Endoplasmic Reticulum Stress and Autophagy Are Involved in Hepatotoxicity Induced by Tributyltin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and TBT Treatment
2.3. Cell Proliferation Assay
2.4. Ultrastructure Observation
2.5. Detection of the Protein Expression Profile Using iTRAQ
2.6. Detection of Endoplasmic Reticulum (ER) Stress
2.7. Detection of Autophagy
2.8. Detection of Apoptosis
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
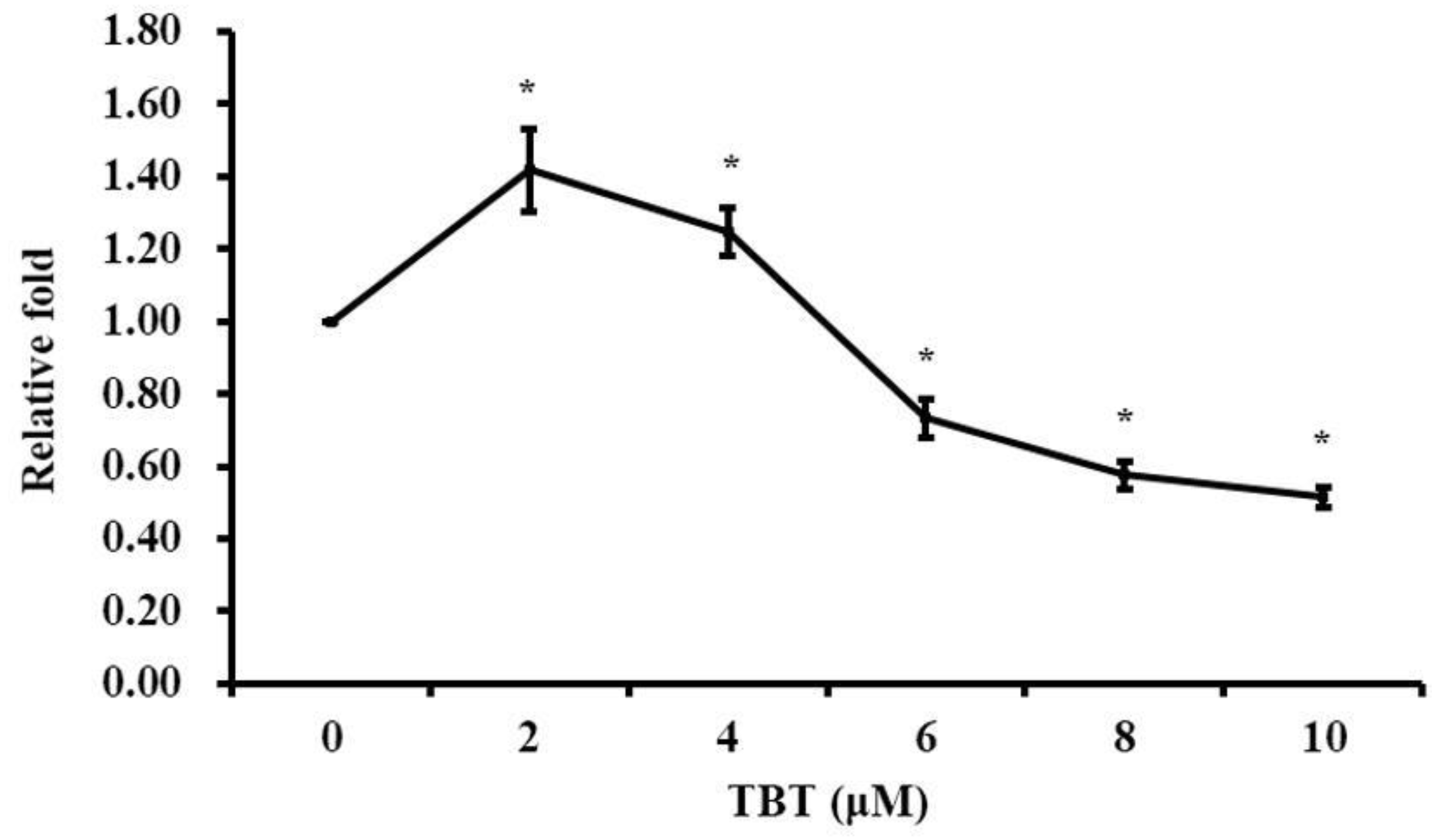
3.1. TBT Induced Alteration of Cell Proliferation
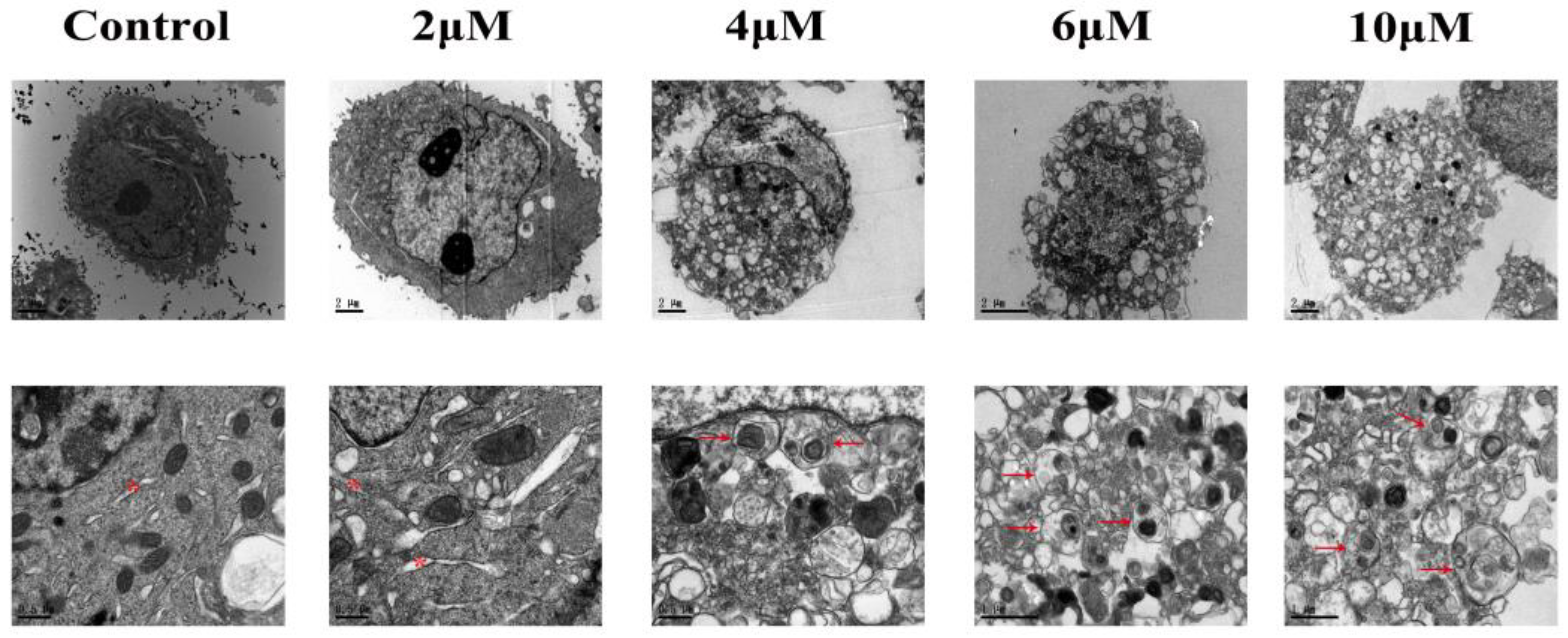
3.2. TBT-Induced Alteration of Cellular Ultrastructure
3.3. TBT-Induced ER Stress
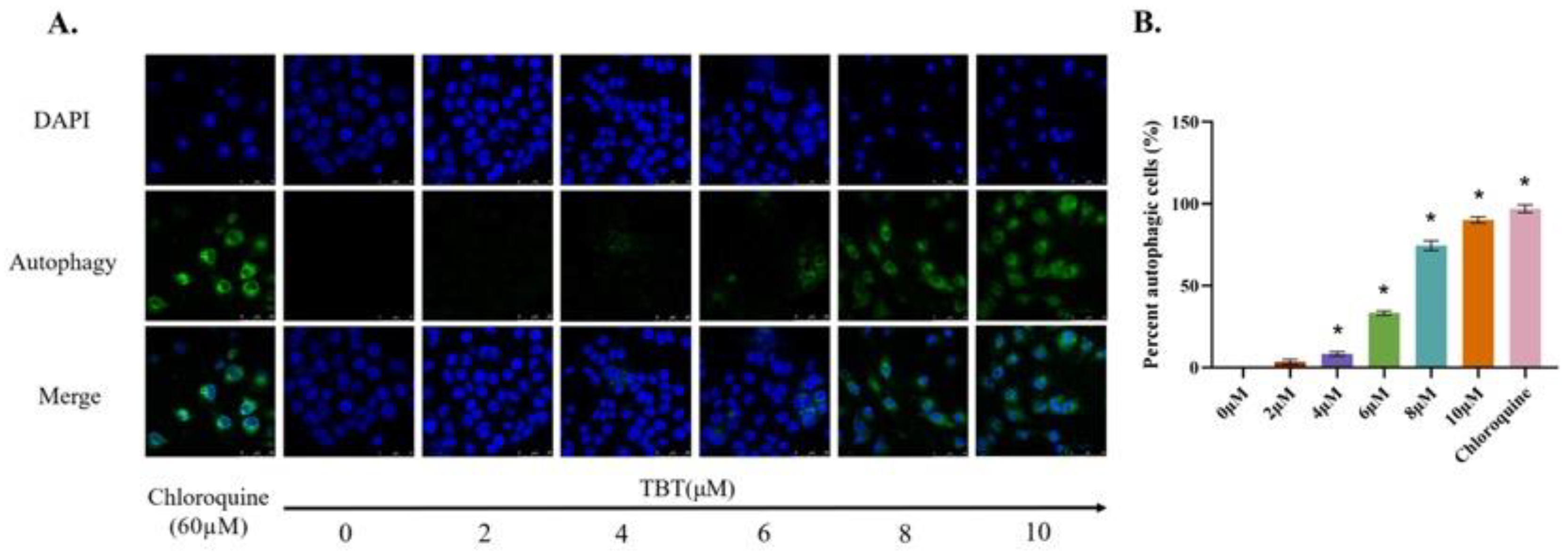
3.4. TBT-Induced Autophagy
3.5. TBT-Induced Apoptosis
3.6. DEPs in Cells Exposed to TBT
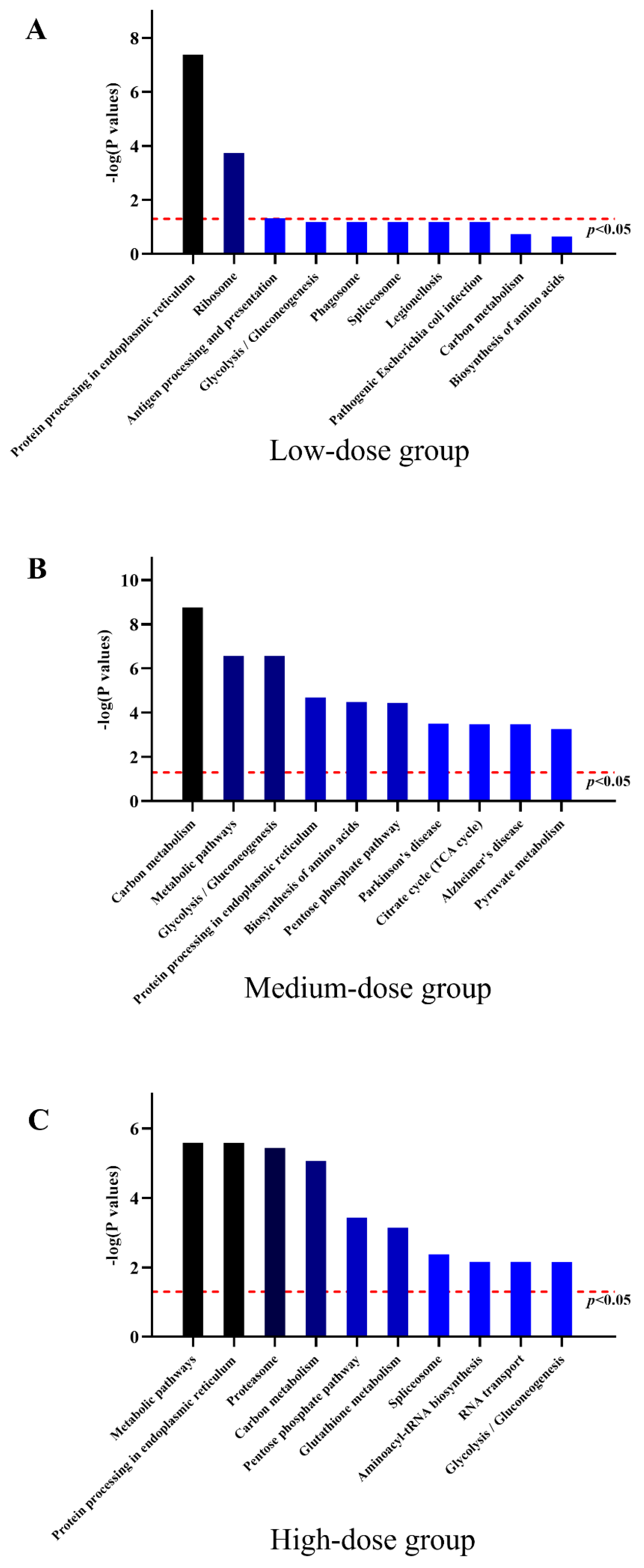
3.7. Protein Expression Profiles of HL7702 Cells with Low-Dose TBT Exposure
3.8. Protein Expression Profiles of HL7702 Cells with Medium-Dose TBT Exposure
3.9. Protein Expression Profiles of HL7702 Cells with High-Dose TBT Exposure
3.10. Effects of TBT on the Expression Levels of ER Stress-Associated Proteins
3.11. Effects of TBT on the Expression Levels of Autophagy-Associated Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furdek, M.; Vahčič, M.; Ščančar, J.; Milačič, R.; Kniewald, G.; Mikac, N. Organotin compounds in seawater and Mytilus galloprovincialis mussels along the Croatian Adriatic Coast. Mar. Pollut. Bull. 2012, 64, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Okoro, H.K.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G.; Opeolu, B. Human exposure, biomarkers, and fate of organotins in the environment. Rev. Environ. Contam. Toxicol. 2011, 213, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environ. Int. 2008, 34, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Furdek, M.; Mikac, N.; Bueno, M.; Tessier, E.; Cavalheiro, J.; Monperrus, M. Organotin persistence in contaminated marine sediments and porewaters: In situ degradation study using species-specific stable isotopic tracers. J. Hazard. Mater. 2016, 307, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Gipperth, L. The legal design of the international and European Union ban on tributyltin antifouling paint: Direct and indirect effects. J. Environ. Manag. 2009, 90 (Suppl. S1), S86–S95. [Google Scholar] [CrossRef] [PubMed]
- Omae, I. Organotin Antifouling Paints and Their Alternatives. ChemInform 2003, 34, 81–105. [Google Scholar] [CrossRef]
- Horiguchi, T. Masculinization of female gastropod mollusks induced by organotin compounds, focusing on mechanism of actions of tributyltin and triphenyltin for development of imposex. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2006, 13, 77–87. [Google Scholar]
- Marshall, D.J.; Rajkumar, A. Imposex in the indigenous Nassarius kraussianus (Mollusca: Neogastropoda) from South African harbours. Mar. Pollut. Bull. 2003, 46, 1150–1155. [Google Scholar] [CrossRef]
- Hagger, J.A.; Depledge, M.H.; Galloway, T.S. Toxicity of tributyltin in the marine mollusc Mytilus edulis. Mar. Pollut. Bull. 2005, 51, 811–816. [Google Scholar] [CrossRef]
- Hoch, M. Organotin compounds in the environment—An overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Viglino, L.; Pelletier, E.; St-Louis, R. Highly persistent butyltins in northern marine sediments: A long-term threat for the Saguenay Fjord (Canada). Environ. Toxicol. Chem. 2004, 23, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Yi, A.X.; Leung, K.M.Y.; Lam, M.H.W.; Lee, J.-S.; Giesy, J.P. Review of measured concentrations of triphenyltin compounds in marine ecosystems and meta-analysis of their risks to humans and the environment. Chemosphere 2012, 89, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M.; Enes, P.; Reis-Henriques, M.A.; Kuballa, J.; Castro, L.F.C.; Vieira, M.N. Organotin levels in seafood from Portuguese markets and the risk for consumers. Chemosphere 2009, 75, 661–666. [Google Scholar] [CrossRef]
- Rantakokko, P.; Turunen, A.; Verkasalo, P.K.; Kiviranta, H.; Männistö, S.; Vartiainen, T. Blood levels of organotin compounds and their relation to fish consumption in Finland. Sci. Total Environ. 2008, 399, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Strand, J.; Jacobsen, J.A. Accumulation and trophic transfer of organotins in a marine food web from the Danish coastal waters. Sci. Total Environ. 2005, 350, 72–85. [Google Scholar] [CrossRef]
- De Lemos Barbosa, C.M.; Ferrão, F.M.; Graceli, J.B. Organotin Compounds Toxicity: Focus on Kidney. Front. Endocrinol. 2018, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Grondin, M.; Marion, M.; Denizeau, F.; Averill-Bates, D.A. Tributyltin induces apoptotic signaling in hepatocytes through pathways involving the endoplasmic reticulum and mitochondria. Toxicol. Appl. Pharmacol. 2007, 222, 57–68. [Google Scholar] [CrossRef]
- Suzuki, J.S.; Ishido, M. Transcriptome of tributyltin-induced apoptosis of the cultured rat mesencephalic neural stem cells. Toxicology 2011, 287, 61–68. [Google Scholar] [CrossRef]
- Tu, W.-W.; Ji, L.-D.; Qian, H.-X.; Zhou, M.; Zhao, J.-S.; Xu, J. Tributyltin induces disruption of microfilament in HL7702 cells via MAPK-mediated hyperphosphorylation of VASP. Environ. Toxicol. 2016, 31, 1530–1538. [Google Scholar] [CrossRef]
- Zhou, M.; Feng, M.; Fu, L.-L.; Ji, L.-D.; Zhao, J.-S.; Xu, J. Toxicogenomic analysis identifies the apoptotic pathway as the main cause of hepatotoxicity induced by tributyltin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2016, 97, 316–326. [Google Scholar] [CrossRef]
- Burton, E.D.; Phillips, I.R.; Hawker, D.W. Sorption and desorption behavior of tributyltin with natural sediments. Environ. Sci. Technol. 2004, 38, 6694–6700. [Google Scholar] [CrossRef]
- Ciesielski, T.; Wasik, A.; Kuklik, I.; Skóra, K.; Namieśnik, J.; Szefer, P. Organotin compounds in the liver tissue of marine mammals from the Polish coast of the Baltic Sea. Environ. Sci. Technol. 2004, 38, 1415–1420. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Santos, G.S.; Cestari, M.M.; de Oliveira Ribeiro, C.A.; de Assis, H.C.S.; Yamamoto, F.; Guiloski, I.C.; de Marchi, M.R.R.; Montone, R.C. Bioaccumulation of butyltins and liver damage in the demersal fish Cathorops spixii (Siluriformes, Ariidae). Environ. Sci. Pollut. Res. Int. 2014, 21, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Strand, J. Butyltin compounds in human liver. Environ. Res. 2002, 88, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Duda, C.A.; Villeneuve, D.L.; Kannan, K.; Falandysz, J.; Giesy, J.P. Butyltin compounds in sediment and fish from the Polish Coast of the Baltic Sea. Environ. Sci. Pollut. Res. Int. 1999, 6, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Mukai, H.; Tanabe, S.; Sakayama, K.; Miyazaki, T.; Masuno, H. Butyltin residues in livers of humans and wild terrestrial mammals and in plastic products. Environ. Pollut. 1999, 106, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W.; Guillette, L.J., Jr. Contaminants and impoSEX: Transcriptomics of contaminant-induced sex change. Mol. Ecol. 2013, 22, 1485–1487. [Google Scholar] [CrossRef]
- Lam, N.H.; Jeong, H.-H.; Kang, S.-D.; Kim, D.-J.; Ju, M.-J.; Horiguchi, T.; Cho, H.-S. Organotins and new antifouling biocides in water and sediments from three Korean Special Management Sea Areas following ten years of tributyltin regulation: Contamination profiles and risk assessment. Mar. Pollut. Bull. 2017, 121, 302–312. [Google Scholar] [CrossRef]
- Tryphonas, H.; Cooke, G.; Caldwell, D.; Bondy, G.; Parenteau, M.; Hayward, S.; Pulido, O. Oral (gavage), in utero and post-natal exposure of Sprague-Dawley rats to low doses of tributyltin chloride. Part II: Effects on the immune system. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2004, 42, 221–235. [Google Scholar] [CrossRef]
- Huang, C.F.; Yang, C.Y.; Tsai, J.R.; Wu, C.T.; Liu, S.H.; Lan, K.C. Low-dose tributyltin exposure induces an oxidative stress-triggered JNK-related pancreatic β-cell apoptosis and a reversible hypoinsulinemic hyperglycemia in mice. Sci. Rep. 2018, 8, 5734. [Google Scholar] [CrossRef]
- Katika, M.R.; Hendriksen, P.J.M.; van Loveren, H.; Peijnenburg, A. Exposure of Jurkat cells to bis (tri-n-butyltin) oxide (TBTO) induces transcriptomics changes indicative for ER- and oxidative stress, T cell activation and apoptosis. Toxicol. Appl. Pharmacol. 2011, 254, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Krug, H.F. Caspase-10 is the key initiator caspase involved in tributyltin-mediated apoptosis in human immune cells. J. Toxicol. 2012, 2012, 395482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Luo, X.; Yang, Y.; Jian, F.; Wang, X.; Xie, L. The roles of DNA damage-dependent signals and MAPK cascades in tributyltin-induced germline apoptosis in Caenorhabditis elegans. Chemosphere 2014, 108, 231–238. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 6, 469–471. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Gao, L.; Liu, H.; Liu, C. iTRAQ-Based Proteomic Analysis of Neonatal Kidney from Offspring of Protein Restricted Rats Reveals Abnormalities in Intraflagellar Transport Proteins. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 185–199. [Google Scholar] [CrossRef]
- Cox, D.J.; Strudwick, N.; Ali, A.A.; Paton, A.W.; Paton, J.C.; Schröder, M. Measuring signaling by the unfolded protein response. Methods Enzym. 2011, 491, 261–292. [Google Scholar] [CrossRef]
- Brinke, A.; Buchinger, S. Toxicogenomics in Environmental Science. Adv. Biochem. Eng. Biotechnol. 2017, 157, 159–186. [Google Scholar] [CrossRef]
- Perkel, J.M. Single-cell proteomics takes centre stage. Nature 2021, 597, 580–582. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, K.; Kaufman, R.J. The unfolded protein response—A stress signaling pathway of the endoplasmic reticulum. J. Chem. Neuroanat. 2004, 28, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Stefan, C.J.; Manford, A.G.; Baird, D.; Yamada-Hanff, J.; Mao, Y.; Emr, S.D. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 2011, 144, 389–401. [Google Scholar] [CrossRef]
- Ochoa, C.D.; Wu, R.F.; Terada, L.S. ROS signaling and ER stress in cardiovascular disease. Mol. Asp. Med. 2018, 63, 18–29. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Chambers, J.E.; Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2022, 21, 115–140. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Oikawa, D.; Kimata, Y.; Kohno, K.; Iwawaki, T. Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp. Cell Res. 2009, 315, 2496–2504. [Google Scholar] [CrossRef]
- Isomura, M.; Kotake, Y.; Masuda, K.; Miyara, M.; Okuda, K.; Samizo, S.; Sanoh, S.; Hosoi, T.; Ozawa, K.; Ohta, S. Tributyltin-induced endoplasmic reticulum stress and its Ca(2+)-mediated mechanism. Toxicol. Appl. Pharmacol. 2013, 272, 137–146. [Google Scholar] [CrossRef]
- Lai, E.; Teodoro, T.; Volchuk, A. Endoplasmic reticulum stress: Signaling the unfolded protein response. Physiology 2007, 22, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.W.; Diehl, J.A. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. USA 2000, 97, 12625–12630. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Schröder, M.; Kaufman, R.J. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 2000, 275, 24881–24885. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Duscha, S.; Boukari, H.; Meyer, M.; Xie, J.; Wei, G.; Schrepfer, T.; Roschitzki, B.; Boettger, E.C.; Schacht, J. XBP1 mitigates aminoglycoside-induced endoplasmic reticulum stress and neuronal cell death. Cell Death Dis. 2015, 6, e1763. [Google Scholar] [CrossRef]
- Malhi, H.; Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011, 54, 795–809. [Google Scholar] [CrossRef]
- Song, S.; Tan, J.; Miao, Y.; Li, M.; Zhang, Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell. Physiol. 2017, 232, 2977–2984. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Yang, C.; Chen, X.; Zheng, W.; Yang, Y.; Tang, Y. Structure-function analysis of human protein Ero1-Lalpha. Biochem. Biophys. Res. Commun. 2009, 389, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.E.; Limbird, L.E. G protein-coupled receptor interacting proteins: Emerging roles in localization and signal transduction. Cell. Signal. 2002, 14, 297–309. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L. Endoplasmic Reticulum Stress and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 167–177. [Google Scholar] [CrossRef]
- Ding, W.X.; Ni, H.M.; Gao, W.; Yoshimori, T.; Stolz, D.B.; Ron, D.; Yin, X.M. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am. J. Pathol. 2007, 171, 513–524. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef]
- Hanada, T.; Noda, N.N.; Satomi, Y.; Ichimura, Y.; Fujioka, Y.; Takao, T.; Inagaki, F.; Ohsumi, Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007, 282, 37298–37302. [Google Scholar] [CrossRef]
- Li, S.-P.; He, J.-D.; Wang, Z.; Yu, Y.; Fu, S.-Y.; Zhang, H.-M.; Zhang, J.-J.; Shen, Z.-Y. miR-30b inhibits autophagy to alleviate hepatic ischemia-reperfusion injury via decreasing the Atg12-Atg5 conjugate. World J. Gastroenterol. 2016, 22, 4501–4514. [Google Scholar] [CrossRef]
- Otomo, C.; Metlagel, Z.; Takaesu, G.; Otomo, T. Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat. Struct. Mol. Biol. 2013, 20, 59–66. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Abada, A.; Elazar, Z. Endocytosis and autophagy: Exploitation or cooperation? Cold Spring Harb. Perspect. Biol. 2014, 6, a018358. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Wen, J.; Tao, L.; Zhao, M.; Ge, W.; Wang, L.; Zhang, J.; Weng, D. RIP1 and RIP3 contribute to Tributyltin-induced toxicity in vitro and in vivo. Chemosphere 2019, 218, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kulbay, M.; Johnson, B.; Bernier, J. DNA fragmentation factor 40 expression in T cells confers sensibility to tributyltin-induced apoptosis. Toxicology 2019, 426, 152255. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Fu, L.; Feng, M.; Wang, X.; Yun, Z.; Xu, J. Endoplasmic Reticulum Stress and Autophagy Are Involved in Hepatotoxicity Induced by Tributyltin. Toxics 2023, 11, 607. https://doi.org/10.3390/toxics11070607
Liang W, Fu L, Feng M, Wang X, Yun Z, Xu J. Endoplasmic Reticulum Stress and Autophagy Are Involved in Hepatotoxicity Induced by Tributyltin. Toxics. 2023; 11(7):607. https://doi.org/10.3390/toxics11070607
Chicago/Turabian StyleLiang, Weiqi, Lingling Fu, Mei Feng, Xiaorong Wang, Zhaohui Yun, and Jin Xu. 2023. "Endoplasmic Reticulum Stress and Autophagy Are Involved in Hepatotoxicity Induced by Tributyltin" Toxics 11, no. 7: 607. https://doi.org/10.3390/toxics11070607
APA StyleLiang, W., Fu, L., Feng, M., Wang, X., Yun, Z., & Xu, J. (2023). Endoplasmic Reticulum Stress and Autophagy Are Involved in Hepatotoxicity Induced by Tributyltin. Toxics, 11(7), 607. https://doi.org/10.3390/toxics11070607




