Benchmark Dose Approach to DNA and Liver Damage by Chlorpyrifos and Imidacloprid in Male Rats: The Protective Effect of a Clove-Oil-Based Nanoemulsion Loaded with Pomegranate Peel Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Insecticides
2.2. Pomegranate-Peel-Extract-Loaded Clove Oil Nanoemulsion
2.3. Animals and Experimental Design
- Group (I): control group, which received only drinking water.
- Group (II): group that received pomegranate-extract-loaded clove nanoemulsion (PELCN), which administered orally (1 mL/100 g/kg bw/day) at a dose equal to 50 mg/kg bw/day of pomegranate extract and 500 mg/kg bw/day of clove oil nanoemulsion.
- Groups (III, IV and V): imidacloprid (IM) groups, which received IM in drinking water at doses of 14, 28, and 54 mg/kg bw/day, respectively. The lowest dose level of IM that was selected was equal to the NOAEL (14 mg/kg bw/day), whereas the two- and four-fold doses were 28 and 54 mg/kg bw/day, respectively [9].
- Groups (VI, VII, and VIII): chlorpyrifos (CPF) groups, which received CPF in drinking water at doses of 1, 2, and 4 mg/kg bw/day, respectively. The lowest dose level of CPF that was selected was equal to the NOAEL (1 mg/kg bw/day), whereas the other doses were 2 and 4 times the NOAEL [10].
- Group (IX): IM-PELCN group, which received oral PELCN at a dose of 50 mg/kg bw/day of pomegranate extract and 500 mg/kg bw/day of clove oil nanoemulsion, as well as IM in drinking water at a dose of 54 mg/kg bw/day.
- Group (X): CPF-PELCN group, which received oral PELCN at a dose of 50 mg/kg bw/day of pomegranate extract and 500 mg/kg bw/day of clove oil nanoemulsion as well as CPF in drinking water at a dose of 4 mg/kg bw/day. All treatments were administered every day for 28 days. The IM and CPF dosages were chosen in accordance with Organisation for Economic Cooperation and Development (OECD)’s guidelines; the highest dose level produced toxic effects but not severe injury or death. The lowest dose was equal to the no-observed-adverse-effects level (NOAEL), whereas the highest dosages were equivalent to two and four times the NOAEL [47]. Animals were fasted overnight at the end of the study. Body weights were recoded daily, and based on the average daily water intake and body weights of the treated male rats, daily adjustments to the IM and CPF doses were made before being administered in drinking water. The dose of pomegranate-extract-loaded clove nanoemulsion was chosen at 50 mg/kg bw/day of extract and 500 mg/kg bw/day of clove oil and was administered orally (1 mL/100-g bw/day of PELCN) according to the results of antioxidant activity studies and previous studies [44,45].
2.4. Sample Collecting
2.5. Biomarkers of Serum Liver Function
2.6. Plasma Total Antioxidant Capacity (TAC)
2.7. Oxidative Stress Biomarkers in Liver Homogenate
2.7.1. Liver Tissue Homogenate
2.7.2. Antioxidant Enzymes in Liver Homogenate
2.8. Comet Assay (Single-Cell Gel Electrophoresis) of Liver Samples
2.9. Benchmark Dose (BMD)
2.10. Histopathological Analysis Techniques
2.11. Statistical Analyses
3. Results
3.1. Pomegranate-Peel-Extract-Loaded Clove Oil Nanoemulsion
3.2. Signs of Toxicity, Body and Liver Weights
3.3. Liver Function Markers
3.4. Oxidative Stress Markers
3.5. DNA Damage
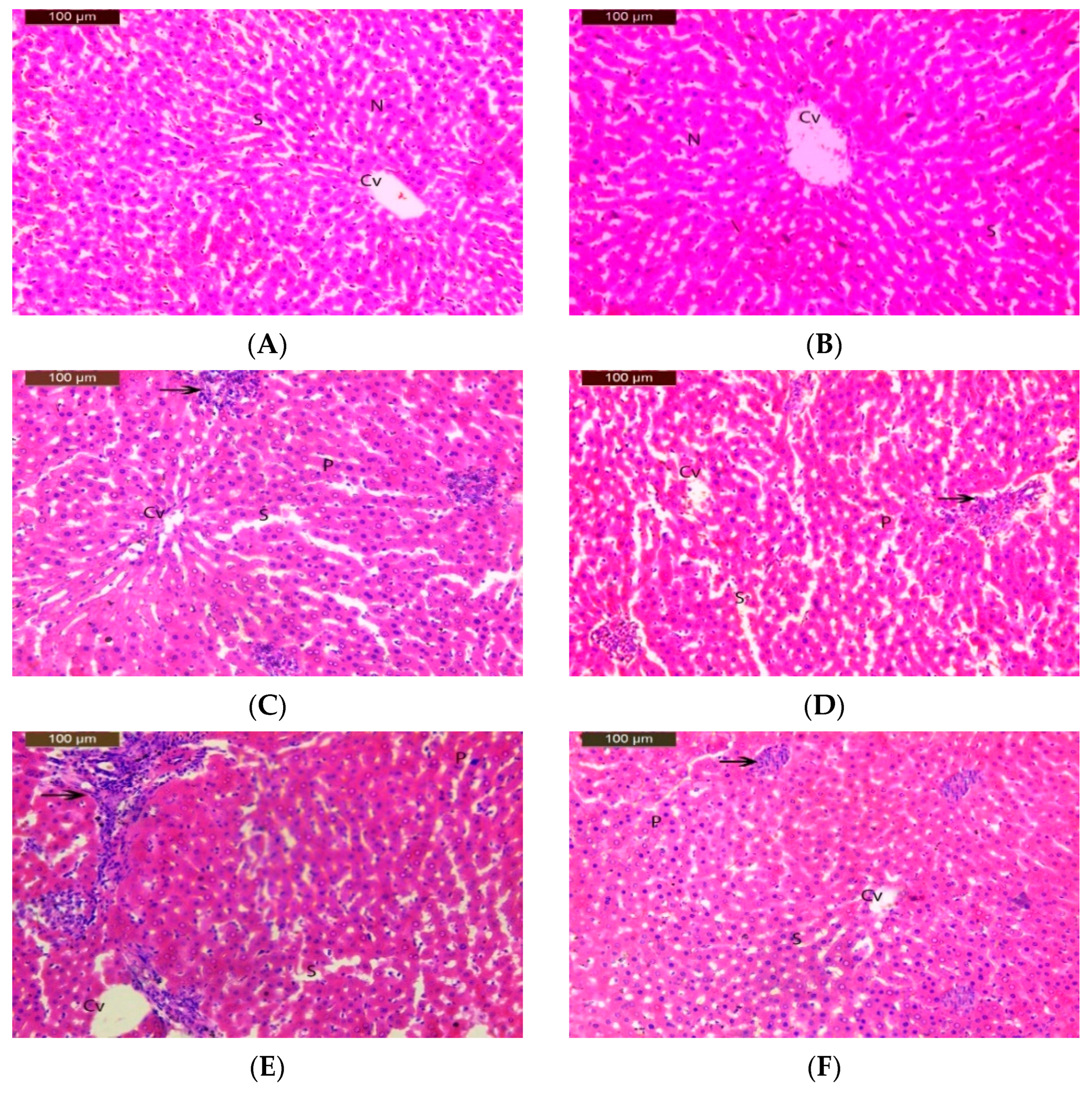
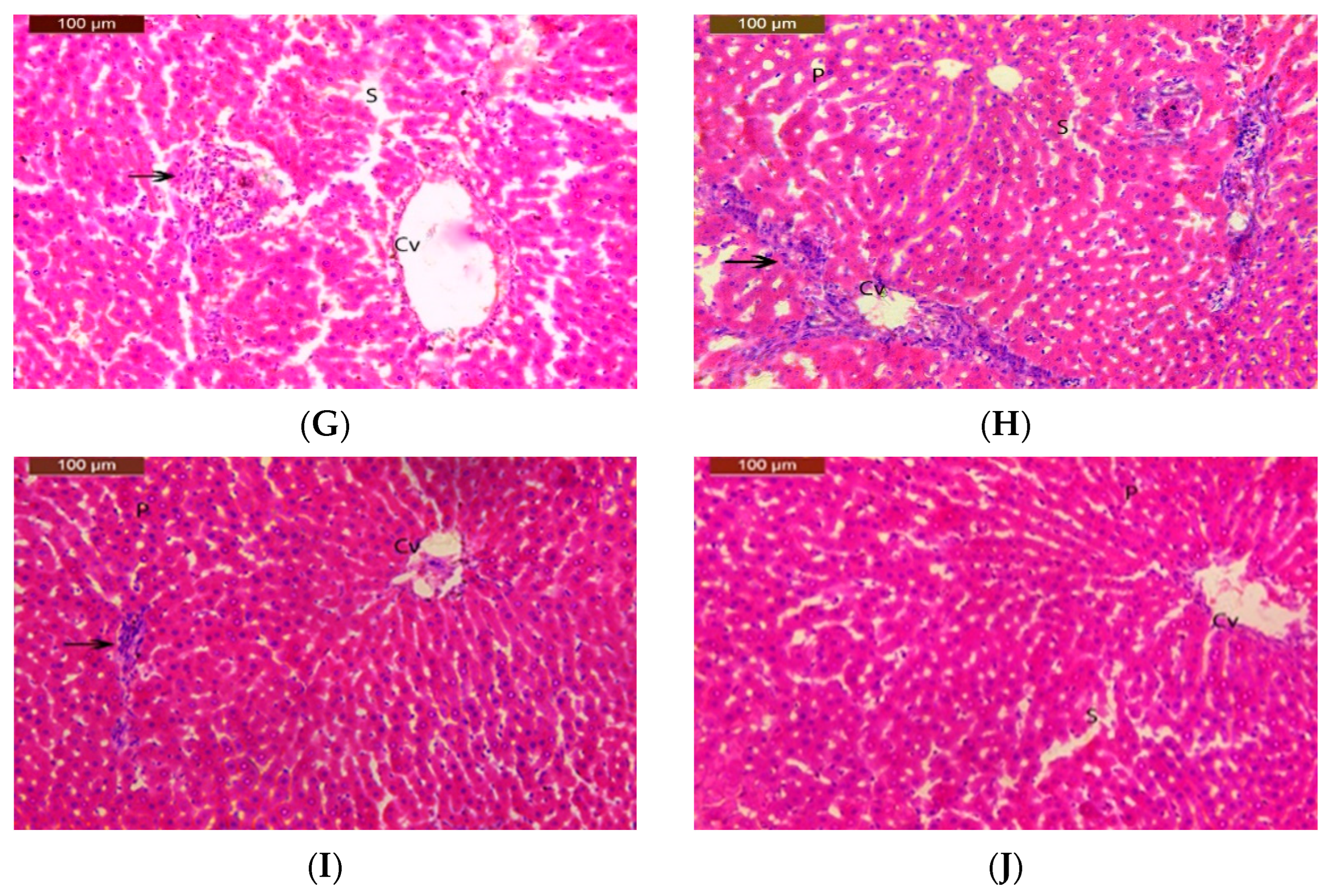
3.6. Histopathological Study
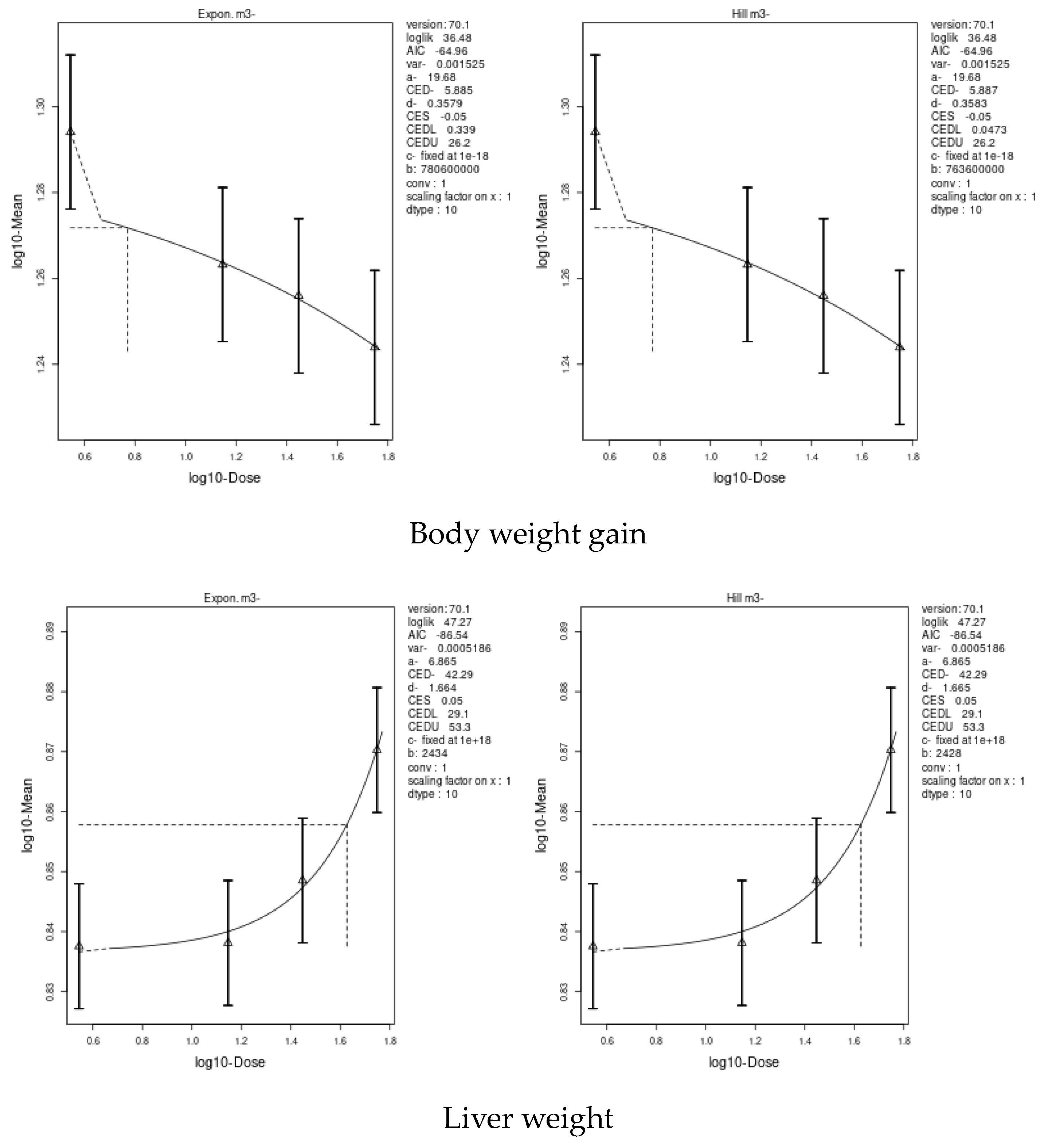
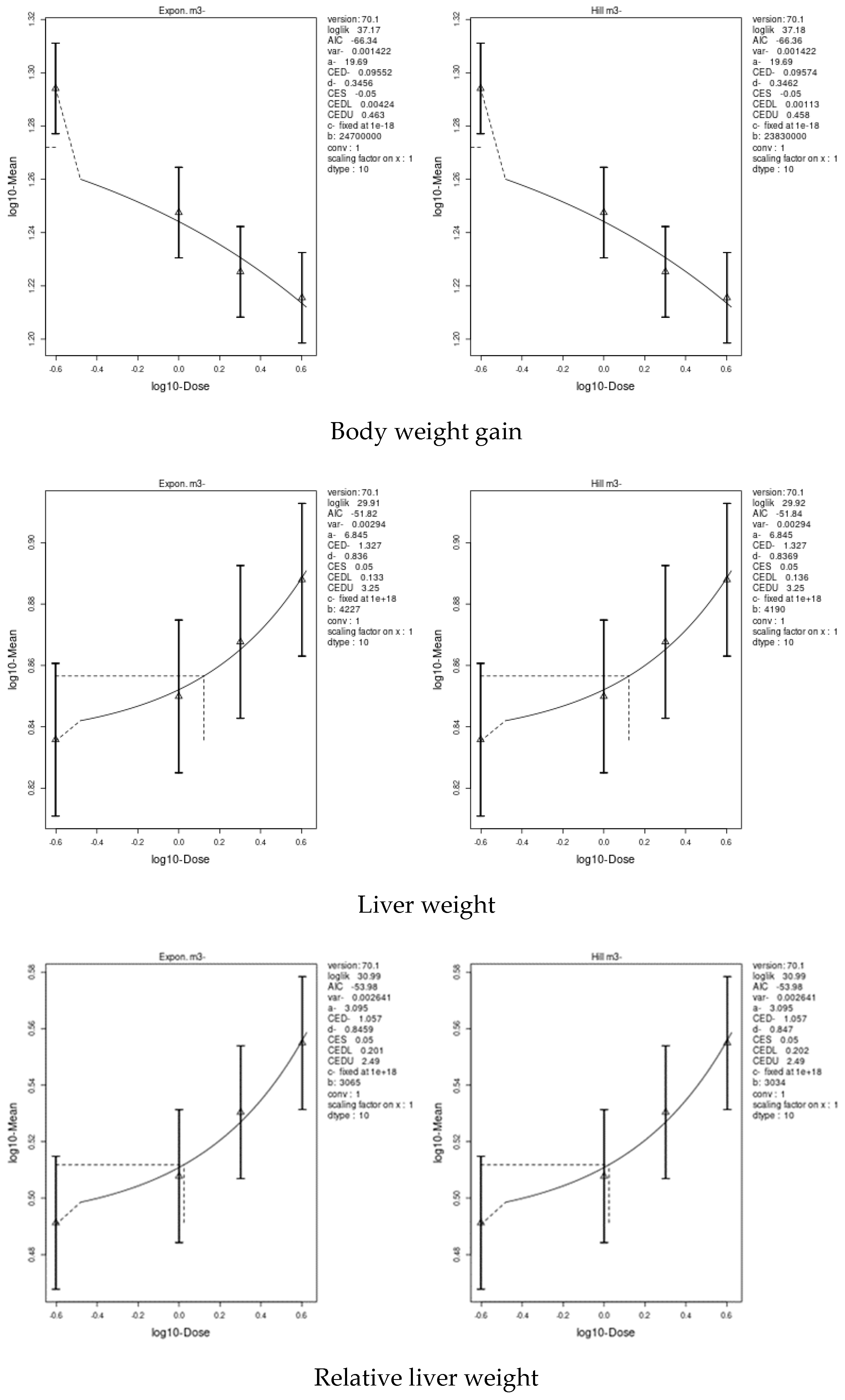
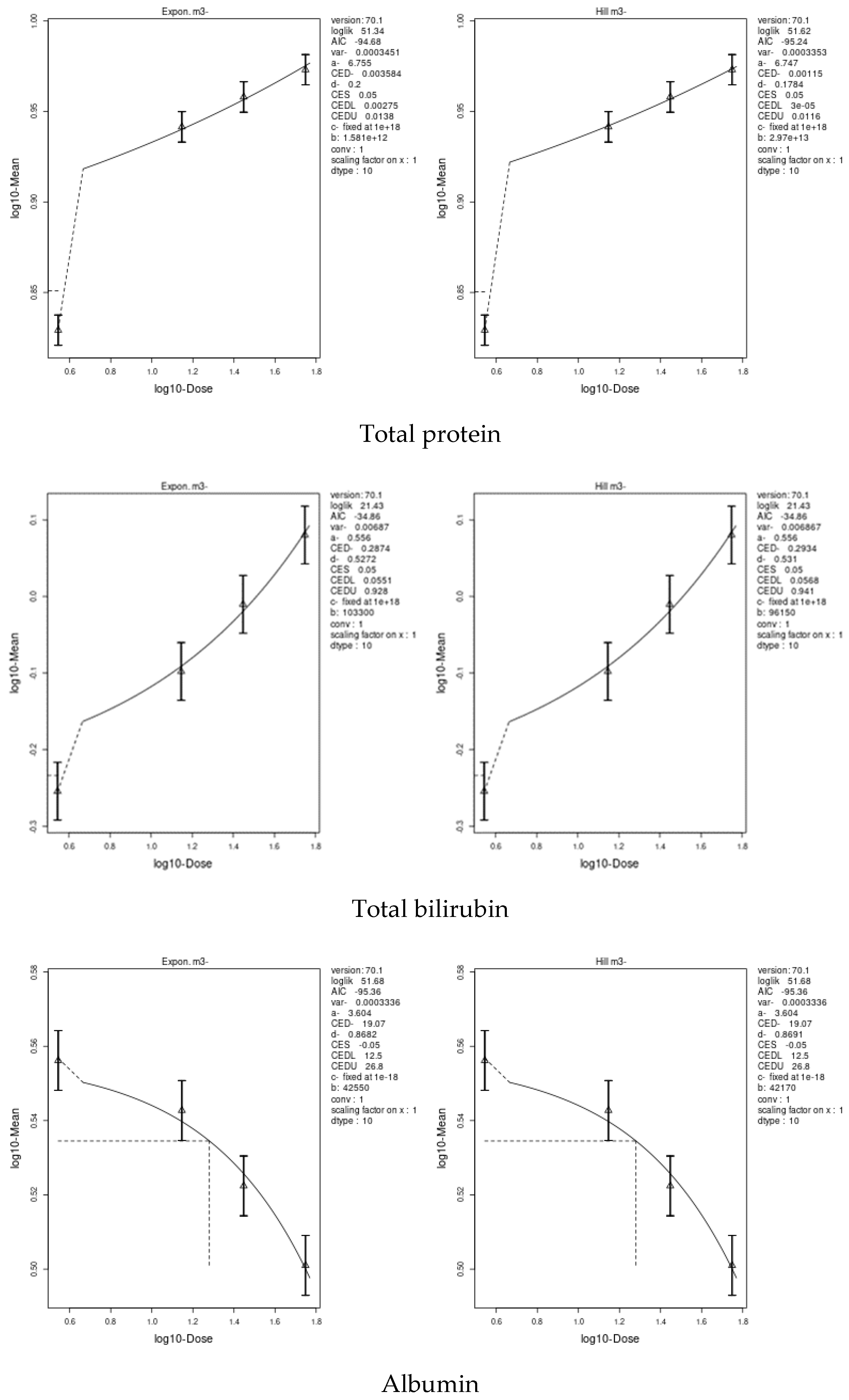
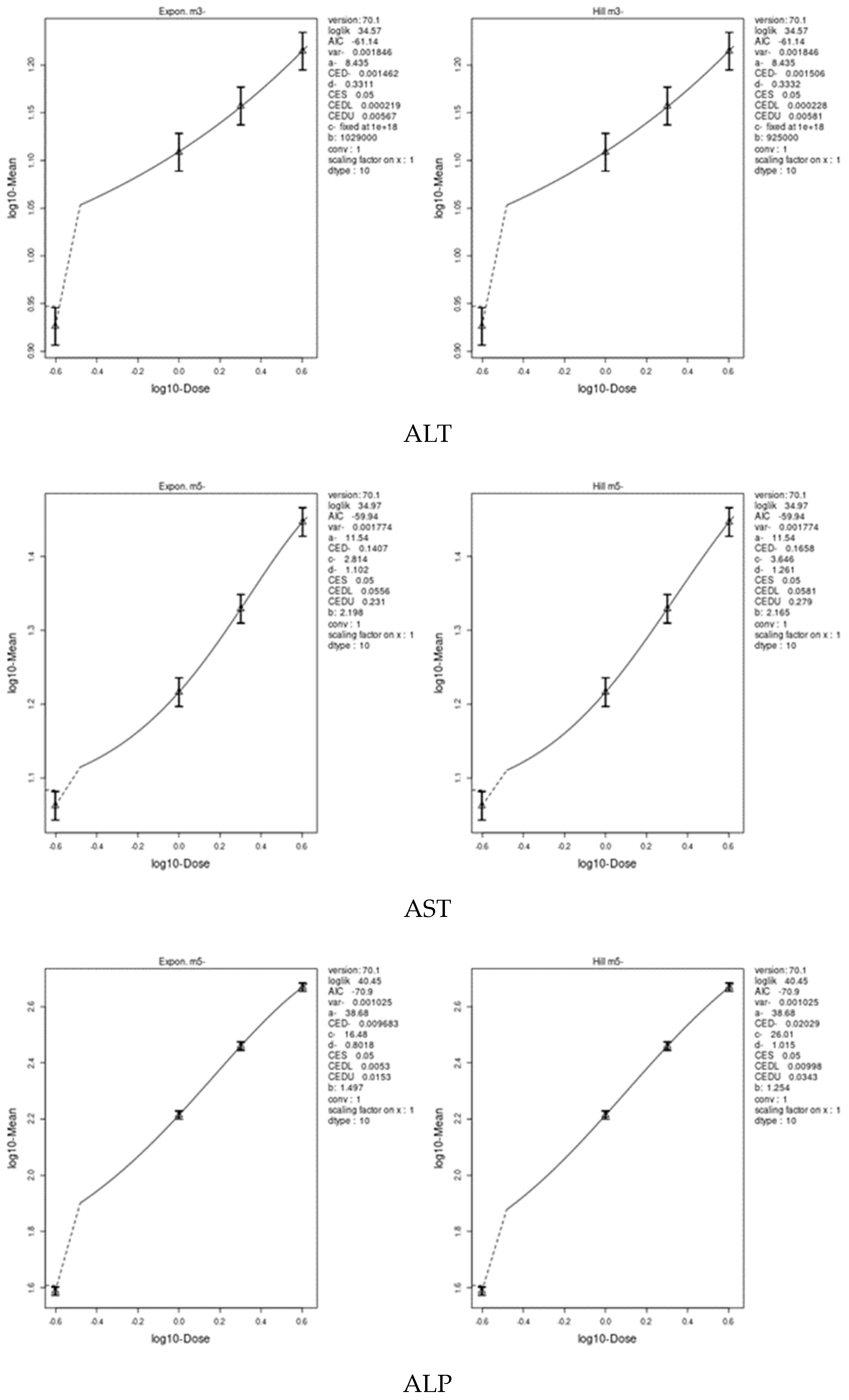
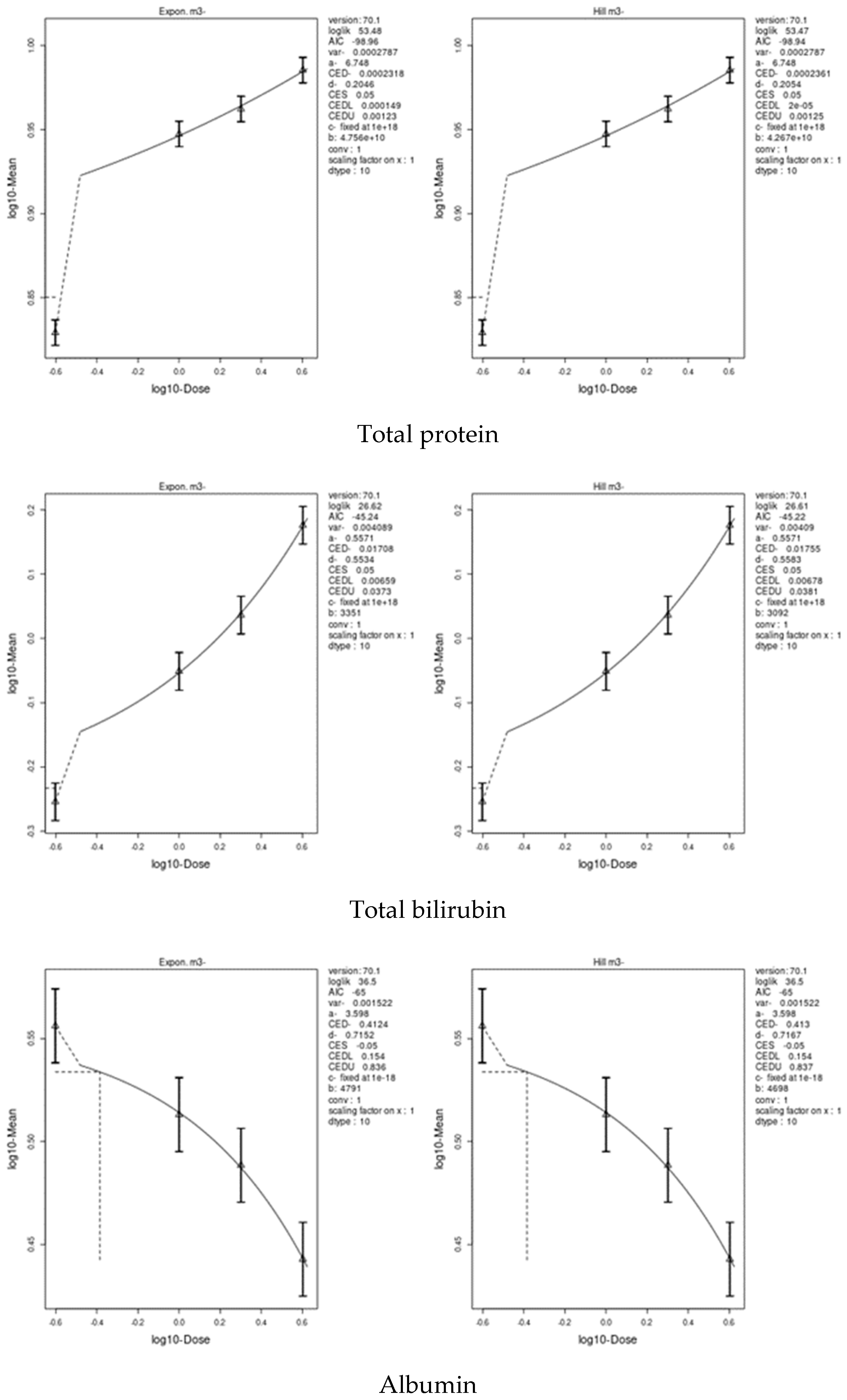
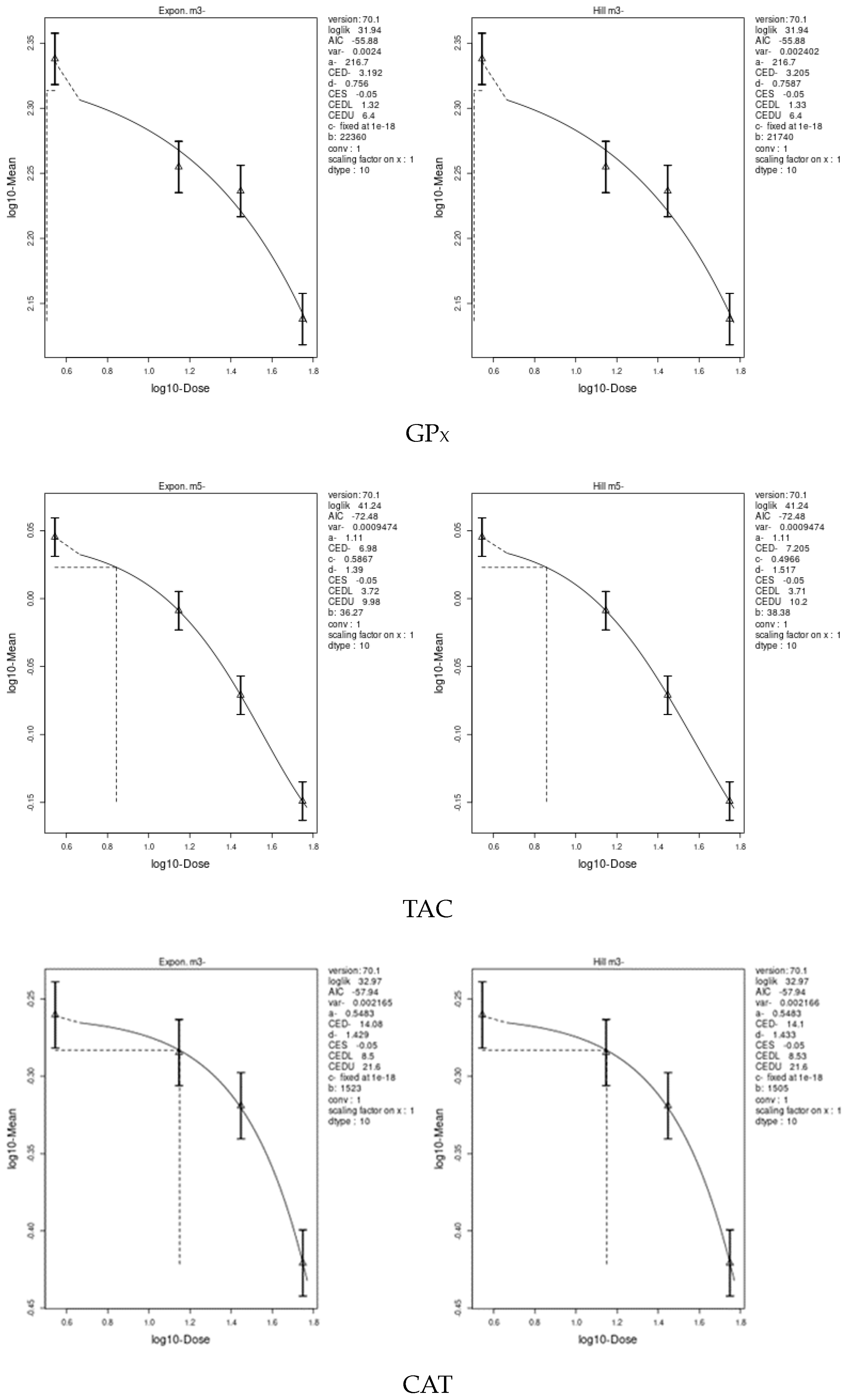
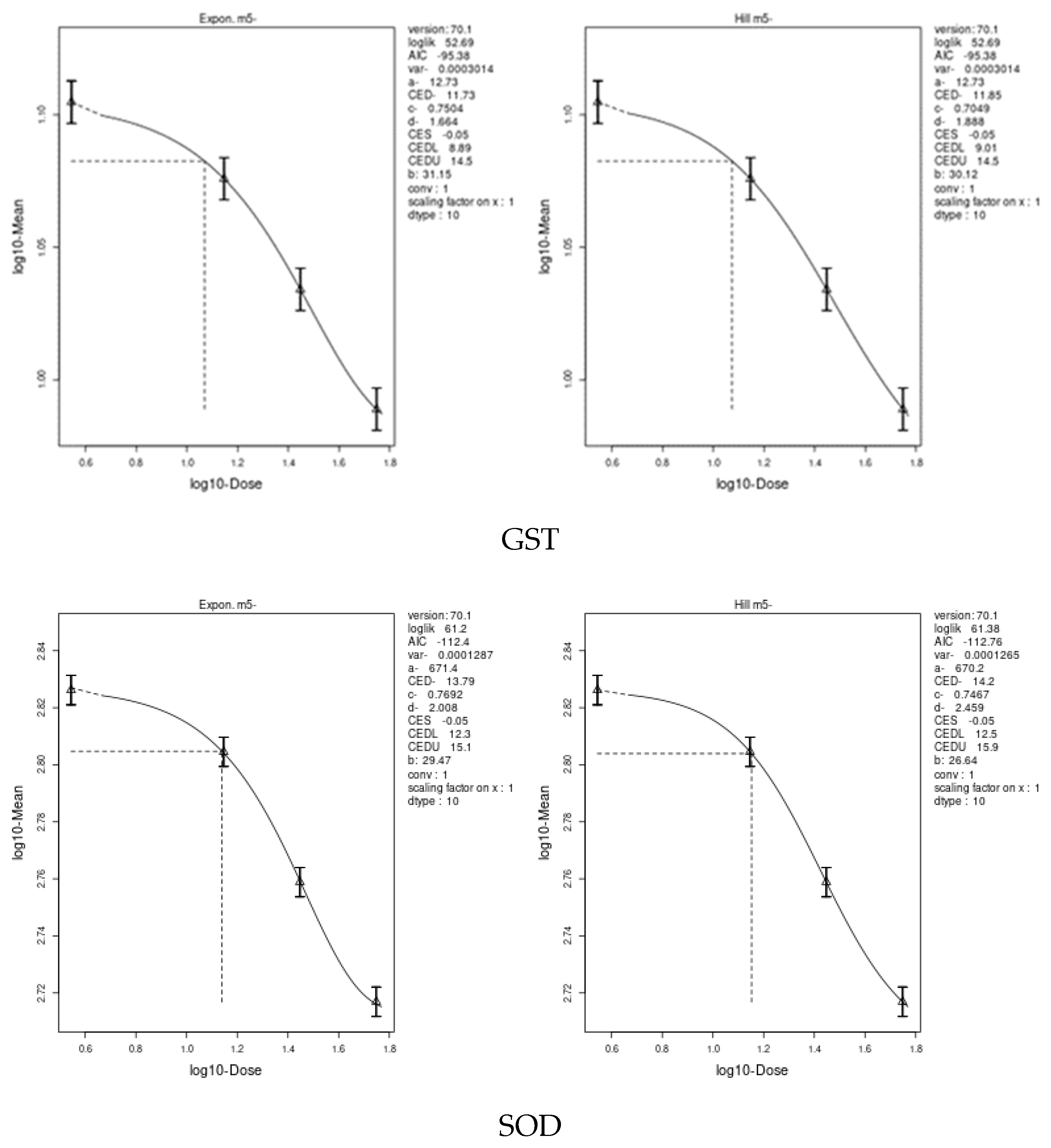
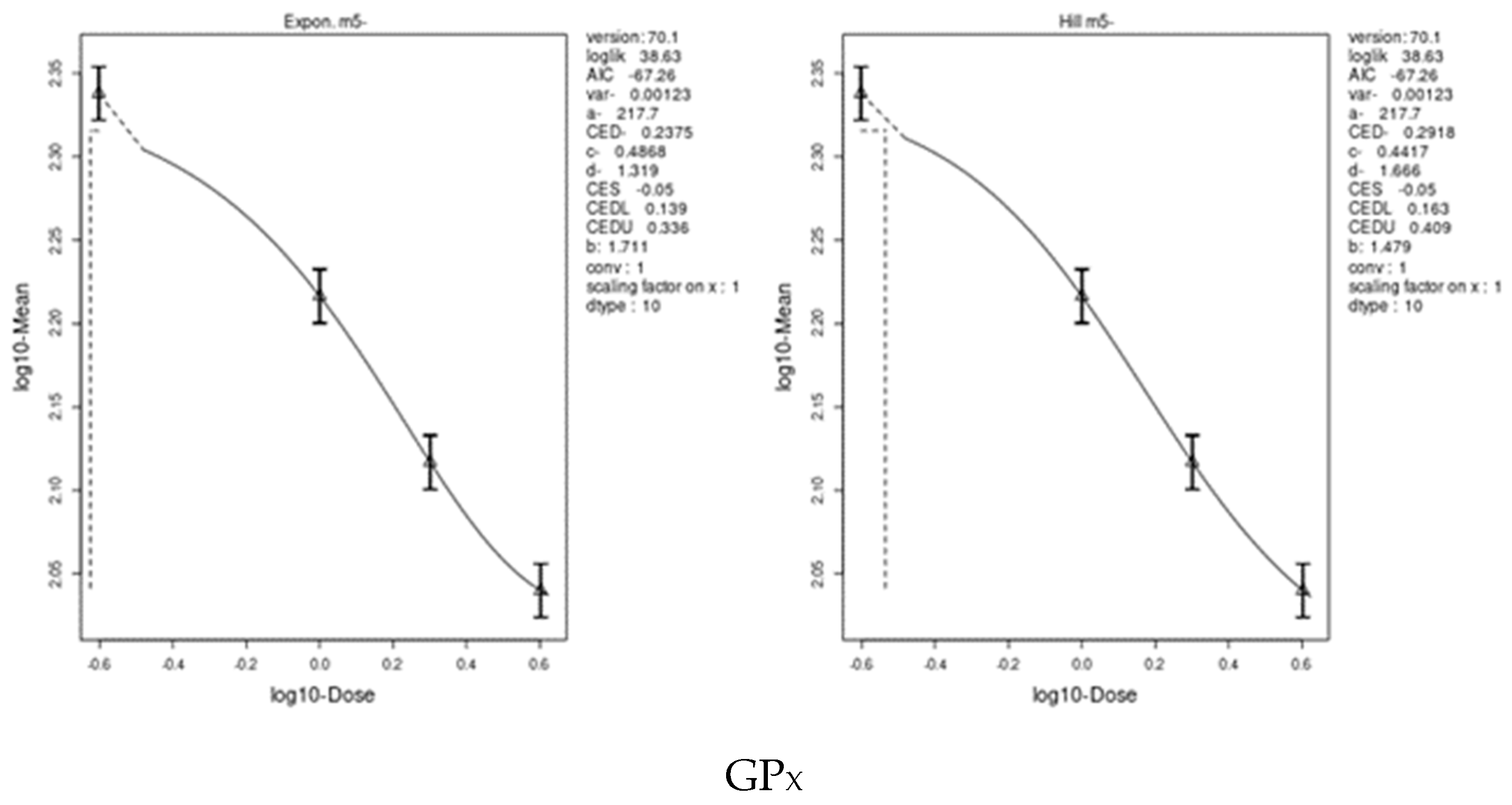
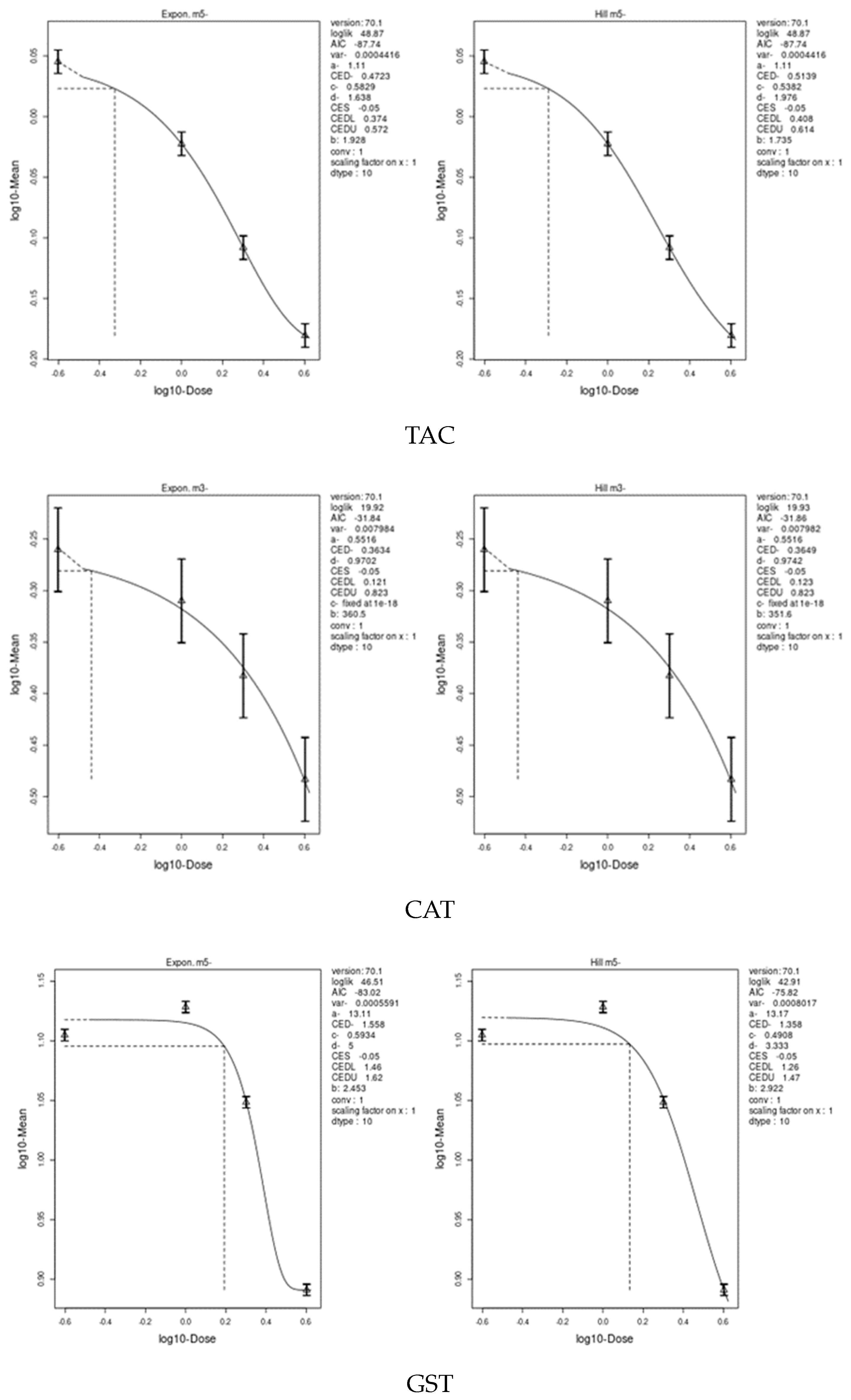
3.7. Benchmark Dose (BMD) Approach
4. Discussion
4.1. Adverse Effects of Exposure to Pesticides
4.2. Clove-Oil-Based Nanoemulsion Loaded with Pomegranate Extract
4.3. Effect of IM and CPF on Body and Liver Weights
4.4. Effect of IM and CPF on Liver Function Biomarkers
4.5. Effect of IM and CPF on Oxidative Stress Biomarkers
4.6. Effect of IM and CPF on DNA Damage via Comet Assay
4.7. Effect of IM and CPF on Liver Tissue Architecture
4.8. Benchmark Dose (BMD) Approach and Dose–Response
4.9. Mechanism of Hepatoprotective of Clove-Oil-Based Nanoemulsion Loaded with Pomegranate Extract
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 2833. [Google Scholar] [CrossRef]
- Sarkar, S.; Dias Bernardes Gil, J.; Keeley, J.; Möhring, N.; Jansen, K. The Use of Pesticides in Developing Countries and Their Impact on Health and the Right to Food Policy Department for External Relations. Eur. Union 2021, 1–56. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Marei, A.E.S.M.; Al-Ashkar, M.A.M.; Mossa, A.T.H. Adverse Biochemical Effects of Various Pesticides on Sprayers of Cotton Fields in El-Behira Governorate, Egypt. Biomed. Aging Pathol. 2014, 4, 251–256. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.-T.H. Oxidative Damage, Biochemical and Histopathological Alterations in Rats Exposed to Chlorpyrifos and the Antioxidant Role of Zinc. Pestic. Biochem. Physiol. 2010, 96, 14–23. [Google Scholar] [CrossRef]
- Vohra, P.; Khera, K.S.; Sangha, G.K. Physiological, Biochemical and Histological Alterations Induced by Administration of Imidacloprid in Female Albino Rats. Pestic. Biochem. Physiol. 2014, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Mossa, A.T.H. Lipid Peroxidation and Oxidative Stress in Rat Erythrocytes Induced by Chlorpyrifos and the Protective Effect of Zinc. Pestic. Biochem. Physiol. 2009, 93, 34–39. [Google Scholar] [CrossRef]
- Jia, Z.; Misra, H.P. Reactive Oxygen Species in in Vitro Pesticide-Induced Neuronal Cell (SH-SY5Y) Cytotoxicity: Role of NFκB and Caspase-3. Free Radic. Biol. Med. 2007, 42, 288–298. [Google Scholar] [CrossRef]
- Eiben, R.; Rinke, M. Subchronic Toxicity on Wistar Rats. Administration in the Feed for 96 Days; Bayer AG. Department of Toxicology. Fachbereich Toxikologie: Wuppertal, Germany, 1989; p. 18187. [Google Scholar]
- e-Pesticide-Manual Pesticide Manual Online—BCPC British Crop Production Council: BCPC British Crop Production Council. Available online: https://www.bcpc.org/product/bcpc-online-pesticide-manual-latest-version (accessed on 11 April 2023).
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Mohd Effendy, N.; Mohamed, N.; Muhammad, N.; Naina Mohamad, I.; Shuid, A.N. Eurycoma Longifolia: Medicinal Plant in the Prevention and Treatment of Male Osteoporosis Due to Androgen Deficiency. Evid. -Based Complement. Altern. Med. 2012, 2012, 125761. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- González Peña, O.I.; López Zavala, M.Á.; Cabral Ruelas, H. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Azzini, E.; Zucca, P.; Varoni, E.M.; Kumar, N.V.A.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Haggag, M.I.; Elhaw, M.H. Estimation of Some Phytochemical Materials and Isolation of Two Flavonoids from Pomegranate Peel Using Different Chromatographic Techniques. Mater. Today Proc. 2022, 57, 362–367. [Google Scholar] [CrossRef]
- Mishra, B.B.; Tiwari, V.K. Natural Products: An Evolving Role in Future Drug Discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- Valero-Mendoza, A.G.; Meléndez-Rentería, N.P.; Chávez-González, M.L.; Flores-Gallegos, A.C.; Wong-Paz, J.E.; Govea-Salas, M.; Zugasti-Cruz, A.; Ascacio-Valdés, J.A. The Whole Pomegranate (Punica granatum L.), Biological Properties and Important Findings: A Review. Food Chem. Adv. 2023, 2, 100153. [Google Scholar] [CrossRef]
- Abbas Syed, Q. Nutritional and Therapeutic Properties of Pomegranate. Sch. J. Food Nutr. 2018, 1, 115–120. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, Antioxidant and Tyrosinase-Inhibition Activities of Pomegranate Fruit Peel Methanolic Extract. BMC Complement. Altern. Med. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Hanafy, S.M.; Abd El-Shafea, Y.M.; Saleh, W.D.; Fathy, H.M. Chemical Profiling, in Vitro Antimicrobial and Antioxidant Activities of Pomegranate, Orange and Banana Peel-Extracts against Pathogenic Microorganisms. J. Genet. Eng. Biotechnol. 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Fahmy, M.A.; Farghaly, A.A.; Hassan, E.E.; Hassan, E.M.; Hassan, Z.M.; Mahmoud, K.; Omara, E.A. Evaluation of the Anti-Cancer/Anti-Mutagenic Efficiency of Lavandula officinalis Essential Oil. Asian Pacific J. Cancer Prev. 2022, 23, 1215–1222. [Google Scholar] [CrossRef]
- Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.A.I.E.; Naja, F.; Radwan, H.; Ismail, L.C.; Hasan, H.; Hashim, M.; Obaid, R.S. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules 2023, 28, 1809. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A Precious Spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evid. -Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Chericoni, S.; Prieto, J.M.; Iacopini, P.; Cioni, P.; Morelli, I. In Vitro Activity of the Essential Oil of Cinnamomum zeylanicum and Eugenol in Peroxynitrite-Induced Oxidative Processes. J. Agric. Food Chem. 2005, 53, 4762–4765. [Google Scholar] [CrossRef]
- Guerra, F.Q.S.; Mendes, J.M.; De Sousa, J.P.; Morais-Braga, M.F.B.; Santos, B.H.C.; Melo Coutinho, H.D.; Lima, E.D.O. Increasing Antibiotic Activity against a Multidrug-Resistant Acinetobacter spp by Essential Oils of Citrus limon and Cinnamomum zeylanicum. Nat. Prod. Res. 2012, 26, 2235–2238. [Google Scholar] [CrossRef]
- Cheng, S.S.; Liu, J.Y.; Huang, C.G.; Hsui, Y.R.; Chen, W.J.; Chang, S.T. Insecticidal Activities of Leaf Essential Oils from Cinnamomum osmophloeum against Three Mosquito Species. Bioresour. Technol. 2009, 100, 457–464. [Google Scholar] [CrossRef]
- Shahid, M.Z.; Saima, H.; Yasmin, A.; Nadeem, M.T.; Imran, M.; Afzaal, M. Antioxidant Capacity of Cinnamon Extract for Palm Oil Stability. Lipids Health Dis. 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Screening Assessment Phenol, 2-Methoxy-5-(2-propenyl)- (eugenol) and Rose, Rosa Canina, ext. Chemical Abstracts Service Registry Numbers 97-53-0 and 84696-47-9. Available online: https://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=360A57FE-1 (accessed on 20 June 2023).
- Patel, C.; Dadhaniya, P.; Hingorani, L.; Soni, M.G. Safety Assessment of Pomegranate Fruit Extract: Acute and Subchronic Toxicity Studies. Food Chem. Toxicol. 2008, 46, 2728–2735. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and Nano-Encapsulation of Vegetable and Essential Oils to Develop Functional Food Products with Improved Nutritional Profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, M.; Paul, A.K.; Lee, M.T.; Joykin, A.R.; Por, C.S.; Mahboob, T.; Salibay, C.C.; Torres, M.S.; Guiang, M.M.M.; Rahmatullah, M.; et al. Phytochemicals and Nano-Phytopharmaceuticals Use in Skin, Urogenital and Locomotor Disorders: Are We There? Plants 2022, 11, 1265. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Mossa, A.T.H.; Mohafrash, S.M.M. Green Nanoemulsion Insecticides: Toxicity, Safety, and Applications. In Bio-Based Nanoemulsions for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 197–206. ISBN 9780323898461. [Google Scholar]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Crump, K. A New Method for Determining Allowable Daily Intakes*1. Fundam. Appl. Toxicol. 1984, 4, 854–871. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.F.; Mossa, A.T.H.; Refaie, A.A.; Ibrahim, N.E.; Mohafrash, S.M.M. Benchmark Dose and the Adverse Effects of Exposure to Pendimethalin at Low Dose in Female Rats. Basic Clin. Pharmacol. Toxicol. 2022, 130, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.W.; Bailer, A.J. An Empirical Comparison of Low-Dose Extrapolation from Points of Departure (PoD) Compared to Extrapolations Based upon Methods That Account for Model Uncertainty. Regul. Toxicol. Pharmacol. 2013, 67, 75–82. [Google Scholar] [CrossRef]
- Miller, M.A. Gender-Based Differences in the Toxicity of Pharmaceuticals—The Food and Drug Administration’s Perspective. Int. J. Toxicol. 2001, 20, 149–152. [Google Scholar] [CrossRef]
- Gohel, M.; Soni, T.; Hingorani, L.; Patel, A.; Patel, N. Development and Optimization of Plant Extract Loaded Nanoemulsion Mixtures for the Treatment of Inflammatory Disorder. Curr. Res. Drug Discov. 2014, 1, 29–38. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Sehirli, O.; Sener, G.; Dumlu, M.U.; Ercan, F.; Gedik, N.; Gökmen, V. Pomegranate Peel Extract Prevents Liver Fibrosis in Biliary-Obstructed Rats. J. Pharm. Pharmacol. 2010, 59, 1287–1295. [Google Scholar] [CrossRef]
- Mohafrash, S.M.M.; Mossa, A.T.H. Herbal Syrup from Chicory and Artichoke Leaves Ameliorate Liver Damage Induced by Deltamethrin in Weanling Male Rats. Environ. Sci. Pollut. Res. 2020, 27, 7672–7682. [Google Scholar] [CrossRef] [PubMed]
- NRC. Guide for the Care and Use of Laboratory Animals, Council, National Research; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0.
- OECD. OECD Guidelines for the Testing of Chemicals / Section 4: Health Effects Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents (Google EBook); OECD: Paris, France, 2008; ISBN 9264070680. [Google Scholar]
- Rettman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kind, P.R.; King, E.J. Estimation of Plasma Phosphatase by Determination of Hydrolysed Phenol with Amino-Antipyrine. J. Clin. Pathol. 1954, 7, 322–326. [Google Scholar] [CrossRef]
- Malloy, H.T.; Evelyn, K.A. The Determination of Bilirubin with the Photoelectric Colorimeter. J. Biol. Chem. 1937, 119, 481–490. [Google Scholar] [CrossRef]
- Schultze, H.E.; Heremans, J.F. Molecular Biology of Human Proteins, with Special Reference to Plasma Proteins. Volume 1: Nature and Metabolism of Extracellular Proteins. Q. Rev. Biol. 1967, 42, 80–81. [Google Scholar] [CrossRef]
- Doumas, B.T.; Ard Watson, W.; Biggs, H.G. Albumin Standards and the Measurement of Serum Albumin with Bromcresol Green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the Measurement of Antioxidant Activity in Human Fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- OECD. Test No. 492: Reconstructed Human Cornea-like Epithelium (RhCE) Test Method for Identifying Chemicals Not Requiring Classification and Labelling for Eye Irritation or Serious Eye Damage. Guidel. Test. Chem. 2019, 1–27. [Google Scholar] [CrossRef]
- Mohafrash, S.M.M.; Fallatah, S.A.; Farag, S.M.; Mossa, A.T.H. Mentha Spicata Essential Oil Nanoformulation and Its Larvicidal Application against Culex Pipiens and Musca Domestica. Ind. Crops Prod. 2020, 157, 112944. [Google Scholar] [CrossRef]
- Michael, J.D. Toxicologist’s Pocket Handbook, 2nd ed.; Informa Healthcare: New York, NY, USA, 2008. [Google Scholar]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Hernández, A.F.; Gil, F.; Lacasaña, M.; Rodríguez-Barranco, M.; Tsatsakis, A.M.; Requena, M.; Parrón, T.; Alarcón, R. Pesticide Exposure and Genetic Variation in Xenobiotic-Metabolizing Enzymes Interact to Induce Biochemical Liver Damage. Food Chem. Toxicol. 2013, 61, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Mossa, A.-T.H.; Abbassy, M.A. Adverse Haematological and Biochemical Effects of Certain Formulated Insecticides in Male Rats. Res. J. Environ. Toxicol. 2012, 6, 160–168. [Google Scholar] [CrossRef]
- De Freitas Cuba, L.; Salum, F.G.; Cherubini, K.; de Figueiredo, M.A. Antioxidant Agents: A Future Alternative Approach in the Prevention and Treatment of Radiation-Induced Oral Mucositis. Altern. Ther. Health Med. 2015, 21, 36–41. [Google Scholar]
- Patlevič, P.; Vašková, J.; Švorc, P.; Vaško, L.; Švorc, P. Reactive Oxygen Species and Antioxidant Defense in Human Gastrointestinal Diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar] [CrossRef]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The Global Distribution of Acute Unintentional Pesticide Poisoning: Estimations Based on a Systematic Review. BMC Public Health 2020, 20, 1–19. [Google Scholar] [CrossRef]
- Maroni, M.; Fait, A.; Colosio, C. Risk Assessment and Management of Occupational Exposure to Pesticides. Toxicol. Lett. 1999, 107, 145–153. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.-T.H.; Mohafrash, S.M.M.; Ziedan, E.-S.H.E.; Abdelsalam, I.S.; Sahab, A.F. Development of Eco-Friendly Nanoemulsions of Some Natural Oils and Evaluating of Its Efficiency against Postharvest Fruit Rot Fungi of Cucumber. Ind. Crops Prod. 2021, 159, 113049. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K.; Pitsch, S. Evaluation of Organ Weights for Rodent and Non-Rodent Toxicity Studies: A Review of Regulatory Guidelines and a Survey of Current Practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef]
- Mossa, A.T.H.; Mohafrash, S.M.M.; Chandrasekaran, N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. BioMed Res. Res. Int. 2018, 2018, 4308054. [Google Scholar] [CrossRef]
- Mossa, A.T.H.; Swelam, E.S.; Mohafrash, S.M.M. Sub-Chronic Exposure to Fipronil Induced Oxidative Stress, Biochemical and Histotopathological Changes in the Liver and Kidney of Male Albino Rats. Toxicol. Rep. 2015, 2, 775–784. [Google Scholar] [CrossRef]
- Hossain, M.A.; Sutradhar, L.; Sarker, T.R.; Saha, S.; Iqbal, M.M. Toxic Effects of Chlorpyrifos on the Growth, Hematology, and Different Organs Histopathology of Nile tilapia, Oreochromis niloticus. Saudi J. Biol. Sci. 2022, 29, 103316. [Google Scholar] [CrossRef]
- Heikal, T.M.; Mossa, H.A.-T. Cyromazine and Chlorpyrifos Induced Renal Toxicity in Rats: The Ameliorated Effects of Green Tea Extract. J. Environ. Anal. Toxicol. 2012, 2, 5. [Google Scholar] [CrossRef]
- Bal, R.; Türk, G.; Tuzcu, M.; Yilmaz, O.; Kuloglu, T.; Gundogdu, R.; Gür, S.; Agca, A.; Ulas, M.; Çambay, Z.; et al. Assessment of Imidacloprid Toxicity on Reproductive Organ System of Adult Male Rats. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2012, 47, 434–444. [Google Scholar] [CrossRef]
- Sevim, Ç.; Akpınar, E.; Aksu, E.H.; Ömür, A.D.; Yıldırım, S.; Kara, M.; Bolat, İ.; Tsatsakis, A.; Mesnage, R.; Golokhvast, K.S.; et al. Reproductive Effects of S. boulardii on Sub-Chronic Acetamiprid and Imidacloprid Toxicity in Male Rats. Toxics 2023, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Drury, R. Theory and Practice of Histological Techniques. JAMA 1983, 250, 1909. [Google Scholar] [CrossRef]
- Demir, F.; Uzun, F.G.; Durak, D.; Kalender, Y. Subacute Chlorpyrifos-Induced Oxidative Stress in Rat Erythrocytes and the Protective Effects of Catechin and Quercetin. Pestic. Biochem. Physiol. 2011, 99, 77–81. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.T.H. Adverse Effects of Exposure to Low Doses of Chlorpyrifos in Lactating Rats. Toxicol. Ind. Health 2011, 27, 213–224. [Google Scholar] [CrossRef]
- Lazic, S.E.; Semenova, E.; Williams, D.P. Determining Organ Weight Toxicity with Bayesian Causal Models: Improving on the Analysis of Relative Organ Weights. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Cattley, R.C.; Cullen, J.M. Liver and Gall Bladder. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1–3, pp. 1509–1566. ISBN 9780124157590. [Google Scholar]
- Meggs, W.J.; Brewer, K.L. Weight Gain Associated with Chronic Exposure to Chlorpyrifos in Rats. J. Med. Toxicol. 2007, 3, 89–93. [Google Scholar] [CrossRef]
- Peiris, D.C.; Dhanushka, T. Low Doses of Chlorpyrifos Interfere with Spermatogenesis of Rats through Reduction of Sex Hormones. Environ. Sci. Pollut. Res. 2017, 24, 20859–20867. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Srivastava, M.K.; Kapoor, U.; Srivastava, L.P. A 90 Days Oral Toxicity of Imidacloprid in Female Rats: Morphological, Biochemical and Histopathological Evaluations. Food Chem. Toxicol. 2010, 48, 1185–1190. [Google Scholar] [CrossRef]
- Hall, A.P.; Elcombe, C.R.; Foster, J.R.; Harada, T.; Kaufmann, W.; Knippel, A.; Küttler, K.; Malarkey, D.E.; Maronpot, R.R.; Nishikawa, A.; et al. Liver Hypertrophy: A Review of Adaptive (Adverse and Non-Adverse) Changes-Conclusions from the 3rd International ESTP Expert Workshop. Toxicol. Pathol. 2012, 40, 971–994. [Google Scholar] [CrossRef]
- Sellers, R.S.; Morton, D.; Michael, B.; Roome, N.; Johnson, J.K.; Yano, B.L.; Perry, R.; Schafer, K. Society of Toxicologic Pathology Position Paper: Organ Weight Recommendations for Toxicology Studies. Toxicol. Pathol. 2007, 35, 751–755. [Google Scholar] [CrossRef]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Mohafrash, S.M.M.; Hassan, E.E.; El-shaer, N.H.; Mossa, A.T.H. Detoxification Gene Expression, Genotoxicity, and Hepatorenal Damage Induced by Subacute Exposure to the New Pyrethroid, Imiprothrin, in Rats. Environ. Sci. Pollut. Res. 2021, 28, 33505–33521. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver Enzyme Alteration: A Guide for Clinicians. C. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.Y.F.; Aw, T.C. Liver Function Tests (LFTs). Proc. Singapore Healthc. 2010, 19, 80–82. [Google Scholar] [CrossRef]
- Sriapha, C.; Trakulsrichai, S.; Intaraprasong, P.; Wongvisawakorn, S.; Tongpoo, A.; Schimmel, J.; Wananukul, W. Imidacloprid Poisoning Case Series: Potential for Liver Injury. Clin. Toxicol. 2020, 58, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Mossa, A.H. Adverse Effects of Lactational Exposure to Chlorpyrifos in Suckling Rats. Hum. Exp. Toxicol. 2010, 29, 77–92. [Google Scholar] [CrossRef]
- Bayomy, M.F.F.; Abdel Samie, H.A.; Gendia, S.E.M. Potential Hepatoprotection Exerted by Ginseng against Chlorpyrifos-Induced Hepatotoxicity in Albino Rats. J. Biosci. Appl. Res. 2016, 2, 51–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, M.; Zhou, P.; Wang, C.; Zhang, Q.; Zhao, M. Multilevel Evaluations of Potential Liver Injury of Bifenthrin. Pestic. Biochem. Physiol. 2015, 122, 29–37. [Google Scholar] [CrossRef]
- Gangemi, S.; Gofita, E.; Costa, C.; Teodoro, M.; Briguglio, G.; Nikitovic, D.; Tzanakakis, G.; Tsatsakis, A.M.; Wilks, M.F.; Spandidos, D.A.; et al. Occupational and Environmental Exposure to Pesticides and Cytokine Pathways in Chronic Diseases (Review). Int. J. Mol. Med. 2016, 38, 1012–1020. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Cortés-Iza, S.C.; Rodríguez, A.I. Oxidative Stress and Pesticide Disease: A Challenge for Toxicology. Rev. Fac. Med. 2018, 66, 261–267. [Google Scholar] [CrossRef]
- Beone, G.M.; Carini, F.; Guidotti, L.; Rossi, R.; Gatti, M.; Fontanella, M.C.; Cenci, R.M. Potentially Toxic Elements in Agricultural Soils from the Lombardia Region of Northern Italy. J. Geochemical Explor. 2018, 190, 436–452. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.C.; Van Doornen, L.J.P.; Kemper, H.C.G. The Role of Antioxidant Vitamins and Enzymes in the Prevention of Exercise-Induced Muscle Damage. Sport. Med. 1996, 21, 213–238. [Google Scholar] [CrossRef]
- Child, R.B.; Wilkinson, D.M.; Fallowfield, J.O.L.; Donnelly, A.E. Elevated Serum Antioxidant Capacity and Plasma Malondialdehyde Concentration in Response to a Simulated Half-Marathon Run. Med. Sci. Sports Exerc. 1998, 30, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Yasmeen, R. Evaluation of Genotoxicity by Comet Assay (Single-Cell Gel Electrophoresis) in Tissues of the Fish Cyprinus Carpio during Sub-Lethal Exposure to Karanjin. J. Basic Appl. Zool. 2018, 79, 1–13. [Google Scholar] [CrossRef]
- De Lapuente, J.; Lourenço, J.; Mendo, S.A.; Borràs, M.; Martins, M.G.; Costa, P.M.; Pacheco, M. The Comet Assay and Its Applications in the Field of Ecotoxicology: A Mature Tool That Continues to Expand Its Perspectives. Front. Genet. 2015, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Frenzilli, G.; Lyons, B.P. The Comet Assay in Marine Animals. Methods Mol. Biol. 2013, 1044, 363–372. [Google Scholar] [CrossRef]
- Valverde, M.; Rojas, E. Environmental and Occupational Biomonitoring Using the Comet Assay. Mutat. Res./Rev. Mutat. Res. 2009, 681, 93–109. [Google Scholar] [CrossRef]
- McCauley, L.; Lasarev, M.; Muniz, J.; Stewart, V.N.; Kisby, G. Analysis of Pesticide Exposure and DNA Damage in Immigrant Farmworkers. J. Agromed. 2008, 13, 237–246. [Google Scholar] [CrossRef]
- Bharti, S.; Rasool, F. Analysis of the Biochemical and Histopathological Impact of a Mild Dose of Commercial Malathion on Channa Punctatus (Bloch) Fish. Toxicol. Rep. 2021, 8, 443–455. [Google Scholar] [CrossRef]
- Ojha, A.; Yaduvanshi, S.K.; Pant, S.C.; Lomash, V.; Srivastava, N. Evaluation of DNA Damage and Cytotoxicity Induced by Three Commonly Used Organophosphate Pesticides Individually and in Mixture, in Rat Tissues. Environ. Toxicol. 2013, 28, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, E.M.; Afroz, R.; Chowdhury, M.A.Z.; Gan, S.H.; Karim, N.; Islam, M.N.; Khalil, M.I. A Model of Chlorpyrifos Distribution and Its Biochemical Effects on the Liver and Kidneys of Rats. Hum. Exp. Toxicol. 2016, 35, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, E.M.; Afroz, R.; Chowdhury, M.A.Z.; Khalil, M.I.; Hossain, M.S.; Rahman, M.A.; Rashid, M.H.; Gan, S.H. Honey Has a Protective Effect against Chlorpyrifos-Induced Toxicity on Lipid Peroxidation, Diagnostic Markers and Hepatic Histoarchitecture. Eur. J. Integr. Med. 2015, 7, 525–533. [Google Scholar] [CrossRef]
- Duzguner, V.; Erdogan, S. Chronic Exposure to Imidacloprid Induces Inflammation and Oxidative Stress in the Liver & Central Nervous System of Rats. Pestic. Biochem. Physiol. 2012, 104, 58–64. [Google Scholar] [CrossRef]
- Mahajan, L.; Verma, P.K.; Raina, R.; Sood, S. Toxic Effects of Imidacloprid Combined with Arsenic: Oxidative Stress in Rat Liver. Toxicol. Ind. Health 2018, 34, 726–735. [Google Scholar] [CrossRef]
- EFSA. Public Consultation on the Draft Guidance on the Use of the Benchmark Dose Approach in Risk Assessment, European Food Safety Authority (EFSA); EFSA: Parma, Italy, 2022; Volume 19. [CrossRef]
- More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on the Use of the Benchmark Dose Approach in Risk Assessment. EFSA J. 2022, 20, e07584. [Google Scholar] [CrossRef]
- USEPA Reference Dose (RfD): Description and Use in Health Risk Assessments | US EPA. Available online: https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments (accessed on 9 May 2023).
- OECD. OECD/OCDE 423 Organisation for Economic Co-Operation and Development (OECD) Guideline for Testing of Chemicals, Acute Oral Toxicity-Acute Toxic Class Method; OECD: Paris, France, 2001. [Google Scholar]
- King, G.S. Handbook of Toxicologic Pathology. In Archives of Pathology & Laboratory Medicine; Academic Press: Cambridge, MA, USA, 2002; Volume 126, pp. 1138–1139. ISBN 0 12 330215 3. [Google Scholar]
- Edler, L. Benchmark Dose Approach in Regulatory Toxicology. In Regulatory Toxicology; Springer: Cham, Switzerland, 2021; pp. 339–374. [Google Scholar]
- Taheri, E.; Amin, M.M.; Daniali, S.S.; Abdollahpour, I.; Fatehizadeh, A.; Kelishadi, R. Health Risk Assessment of Exposure to Chlorpyrifos in Pregnant Women Using Deterministic and Probabilistic Approaches. PLoS ONE 2022, 17, e0262127. [Google Scholar] [CrossRef]
- Wołejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A.; Pietruszyńska, M.; Wydro, U. Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12209. [Google Scholar] [CrossRef]
- Thany, S.H. Molecular Mechanism of Action of Neonicotinoid Insecticides. Int. J. Mol. Sci. 2023, 24, 5484. [Google Scholar] [CrossRef]
- Santos, I.S.; Ponte, B.M.; Boonme, P.; Silva, A.M.; Souto, E.B. Nanoencapsulation of Polyphenols for Protective Effect against Colon-Rectal Cancer. Biotechnol. Adv. 2013, 31, 514–523. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Benchagra, L.; Berrougui, H.; Islam, M.O.; Ramchoun, M.; Boulbaroud, S.; Hajjaji, A.; Fulop, T.; Ferretti, G.; Khalil, A. Antioxidant Effect of Moroccan Pomegranate (Punica granatum L. Sefri Variety) Extracts Rich in Punicalagin against the Oxidative Stress Process. Foods 2021, 10, 2219. [Google Scholar] [CrossRef] [PubMed]
- Saparbekova, A.A.; Kantureyeva, G.O.; Kudasova, D.E.; Konarbayeva, Z.K.; Latif, A.S. Potential of Phenolic Compounds from Pomegranate (Punica granatum L.) by-Product with Significant Antioxidant and Therapeutic Effects: A Narrative Review. Saudi J. Biol. Sci. 2023, 30, 103553. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Eugenol: A Structure-Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-Inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Teniente, S.L.; Flores-Gallegos, A.C.; Esparza-González, S.C.; Campos-Múzquiz, L.G.; Nery-Flores, S.D.; Rodríguez-Herrera, R. Anticancer Effect of Pomegranate Peel Polyphenols against Cervical Cancer. Antioxidants 2023, 12, 127. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Cichoz-Lach, H.; Michalak, A. Oxidative Stress as a Crucial Factor in Liver Diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Hunyadi, A. The Mechanism(s) of Action of Antioxidants: From Scavenging Reactive Oxygen/Nitrogen Species to Redox Signaling and the Generation of Bioactive Secondary Metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-Κβ System. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Wolino, K.S.; Almeida, P.P.; Mafra, D.; Stockler-Pinto, M.B. Bioactive Compounds Modulating Toll-like 4 Receptor (TLR4)-Mediated Inflammation: Pathways Involved and Future Perspectives. Nutr. Res. 2022, 107, 96–116. [Google Scholar] [CrossRef] [PubMed]
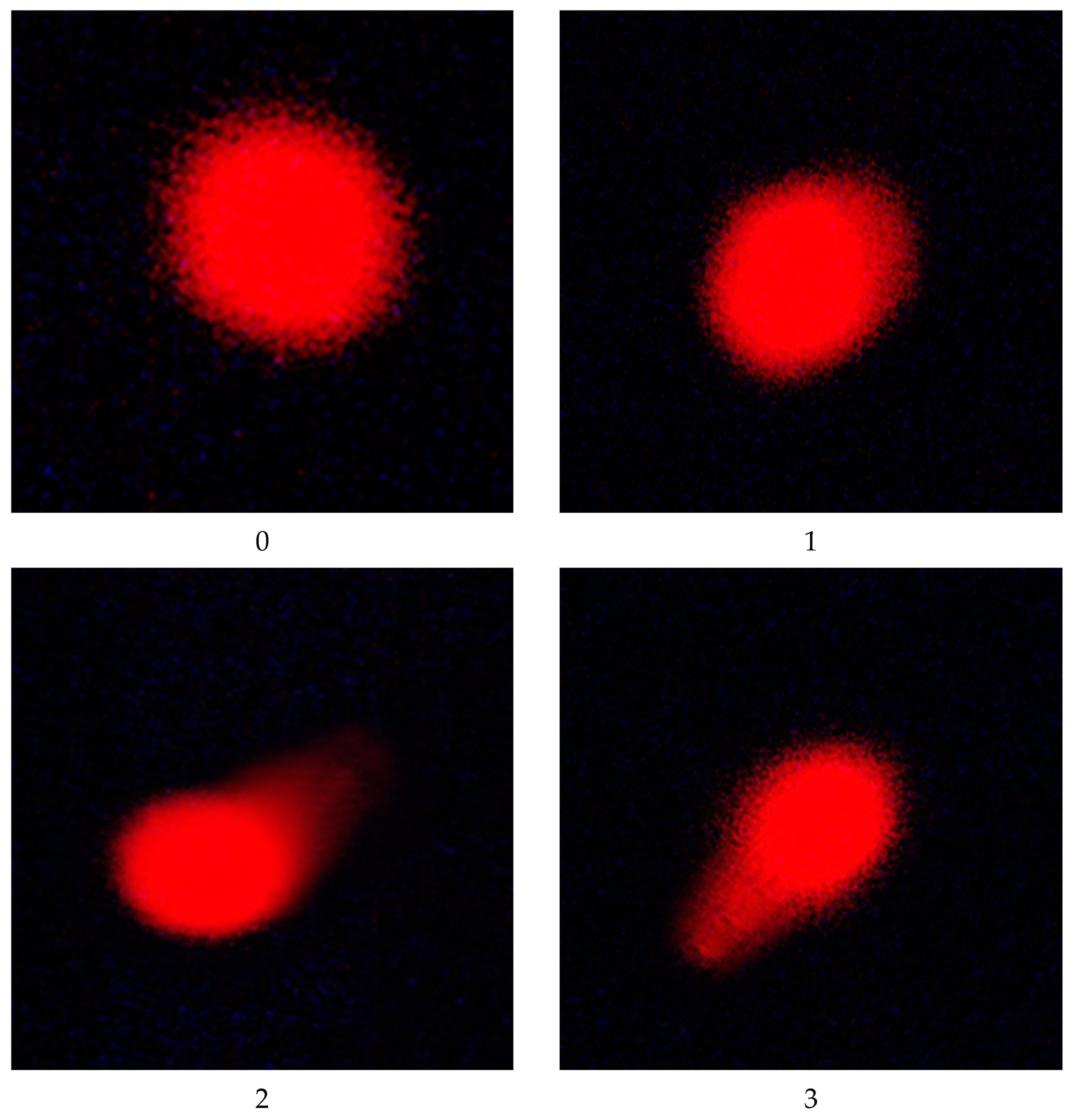


| Group | Initial bw (g) | Final bw (g) | Weekly bw Gain (g) | % of Weekly bw Gain | Liver Weight | Relative Liver Weight |
|---|---|---|---|---|---|---|
| I | 142.00 ± 2.30 | 220.80 ± 2.60 ab | 19.70 ± 0.80 a | 100.00 | 6.88 ± 0.63 cd | 3.11 ± 0.26 cd |
| II | 142.80 ± 1.90 | 220.40 ± 2.40 abc | 19.40 ± 1.04 ab | 98.48 | 6.57 ± 0.24 d | 2.98 ± 0.12 d |
| III | 145.20 ± 2.20 | 218.60 ± 1.90 bc | 18.35 ± 0.80 bc | 93.15 | 6.89 ± 0.36 bcd | 3.15 ± 1.40 cd |
| IV | 146.60 ± 2.10 | 218.80 ± 2.60 bc | 18.05 ± 0.90 c | 91.62 | 7.06 ± 0.43 bcd | 3.23 ± 0.21 bc |
| V | 148.20 ± 1.10 | 218.40 ± 2.30 bc | 17.55 ± 0.70 cd | 89.09 | 7.42 ± 0.29 ab | 3.39 ± 0.13 ab |
| VI | 149.00 ± 2.40 | 219.80 ± 1.50 abc | 17.70 ± 0.80 cd | 89.85 | 7.08 ± 0.17 bcd | 3.22 ± 0.06 bcd |
| VII | 150.40 ± 0.50 | 217.60 ± 1.10 cd | 16.80 ± 0.20 de | 85.28 | 7.39 ± 0.50 abc | 3.40 ± 0.24 ab |
| VIII | 149.60 ± 1.90 | 215.40 ± 1.80 d | 16.45 ± 0.90 e | 83.50 | 7.73 ± 0.26 a | 3.59 ± 0.10 a |
| IX | 148.00 ± 1.00 | 222.00 ± 2.10 a | 18.50 ± 0.60 bc | 93.91 | 6.92 ± 0.29 bcd | 3.12 ± 0.11 cd |
| X | 150.00 ± 1.60 | 221.80 ± 1.60 a | 17.95 ± 0.80 c | 91.12 | 6.71 ± 0.35 d | 3.02 ± 0.17 cd |
| Group | AST (U/L) | ALT (U/L) | ALP (IU/L) | Total Bilirubin (mg/dL) | Total Protein (g/dL) | Albumin (g/dL) |
|---|---|---|---|---|---|---|
| I | 11.55 ± 0.43 h | 8.45 ± 0.49 f | 38.75 ± 2.39 g | 0.56 ± 0.06 g | 6.75 ± 0.18 h | 3.60 ± 0.09 b |
| II | 9.98 ± 0.58 i | 7.27 ± 0.54 g | 39.79 ± 2.73 g | 0.43 ± 0.06 h | 6.96 ± 0.09 h | 3.82 ± 0.18 a |
| III | 13.51 ± 0.39 g | 10.30 ± 0.54 e | 60.32 ± 6.52 f | 0.80 ± 0.05 f | 8.74 ± 0.12 fe | 3.49 ± 0.07 b |
| IV | 17.64 ± 1.58 ed | 12.89 ± 1.08 c | 76.45 ± 5.13 e | 0.98 ± 0.08 ed | 9.08 ± 0.19 dc | 3.33 ± 0.04 c |
| V | 24.81 ± 2.11 b | 15.04 ± 0.75 b | 93.62 ± 6.69 d | 1.21 ± 0.13 b | 9.40 ± 0.17 b | 3.17 ± 0.06 de |
| VI | 16.46 ± 0.42 fe | 12.86 ± 0.70 c | 81.68 ± 7.49 e | 0.89 ± 0.05 fe | 8.86 ± 0.13 ed | 3.26 ± 0.03 cd |
| VII | 21.39 ± 1.50 c | 14.37 ± 0.60 b | 106.81 ± 7.35 c | 1.09 ± 0.08 c | 9.17 ± 0.16 c | 3.08 ± 0.07 e |
| VIII | 28.00 ± 1.23 a | 16.40 ± 0.56 a | 178.13 ± 6.85 a | 1.50 ± 0.03 a | 9.67 ± 0.11 a | 2.78 ± 0.21 f |
| IX | 15.26 ± 1.48 f | 12.03 ± 0.85 dc | 84.40 ± 6.72 e | 1.06 ± 0.11 dc | 8.58 ± 0.29 f | 3.34 ± 0.08 c |
| X | 18.48 ± 0.79 d | 11.58 ± 0.95 d | 157.50 ± 10.22 b | 1.15 ± 0.07 cb | 8.29 ± 0.21 g | 3.14 ± 0.07 de |
| Group | Oxidative Stress Markers | ||||
|---|---|---|---|---|---|
| Liver Tissue | Serum | ||||
| GST (U/g. t.) | GPx (U/g. t.) | SOD (U/g. t.) | CAT (U/g. t.) | TAC (mM/L) | |
| I | 12.73 ± 0.14 b | 217.88 ± 7.7 b | 670.2 ± 6.5 b | 0.55 ± 0.03 b | 1.11 ± 0.02 b |
| II | 12.29 ± 0.15 c | 252.89 ± 23.2 a | 676.95 ± 19.8 a | 0.66 ± 0.02 a | 1.15 ± 0.01 a |
| III | 11.91 ± 0.19 d | 180.27 ± 12.4 dc | 637.61 ± 3.9 c | 0.52 ± 0.03 cb | 0.98 ± 0.02 c |
| IV | 10.82 ± 0.27 f | 172.49 ± 7.4 dc | 574.05 ± 11.5 e | 0.48 ± 0.02 d | 0.85 ± 0.04 d |
| V | 9.75 ± 0.22 h | 137.47 ± 5.1 e | 521.1 ± 5.2 g | 0.38 ± 0.02 e | 0.71 ± 0.03 f |
| VI | 13.44 ± 0.13 a | 164.71 ± 6.6 d | 607.65 ± 9.6 d | 0.49 ± 0.01 dc | 0.95 ± 0.03 c |
| VII | 11.18 ± 0.10 e | 130.98 ± 5.5 e | 497.1 ± 12.1 h | 0.42 ± 0.07 e | 0.78 ± 0.02 e |
| VIII | 7.78 ± 0.12 i | 109.71 ± 4.3 f | 401.44 ± 5.04 i | 0.33 ± 0.03 f | 0.66 ± 0.01 g |
| IX | 10.95 ± 0.26 fe | 182.86 ± 9.9 c | 574.05 ± 11.3 e | 0.51 ± 0.02 dc | 0.88 ± 0.04 d |
| X | 10.18 ± 0.16 g | 169.89 ± 7.5 dc | 543.33 ± 11.9 f | 0.49 ± 0.01 dc | 0.86 ± 0.04 d |
| Group | Cell Number | Comet Class * | Mean ± SEM | ||||
|---|---|---|---|---|---|---|---|
| Total Cells | Comet | 0 | 1 | 2 | 3 | ||
| I | 400 | 39 | 361 | 31 | 8 | 0 | 9.75 ± 0.85 |
| II | 400 | 41 | 359 | 34 | 7 | 0 | 10.25 ± 0.48 |
| III | 400 | 51 | 349 | 32 | 14 | 5 | 12.75 ± 1.11 |
| IV | 400 | 66 | 334 | 36 | 18 | 12 | 16.50 ± 1.04 |
| V | 400 | 95 | 305 | 40 | 24 | 31 | 23.75 ± 1.44 |
| VI | 400 | 53 | 347 | 31 | 13 | 9 | 13.25 ± 1.25 |
| VII | 400 | 72 | 328 | 39 | 17 | 16 | 18.00 ± 1.47 |
| VIII | 400 | 97 | 303 | 43 | 20 | 34 | 24.25 ± 1.55 |
| IX | 400 | 63 | 337 | 28 | 16 | 19 | 15.75 ± 1.65 |
| X | 400 | 66 | 334 | 26 | 15 | 25 | 16.50 ± 1.19 |
| Group | Inflammatory Cell Infiltration | Dilated Sinusoids | Degeneration Changes | Pyknotic Nuclei |
|---|---|---|---|---|
| I | - | - | - | - |
| II | - | - | - | - |
| III | + | + | + | + |
| IV | + | + | + | + |
| V | ++ | ++ | ++ | ++ |
| VI | + | + | + | + |
| VII | + | + | + | + |
| VIII | ++ | ++ | ++ | + |
| IX | + | + | + | + |
| X | + | + | + | + |
| Parameters | Imidacloprid (IM) | Chlorpyrifos (CPF) | ||||
|---|---|---|---|---|---|---|
| BMD | BMD Confidence Interval | BMD | BMD Confidence Interval | |||
| Lowest BMDL | Highest BMDU | Lowest BMDL | Highest BMDU | |||
| Body weight | 5.885 | 0.0473 | 26.2 | 0.09552 | 0.00113 | 0.463 |
| Liver weight | 42.29 | 29.1 | 53.3 | 1.327 | 0.133 | 3.25 |
| Relative liver weight | - | - | - | 1.057 | 0.201 | 2.49 |
| ALP | 1.464 | 1.18 | 2.75 | 0.00968 | 0.0053 | 0.0343 |
| ALT | 5.309 | 2.45 | 9.06 | 0.001462 | 0.000219 | 0.00581 |
| AST | 6.866 | 3.43 | 10.8 | 0.1407 | 0.0556 | 0.279 |
| Total protein | 0.00115 | 0.00003 | 0.0138 | 0.0002361 | 0.00002 | 0.00125 |
| Albumin | 19.07 | 12.5 | 26.8 | 0.4124 | 0.154 | 0.837 |
| Total bilirubin | 0.2874 | 0.0551 | 0.941 | 0.01708 | 0.00659 | 0.0381 |
| GPX | 3.192 | 1.32 | 6.4 | 0.2375 | 0.139 | 0.409 |
| TAC | 6.98 | 3.71 | 10.2 | 0.4723 | 0.374 | 0.614 |
| CAT | 14.08 | 8.5 | 21.6 | 0.3634 | 0.121 | 0.823 |
| GST | 11.73 | 8.89 | 14.5 | 1.358 | 1.26 | 1.62 |
| SOD | 13.79 | 12.3 | 15.9 | 0.7079 | 0.619 | 0.815 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, A.A.A.-H.; Gad, M.F.; Refaie, A.A.; Abdelhafez, H.M.; Mossa, A.-T.H. Benchmark Dose Approach to DNA and Liver Damage by Chlorpyrifos and Imidacloprid in Male Rats: The Protective Effect of a Clove-Oil-Based Nanoemulsion Loaded with Pomegranate Peel Extract. Toxics 2023, 11, 569. https://doi.org/10.3390/toxics11070569
Omar AAA-H, Gad MF, Refaie AA, Abdelhafez HM, Mossa A-TH. Benchmark Dose Approach to DNA and Liver Damage by Chlorpyrifos and Imidacloprid in Male Rats: The Protective Effect of a Clove-Oil-Based Nanoemulsion Loaded with Pomegranate Peel Extract. Toxics. 2023; 11(7):569. https://doi.org/10.3390/toxics11070569
Chicago/Turabian StyleOmar, Alia Ahmed Abdel-Hamid, Marwa Farouk Gad, Amel A. Refaie, Hemmat Mansour Abdelhafez, and Abdel-Tawab H. Mossa. 2023. "Benchmark Dose Approach to DNA and Liver Damage by Chlorpyrifos and Imidacloprid in Male Rats: The Protective Effect of a Clove-Oil-Based Nanoemulsion Loaded with Pomegranate Peel Extract" Toxics 11, no. 7: 569. https://doi.org/10.3390/toxics11070569
APA StyleOmar, A. A. A.-H., Gad, M. F., Refaie, A. A., Abdelhafez, H. M., & Mossa, A.-T. H. (2023). Benchmark Dose Approach to DNA and Liver Damage by Chlorpyrifos and Imidacloprid in Male Rats: The Protective Effect of a Clove-Oil-Based Nanoemulsion Loaded with Pomegranate Peel Extract. Toxics, 11(7), 569. https://doi.org/10.3390/toxics11070569







