Immunological and Histological Studies of Different Concentrations of Rosmarinus officinalis and Thymus vulgaris Extracts on Thymus Gland of Chick Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Reagents, Chemicals, and Materials
2.2. Animals and Incubation
2.3. Dose Preparation and Administration
2.4. Experimental Design
2.5. Histological Study of the Thymus Gland
2.6. Immunological Study of the Serum
2.7. Statical Analysis
3. Results
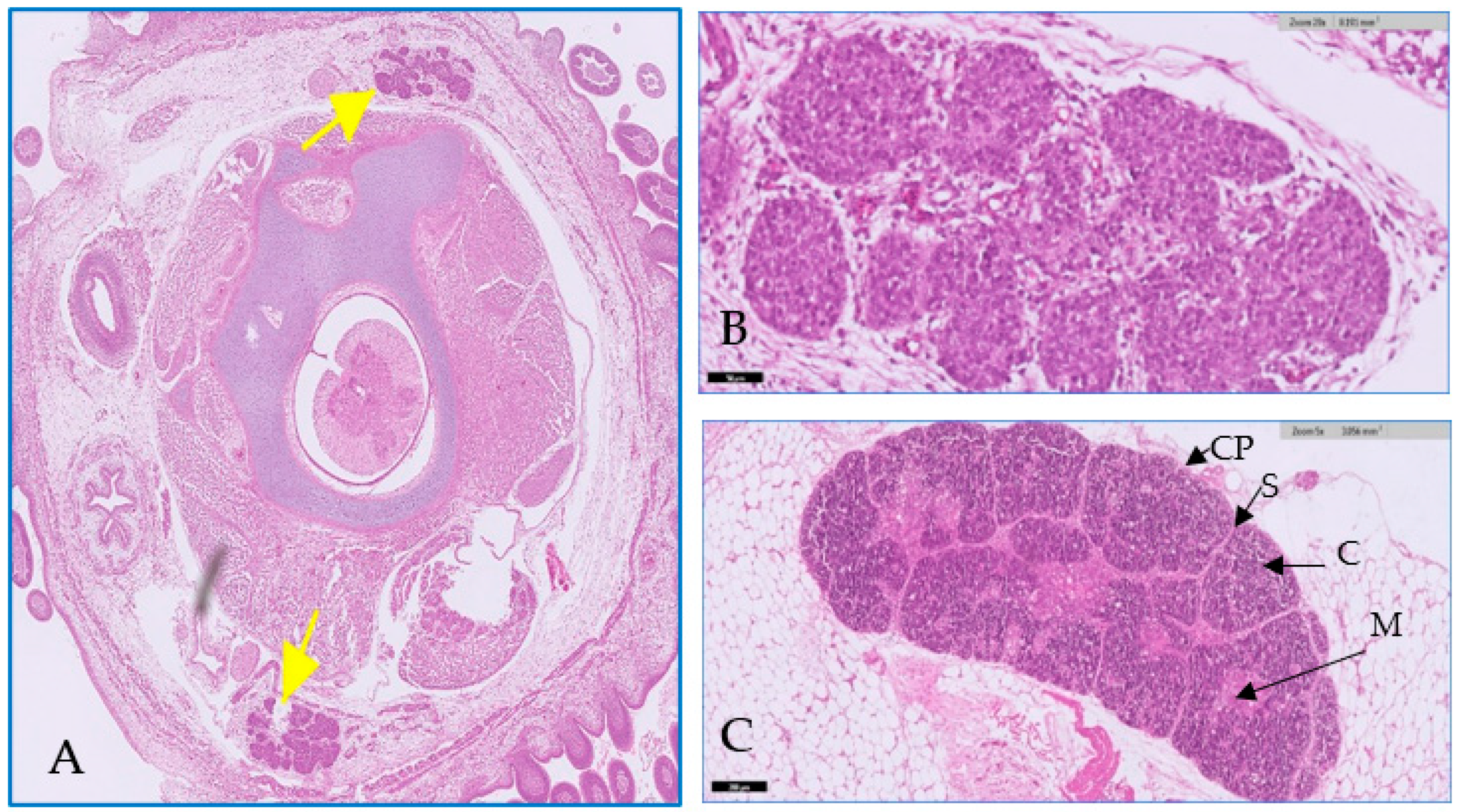
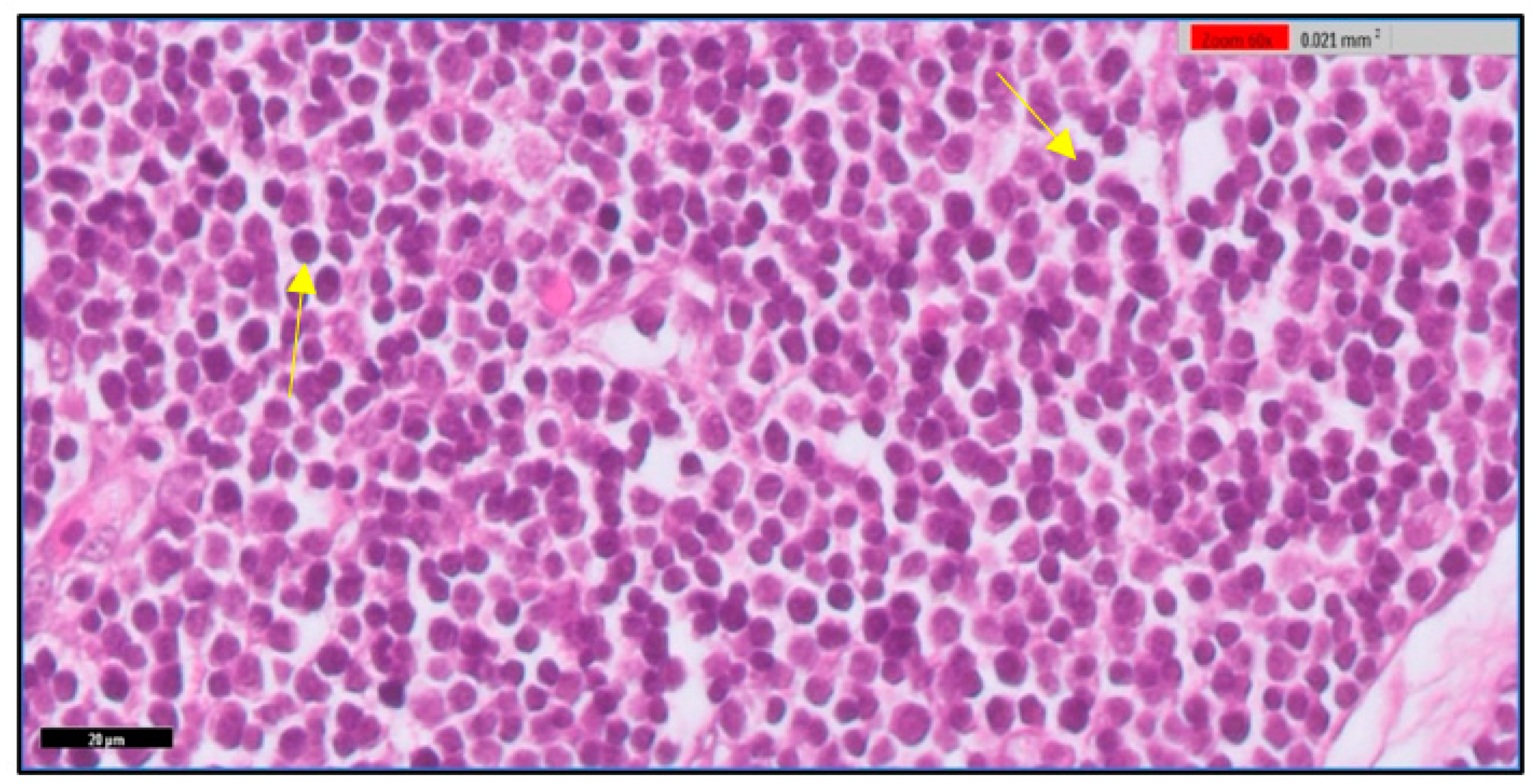
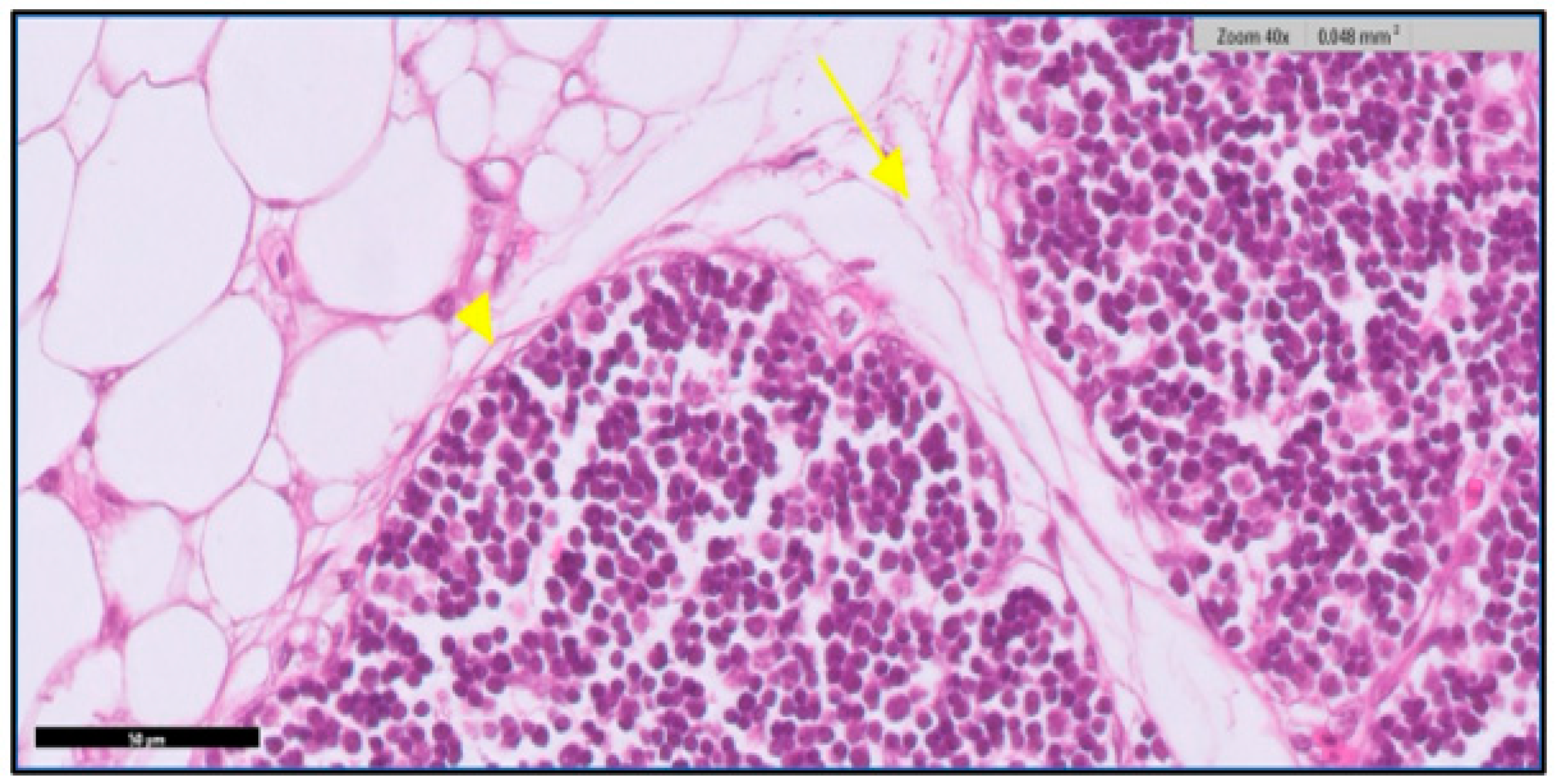
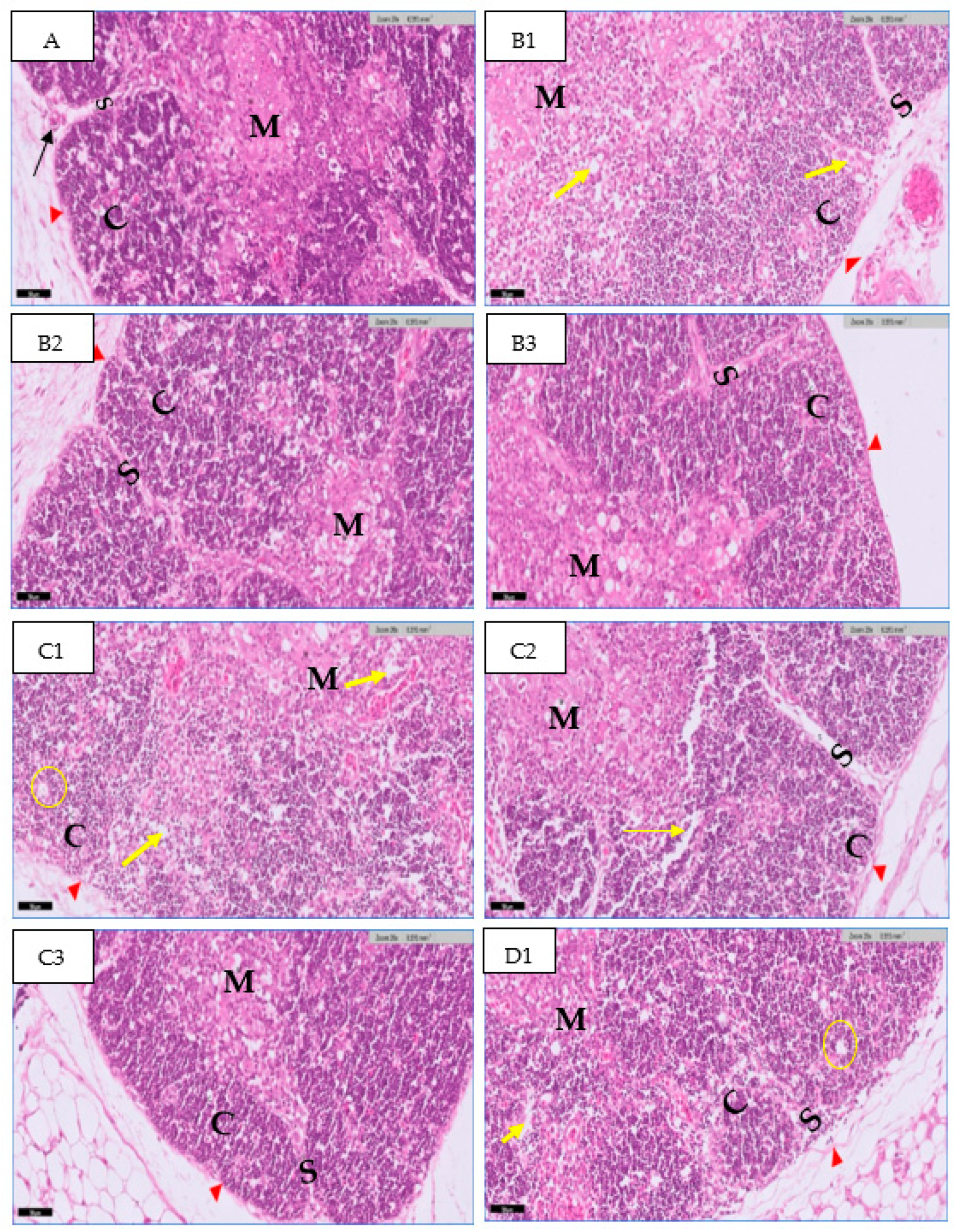
3.1. Microscopic Examination of Thymic Tissue
3.1.1. At 14th Day
3.1.2. At 20th Day
3.2. Immunodiagnostics of Antibodies and Cytokine in Serum
3.2.1. Cytokine Production upon Treatment with Rosemary and Thyme Extract
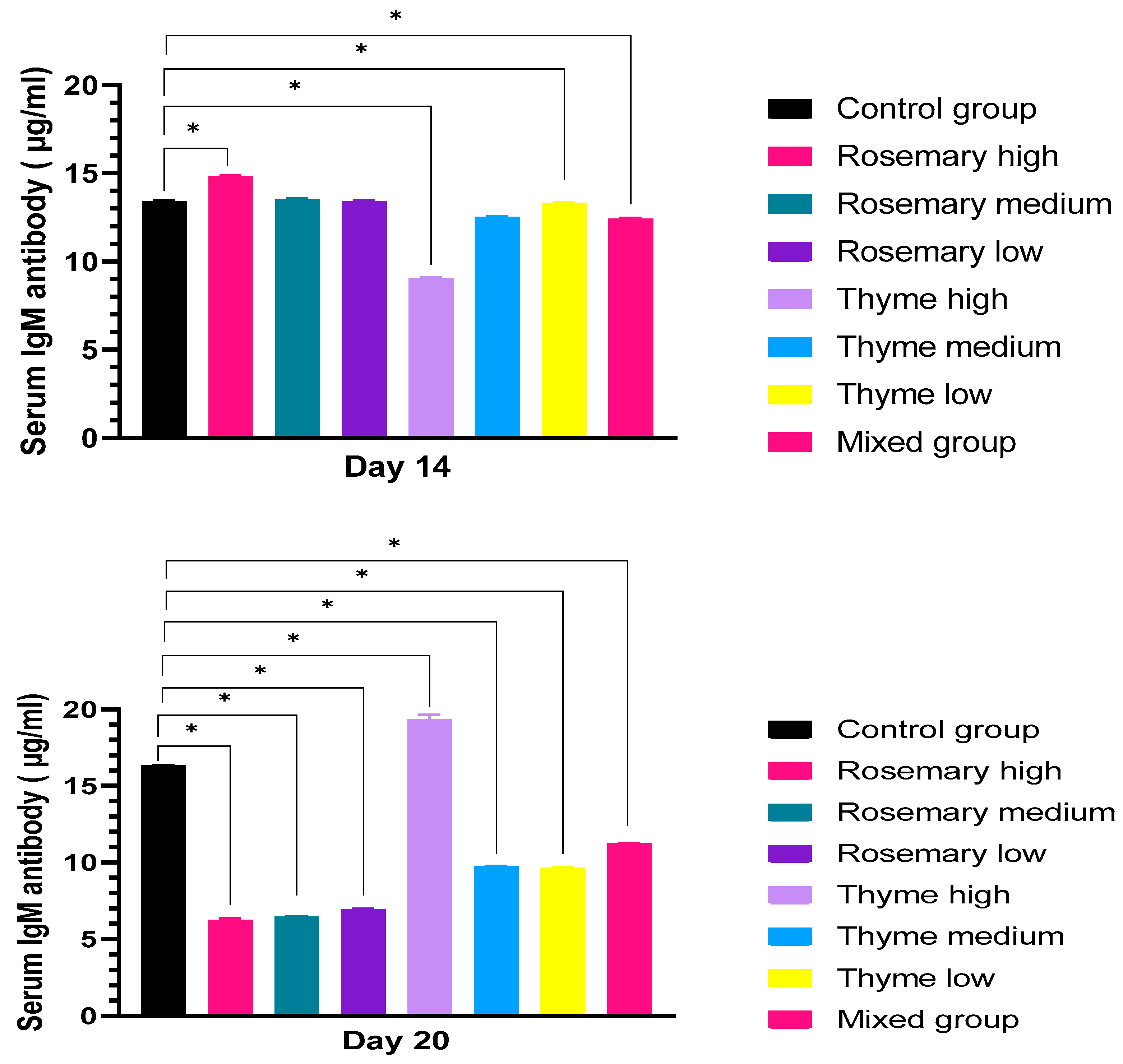
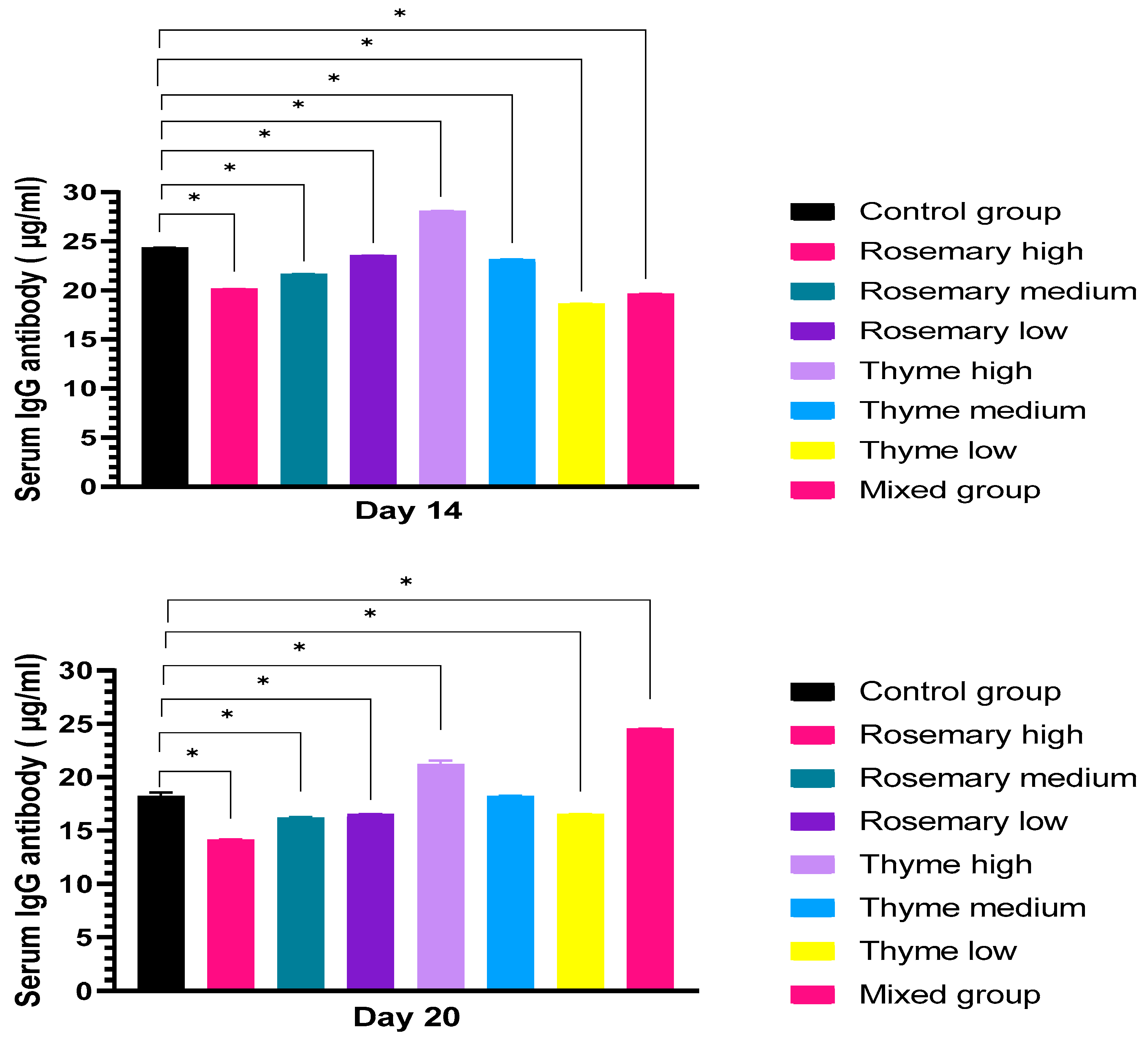
3.2.2. Antibody Production upon Treatment with Rosemary and Thyme Extract
IgM
IgG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Melo Cruvinel, W.; Mesquita Júnior, D.; Araújo, J.A.P.; Catelan, T.T.T.; de Souza, A.W.S.; da Silva, N.P.; Andrade, L.E.C. Immune System—Part I Fundamentals of Innate Immunity with Emphasis on Molecular and Cellular Mechanisms of Inflammatory Response. Rev. Bras. Reumatol. 2010, 50, 434–447. [Google Scholar] [CrossRef]
- Goldstein, A.L.; Low, T.L.; Thurman, G.B.; Zatz, M.M.; Hall, N.; Chen, J.; Hu, S.K.; Naylor, P.B.; McClure, J.E. Current Status of Thymosin and Other Hormones of the Thymus Gland. Recent Prog. Horm. Res. 1981, 37, 369–415. [Google Scholar] [CrossRef]
- Deigin, V.I.; Vinogradova, Y.E.; Vinogradov, D.L.; Krasilshchikova, M.S.; Ivanov, V.T. Thymodepressin—Unforeseen Immunosuppressor. Molecules 2021, 26, 6550. [Google Scholar] [CrossRef]
- Muthukumaran, C.; Kumaravel, A.; Balasundaram, K.; Paramasivan, S. Gross Anatomical Studies on the Thymus Gland in Turkeys (Meleagrisgallopavo). Tamilnadu J. Vet. Anim. Sci. 2011, 7, 6–11. [Google Scholar]
- Zdrojewicz, Z.; Pachura, E.; Pachura, P. The Thymus: A Forgotten, but Very Important Organ. Adv. Clin. Exp. Med. 2016, 25, 369–375. [Google Scholar] [CrossRef]
- Sawad, A.A.; Mohammed, H. Anatomical and histological study of thymus glands in broiler chick s embryo (Gal Galluslus Domesticus). Basrah J. Vet. Res. 2019, 18, 169–177. [Google Scholar]
- Ribatti, D.; Tamma, R.; Elieh Ali Komi, D. The Morphological Basis of the Development of the Chick Embryo Immune System. Exp. Cell Res. 2019, 381, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Ota, A.; Iguchi, T.; Muro, R.; Takayanagi, H. The Fibroblast: An Emerging Key Player in Thymic T Cell Selection. Immunol. Rev. 2021, 302, 68–85. [Google Scholar] [CrossRef]
- Ibrahim, Z.A.; Waheed, I.N. Histopathological Effects of Phytoestrogrn (Genistein) on Thymus Gland of Adult and Post-Natal Female Albino Mice. Sci. J. Univ. Zakho 2022, 10, 29–34. [Google Scholar] [CrossRef]
- Liu, H.; Lu, K.; Macary, P.A.; Wong, K.L.; Heng, A.; Cao, T.; Kemeny, D.M. Soluble Molecules Are Key in Maintaining the Immunomodulatory Activity of Murine Mesenchymal Stromal Cells. J. Cell Sci. 2012, 125, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Mutneja, M.; Mohan, C.; Long, K.D.; Das, C. An Introduction to Antibodies and Their Applications, 3rd ed.; Mutneja, M., Ed.; EMD Millipore: Darmstadt, Germany, 2014; ISBN 9780124160026. [Google Scholar]
- Ciobanu, A.M.; Dumitru, A.E.; Gica, N.; Botezatu, R.; Peltecu, G.; Panaitescu, A.M. Benefits and Risks of IgG Transplacental Transfer. Diagnostics 2020, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Rostenberg, I.; Peñaloza, R. Serum IgG and IgD and Levels in Some Infectious and Noninfectious Diseases. Clin. Chim. Acta 1978, 85, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; Johansson, S.G. Immunoglobulin levels during childhood, with special regard to IgE. Acta Paediatr. 1969, 58, 513–524. [Google Scholar] [CrossRef]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Adaptive Immune Responses to Infection. In Fenner and White’s Medical Virology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–76. [Google Scholar] [CrossRef]
- Ochs, H.D. Patients with Abnormal IgM Levels: Assessment, Clinical Interpretation, and Treatment. Ann. Allergy Asthma Immunol. 2008, 100, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Grégoire Altan-Bonnet; Mukherjee, R. Cytokine-Mediated Communications: A Quantitative Appraisal of Immune Complexity. Nat. Rev. Immunol. 2019, 19, 205–217. [Google Scholar] [CrossRef]
- Justiz, V.A.A.; Ahmad, Q. Interleukin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499840/ (accessed on 22 August 2022).
- O’Farrell, A.; Liu, Y.; Moore, K.W.; Mui, A.L. IL-10 Inhibits Macrophage Activation and Proliferation by Distinct Signaling Mechanisms. EMBO J. 1998, 17, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Metink-Kane, M.M. Cytokines, Inflammation and Pain. Natl. Inst. Health 2012, 23, 1–7. [Google Scholar] [CrossRef]
- Bennett, B.C. Plants as Food. In Economic Botany: Encyclopedia of Life Support Systems (EOLSS); Eolss Publishers: Oxford, UK, 2010. [Google Scholar]
- Olarewaju, O.; Fajinmi, O.; Naidoo, K.; Arthur, G.; Coopoosamy, R. A Review of the Medicinal Plants with Immune-Boosting Potential. J. Med. Plants Econ. Dev. 2022, 6, 15. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Lupattelli, A.; Koren, G.; Nordeng, H. Herbal Medicine Use in Pregnancy: Results of a Multinational Study. BMC Complement. Altern. Med. 2013, 13, 355. [Google Scholar] [CrossRef]
- Rouhi-Boroujeni, H.; Heidarian, E.; Rouhi-Boroujeni, H.; Khoddami, M.; Gharipour, M.; Rafieian-Kopaei, M. Use of Lipid-Lowering Medicinal Herbs during Pregnancy: A Systematic Review on Safety and Dosage. ARYA Atheroscler. 2017, 13, 135–155. [Google Scholar]
- Bernstein, N.; Akram, M.; Yaniv-Bachrach, Z.; Daniyal, M. Is It Safe to Consume Traditional Medicinal Plants during Pregnancy? Phyther. Res. 2021, 35, 1908–1924. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Doyle, P.; Wang, J.-D.; Chang, P.-J.; Lai, J.-N.; Chen, P.-C. Herbal Medicines Used During the First Trimester and Major Congenital Malformations. Drug Saf. 2006, 29, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. Biological Activities of Three Essential Oils of the Lamiaceae Family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Taghizadeh, M.; Sahebkar, E.T.; Amirhossein, M. Rosemary Oil vs Minoxidil 2% for the Treatment of Androgenetic Alopecia: A Randomized Comparative Trial. Skinmed 2015, 13, 15–21. [Google Scholar]
- Damianova, S.; Tasheva, S.; Stoyanova, A.; Damianov, D. Investigation of Extracts from Thyme (Thymus vulgaris L.) for Application in Cosmetics. J. Essent. Oil-Bear. Plants 2008, 11, 443–450. [Google Scholar] [CrossRef]
- Ekoh, S.N.; Akubugwo, E.I.; Ude, V.C.; Edwin, N. Anti-Hyperglycemic and Anti-Hyperlipidemic Effect of Spices (Thymus vulgaris, Murraya koenigii, Ocimum gratissimum and Piper guineense) in Alloxan-Induced Diabetic Rats. Int. J. Biosci. 2014, 4, 179–187. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. ıcia Rosmarinus officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Futur. Sci. 2018, 4, 18. [Google Scholar] [CrossRef]
- Javed, H.; Erum, S.; Tabassum, S.; Ameen, F. An Overview on Medicinal Importance of Thymus vulgaris. J. Asian Sci. Res. 2013, 3, 974–982. [Google Scholar]
- Kazemi, M. Phytochemical Composition of Thymus vulgaris L. Essential Oil. J. Essent. Oil-Bearing Plants 2015, 18, 751–753. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Gammoh, S.; Al-Mahasneh, M.A.; Tranchant, C.C.; Rawshdeh, M. Pharmaceutical, Nutraceutical and Therapeutic Properties of Selected Wild Medicinal Plants: Thyme, Spearmint, and Rosemary; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128146262. [Google Scholar]
- Lanzerstorfer, P.; Sandner, G.; Pitsch, J.; Mascher, B.; Aumiller, T.; Weghuber, J. Acute, Reproductive, and Developmental Toxicity of Essential Oils Assessed with Alternative in Vitro and in Vivo Systems. Arch. Toxicol. 2021, 95, 673–691. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Y.; Wu, C. Chicken Embryonic Toxicity and Potential in Vitro Estrogenic and Mutagenic Activity of Carvacrol and Thymol in Low Dose/Concentration. Food Chem. Toxicol. 2021, 150, 112038. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Heldreth, B. Safety Assessment of Rosmarinus officinalis (Rosemary)-Derived Ingredients as Used in Cosmetics. Cosmet. Ingred. Rev. 2014, 37, 12S–50S. [Google Scholar] [CrossRef]
- Ulbricht, C.; Abrams, T.R.; Brigham, A.; Ceurvels, J.; Clubb, J.; Curtiss, W.; Kirkwood, C.D.; Giese, N.; Hoehn, K.; Iovin, R.; et al. An Evidence-Based Systematic Review of Rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2010, 7, 351–413. [Google Scholar] [CrossRef]
- Rizwan, B.; Zahur, M.; Azhar, N.; Khalid, S.; Sajid, N.; Qadeer, S. Therapeutic Potential of Thymus vulgaris: A Review. Ann. Res. 2020, 3, 147–161. [Google Scholar] [CrossRef]
- Alkarim, S.; Hamid, A. Experimental Embryology; In Arabic; King-Saud University, Ed.; Scientific Publishing and Printing Presses: Mylapore, India, 2008; ISBN 978-85-232-0700-7.
- El-Kholy, K.H.; Sarhan, D.M.A.; El-Said, E.A. Effect of In-Ovo Injection of Herbal Extracts on Post-Hatch Performance, Immunological, and Physiological Responses of Broiler Chickens. J. Worlds Poult. Res. 2021, 11, 183–192. [Google Scholar] [CrossRef]
- Haloui, M.; Louedec, L.; Michel, J.B.; Lyoussi, B. Experimental Diuretic Effects of Rosmarinus officinalis and Centaurium erythraea. J. Ethnopharmacol. 2000, 71, 465–472. [Google Scholar] [CrossRef]
- Kandeil, M.A.; Mohamed, A.E.D.H.; Abdel Gabbar, M.; Ahmed, R.R.; Ali, S.M. Ameliorative Effects of Oral Ginger and/or Thyme Aqueous Extracts on Productive and Reproductive Performance of V-Line Male Rabbits. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1437–1446. [Google Scholar] [CrossRef]
- Amanollahi, R.; Alavi, S.; Shiasi, S. Effects of in Ovo Injection of Rosemary (Rosmarinus officinalis L.) Extract on Gut Development in Broiler Chickens. In Proceedings of the 7th International Veterinary Poultry Congress, Tehran, Iran, 4 February 2020. [Google Scholar]
- Saeed, M.; Babazadeh, D.; Naveed, M.; Alagawany, M.; Abd El-Hack, M.E.; Arain, M.A.; Tiwari, R.; Sachan, S.; Karthik, K.; Dhama, K.; et al. In Ovo Delivery of Various Biological Supplements, Vaccines and Drugs in Poultry: Current Knowledge. J. Sci. Food Agric. 2019, 99, 3727–3739. [Google Scholar] [CrossRef]
- Tóthová, C.; Sesztáková, E.; Bielik, B.; Nagy, O. Changes of Total Protein and Protein Fractions in Broiler Chickens during the Fattening Period. Vet. World 2019, 12, 598–604. [Google Scholar] [CrossRef]
- Lanier, L.L. Shades of Grey-the Blurring View of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2013, 13, 73–74. [Google Scholar] [CrossRef]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac Surg Clin 2019, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C. Embryology and Anatomy of the Thymus Gland. In Thymus Gland Pathology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 13–18. [Google Scholar]
- Van Hai, N. The Use of Medicinal Plants as Immunostimulants in Aquaculture: A Review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Savino, W.; Postel-Vinay, M.C.; Smaniotto, S.; Dardenne, M. The Thymus Gland: A Target Organ for Growth Hormone. Scand. J. Immunol. 2002, 55, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, K.; Utsuyama, M.; Kikuchi, Y. Trade off Situation between Thymus and Growth Hormone: Age-Related Decline of Growth Hormone Is a Cause of Thymic Involution but Favorable for Elongation of Lifespan. Biogerontology 2016, 17, 55–59. [Google Scholar] [CrossRef]
- Bartke, A. Growth Hormone and Aging: Updated Review. World J. Men Health 2019, 37, 19–30. [Google Scholar] [CrossRef]
- Linjawi, S.A. Morphological and Morphmetric Studies on Embryo Development in Camphor Treated Pregnant Rats. J. King Abdulaziz Univ. Sci. 2011, 18, 47–64. [Google Scholar] [CrossRef]
- Freedom, R.M.; Rosen, F.S.; Nadas, A.S. Congenital Cardiovascular Disease and Anomalies of the Third and Fourth Pharyngeal Pouch. Circulation 1972, 46, 165–172. [Google Scholar] [CrossRef]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef]
- Jiang, S.; Shan, F.; Zhang, Y.; Jiang, L.; Cheng, Z. Increased Serum IL-17 and Decreased Serum IL-10 and IL-35 Levels Correlate with the Progression of COPD. Int. J. COPD 2018, 13, 2483–2494. [Google Scholar] [CrossRef]
- Ocaña, A.; Reglero, G. Effects of Thyme Extract Oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on Cytokine Production and Gene Expression of OxLDL-Stimulated THP-1-Macrophages. J. Obes. 2012, 2012, 104706. [Google Scholar] [CrossRef]
- Elshopakey, G.E.; Risha, E.F.; El-Boshy, M.E.; ABdalla, O.A.; HaMed, F.M. Protective Effects of Thymus vulgaris Oil Against CCL4 -Mediated Hepatotoxicity, Oxidative Stress and Immunosuppression in Male Albino Rats. Arch. Anesthesiol. Crit. Care 2018, 4, 527–534. [Google Scholar] [CrossRef]
- Matsubara, T.; Mori, S.; Touchi, A.; Masuda, Y.; Takeuchi, Y. Carbon Tetrachloride-Induced Hepatotoxicity in Rats: Evidence for Different Susceptibilities of Rat Liver Lobes. Jpn. J. Pharmacol. 1983, 33, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Fernandes, D.; Silva, I.; Mateus, V. Potential Anti-Inflammatory Effect of Rosmarinus officinalis in Preclinical In Vivo Models of Inflammation. Molecules 2022, 27, 609. [Google Scholar] [CrossRef]
- Da Rosa, J.S.; Facchin, B.M.; Bastos, J.; Siqueira, M.A.; Micke, G.A.; Dalmarco, E.M.; Pizzolatti, M.G.; Fröde, T.S. Systemic Administration of Rosmarinus officinalis Attenuates the Inflammatory Response Induced by Carrageenan in the Mouse Model of Pleurisy. Planta Med. 2013, 79, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG Placental Transfer in Healthy and Pathological Pregnancies. Clin. Dev. Immunol. 2012, 2012, 13. [Google Scholar] [CrossRef]
- Upton, J. Immunodeficiencies with Hypergammaglobulinemia: A Review. LymphoSign J. 2015, 2, 57–73. [Google Scholar] [CrossRef]
- Shetty, R.; Sureka, S.; Kusumgar, P.; Sethu, S.; Sainani, K. Allergen-specific Exposure Associated with High Immunoglobulin E and Eye Rubbing Predisposes to Progression of Keratoconus. Indian J. Ophthalmol. 2017, 65, 399–402. [Google Scholar] [CrossRef]
- Hiya, S.; Fujiwara, S.; Tanaka, F.; Kohara, N.; Kawamoto, M. High-Dose Immunoglobulin-Dependent Chronic Demyelinating Inflammatory Polyneuropathy Successfully Managed with Subcutaneous Immunoglobulin Using Pharmacokinetic Analysis. eNeurologicalSci 2022, 27, 100404. [Google Scholar] [CrossRef]
- Ewert, D.L.; Eidson, C.S. Effect of Bursectomy and Depletion of Immunoglobulin A on Antibody Production and Resistance to Respiratory Challenge After Local or Systemic Vaccination of Chickens with Newcastle Disease Virus. Infect. Immun. 1977, 18, 146–150. [Google Scholar] [CrossRef]
- Vale, A.M. Clinical Consequences of Defects in B Cell Development. Am. Acad. Allergy Asthma Immunol. 2010, 125, 778–787. [Google Scholar] [CrossRef]
- Bancos, S.; Bernard, M.P.; Topham, D.J.; Phipps, R.P. Ibuprofen and Other Widely Used Non-Steroidal Anti-Inflammatory Drugs Inhibit Antibody Production in Human Cells. Cell Immunol. 2009, 258, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, A.A.J.; Ramphul, K. Antibody Deficiency Disorder; StatPearls Publishing: Treasure Island, FL, USA, 2022; ISBN 29939682. [Google Scholar]
- Stoop, J.W.; Zegers, B.J.; Sander, P.C.; Ballieux, R.E. Serum Immunoglobulin Levels in Healthy Children and Adults. Clin. Exp. Immunol. 1969, 4, 101–112. [Google Scholar] [PubMed]
- Soliman, M.M.; Aldhahrani, A.; Alghamdi, Y.S.; Said, A.M. Impact of Thymus vulgaris Extract on Sodium Nitrite-Induced Alteration of Renal Redox and Oxidative Stress: Biochemical, Molecular, and Immunohistochemical Study. J. Food Biochem. 2022, 46, e13630. [Google Scholar] [CrossRef]
- Al Sheyab, F.M.; Abuharfeil, N.; Salloum, L.; Bani Hani, R.; Awad, D.S. The Effect of Rosemary (Rosmarinus officinalis. L) Plant Extracts on the Immune Response and Lipid Profile in Mice. J. Biol. Life Sci. 2012, 3, 26–29. [Google Scholar] [CrossRef]
- Sulaiman, K.M.; Tayeb, I.T. Response of Broiler Chicken to Inovo Administration of Different Levels of Rosemary Oil (Rosmarinus officinalis). Iraqi J. Agric. Sci. 2021, 52, 896–903. [Google Scholar] [CrossRef]
- Litvack, M.L.; Post, M.; Palaniyar, N. IgM Promotes the Clearance of Small Particles and Apoptotic Microparticles by Macrophages. PLoS ONE 2011, 6, e17223. [Google Scholar] [CrossRef]
- Lee, H.; Kovacs, C.; Mattman, A.; Hollander, Z.; Chen, V.; Ng, R.; Leung, J.M.; Sin, D.D. The Impact of IgG Subclass Deficiency on the Risk of Mortality in Hospitalized Patients with COPD. Respir. Res. 2022, 23, 141. [Google Scholar] [CrossRef]
- Kocaoğlu, M.; Kocaoğlu, B.E.; Erol Aytekin, S.; Keskin, D.M.; Güner, Ş.N.; Keleş, S.; Reisli, İ. Clinical and Laboratory Evaluation of Turkish Children with IgG Subclass Deficiency. Pediatr. Neonatol. 2023, 64, 38–45. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahri, R.Y.; Al-Ghamdi, F.A.; Al-Harbi, S.S. Immunological and Histological Studies of Different Concentrations of Rosmarinus officinalis and Thymus vulgaris Extracts on Thymus Gland of Chick Embryos. Toxics 2023, 11, 625. https://doi.org/10.3390/toxics11070625
Alzahri RY, Al-Ghamdi FA, Al-Harbi SS. Immunological and Histological Studies of Different Concentrations of Rosmarinus officinalis and Thymus vulgaris Extracts on Thymus Gland of Chick Embryos. Toxics. 2023; 11(7):625. https://doi.org/10.3390/toxics11070625
Chicago/Turabian StyleAlzahri, Reem Yahya, Fawzyah Abdullah Al-Ghamdi, and Seetah Saleem Al-Harbi. 2023. "Immunological and Histological Studies of Different Concentrations of Rosmarinus officinalis and Thymus vulgaris Extracts on Thymus Gland of Chick Embryos" Toxics 11, no. 7: 625. https://doi.org/10.3390/toxics11070625
APA StyleAlzahri, R. Y., Al-Ghamdi, F. A., & Al-Harbi, S. S. (2023). Immunological and Histological Studies of Different Concentrations of Rosmarinus officinalis and Thymus vulgaris Extracts on Thymus Gland of Chick Embryos. Toxics, 11(7), 625. https://doi.org/10.3390/toxics11070625






