset, P.; Platel, A.; Happillon, M.; Alleman, L.Y.; Perdrix, E.; Riffault, V.; Chassat, T.; et al. Oxidative stress and inflammation induced by air pollution-derived PM(2.5) persist in the lungs of mice after cessation of their sub-chronic exposure. Env. Int. 2023, 181, 108248. [Google Scholar] [CrossRef] [PubMed]

| Cell Viability | ROS | TNF–α | IL–6 | Apoptosis Rate | DNA Damage | |
|---|---|---|---|---|---|---|
| Na+ | –0.646 | 0.716 | 0.730 | 0.771 | 0.632 | 0.718 |
| NH4+ | –0.858 ** | 0.983 ** | 0.946 ** | 0.928 ** | 0.977 ** | 0.973 ** |
| K+ | –0.575 | 0.673 | 0.538 | 0.631 | 0.506 | 0.636 |
| Mg2+ | –0.662 | 0.757 | 0.677 | 0.755 | 0.612 | 0.734 |
| Ca2+ | –0.781 | 0.876 ** | 0.880 ** | 0.898 ** | 0.822 ** | 0.876 ** |
| F− | –0.788 | 0.885 ** | 0.887 ** | 0.903 ** | 0.831 ** | 0.884 ** |
| Cl− | –0.863 ** | 0.982 ** | 0.962 ** | 0.949 ** | 0.969 ** | 0.977 ** |
| SO42− | –0.862 ** | 0.984 ** | 0.943 ** | 0.947 ** | 0.944 ** | 0.973 ** |
| NO3− | –0.858 ** | 0.981 ** | 0.951 ** | 0.929 ** | 0.980 ** | 0.973 ** |
| OC | –0.721 | 0.807 ** | 0.803 ** | 0.841 ** | 0.725 | 0.805 ** |
| EC | –0.778 | 0.872 ** | 0.875 ** | 0.894 ** | 0.814 ** | 0.872 ** |
| PAHs | –0.829 ** | 0.935 ** | 0.957 ** | 0.921 ** | 0.959 ** | 0.941 ** |
| Endotoxin | –0.824 ** | 0.939 ** | 0.937 ** | 0.890 ** | 0.978 ** | 0.939 ** |
| Cell Viability | ROS | TNF–α | IL–6 | Apoptosis Rate | DNA Damage | |
|---|---|---|---|---|---|---|
| Al | –0.828 ** | 0.958 ** | 0.870 ** | 0.888 ** | 0.896 ** | 0.934 ** |
| V | –0.798 | 0.929 ** | 0.826 ** | 0.845 ** | 0.866 ** | 0.902 ** |
| Cr | –0.646 | 0.718 | 0.722 | 0.769 | 0.624 | 0.717 |
| Mn | –0.829 ** | 0.960 ** | 0.873 ** | 0.888 ** | 0.903 ** | 0.937 ** |
| Fe | –0.823 ** | 0.950 ** | 0.864 ** | 0.893 ** | 0.872 ** | 0.927 ** |
| Co | –0.789 | 0.918 ** | 0.810 ** | 0.844 ** | 0.832 ** | 0.889 ** |
| Ni | –0.651 | 0.773 | 0.621 | 0.672 | 0.664 | 0.732 |
| Cu | –0.327 | 0.374 | 0.284 | 0.401 | 0.183 | 0.348 |
| Zn | –0.731 | 0.847 ** | 0.736 | 0.800 ** | 0.718 | 0.816 ** |
| As | –0.857 ** | 0.983 ** | 0.939 ** | 0.924 ** | 0.971 ** | 0.972 ** |
| Se | –0.690 | 0.797 | 0.692 | 0.766 | 0.655 | 0.768 |
| Cd | –0.810 ** | 0.941 ** | 0.847 ** | 0.857 ** | 0.891 ** | 0.916 ** |
| Ba | –0.513 | 0.589 | 0.493 | 0.594 | 0.414 | 0.562 |
| Pb | –0.809 ** | 0.939 ** | 0.839 ** | 0.865 ** | 0.865 ** | 0.912 ** |
| Sr | –0.473 | 0.535 | 0.471 | 0.569 | 0.368 | 0.516 |
| Sb | –0.828 ** | 0.959 ** | 0.883 ** | 0.878 ** | 0.931 ** | 0.939 ** |
| Ti | –0.827 ** | 0.930 ** | 0.952 ** | 0.928 ** | 0.937 ** | 0.936 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Ge, P.; Lu, Z.; Liu, X.; Cao, M.; Chen, W.; Chen, M. The Cytotoxic Effects of Fine Particulate Matter (PM2.5) from Different Sources at the Air–Liquid Interface Exposure on A549 Cells. Toxics 2024, 12, 21. https://doi.org/10.3390/toxics12010021
Yan Z, Ge P, Lu Z, Liu X, Cao M, Chen W, Chen M. The Cytotoxic Effects of Fine Particulate Matter (PM2.5) from Different Sources at the Air–Liquid Interface Exposure on A549 Cells. Toxics. 2024; 12(1):21. https://doi.org/10.3390/toxics12010021
Chicago/Turabian StyleYan, Zhansheng, Pengxiang Ge, Zhenyu Lu, Xiaoming Liu, Maoyu Cao, Wankang Chen, and Mindong Chen. 2024. "The Cytotoxic Effects of Fine Particulate Matter (PM2.5) from Different Sources at the Air–Liquid Interface Exposure on A549 Cells" Toxics 12, no. 1: 21. https://doi.org/10.3390/toxics12010021
APA StyleYan, Z., Ge, P., Lu, Z., Liu, X., Cao, M., Chen, W., & Chen, M. (2024). The Cytotoxic Effects of Fine Particulate Matter (PM2.5) from Different Sources at the Air–Liquid Interface Exposure on A549 Cells. Toxics, 12(1), 21. https://doi.org/10.3390/toxics12010021






