Toxicity of Nanoscale Zero-Valent Iron to Soil Microorganisms and Related Defense Mechanisms: A Review
Abstract
1. Introduction
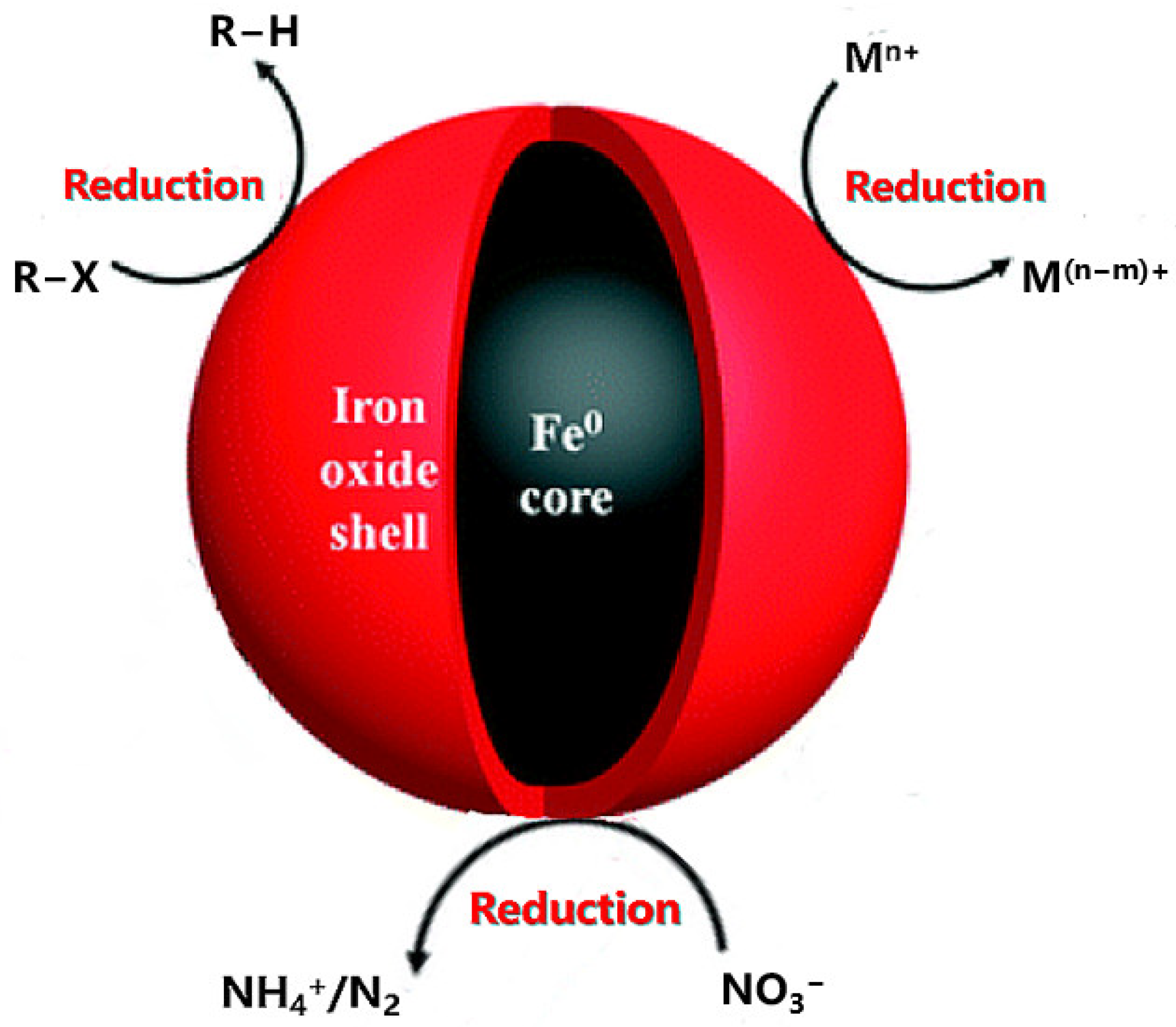
2. Application of Nano Zero-Valent Iron
Application of Nano Zero-Valent Iron in Degradation of Soil Organic Pollutants
3. Behavior of Nano-Zero-Valent Iron in Soil Environment
4. Toxic Effects of nZVI on Microorganisms

| Species of Bacteria | Characteristics of nZVI | Toxic Effect | Reference |
|---|---|---|---|
| Escherichia coli | 10–70 mm 1000 mg·L−1 | The growth of E.coli was inhibited by nZVI with a particle size of 10–70 mm and a concentration of 1000 mg/L, and the toxicity of E. coli was more serious during the exponential growth phase and the decline growth phase. | [17] |
| nZVI-B (precipitated with borohydride); size: 20–100 nm. Concentration: 1000 mg·L−1 nZVI-T (produced by gas phase reduction of iron oxide under H2); size: <100 nm Concentration: 4000 mg·L−1 | The significant negative effects on bacteria were only found in the first two leachates. The highest nZVI toxicity to E. coli was detected in the aqueous phase of the slurry treated with nZVI-B. nZVI-T did not show a negative impact. | [49] | |
| Pseudomonas putida | 10–70 mm 1000 mg·L−1 | nZVI showed more serious toxicity to the exponential phase and the decline phase of Pseudomonas putida. | [17] |
| 50–100 nm 10 μg/L–1.0 g/L | When P. putida cells were exposed to nZVI at concentrations as low as 100 μg/L, they died significantly. Negative reactions could not be detected after the nanoparticles were continuously exposed to oxygen. | [50] | |
| Bacillus cereus | <50 nm 150 g/kg | No toxic effect was detected 90 days after injection of nZVI. | [37] |
| Pseudomona saeruginosa | 50 g/kg | No toxic effect was detected 90 days after injection of nZVI. | [37] |
| Klebsiella oxytoca | 80–120 nm 1000, 5000 and 10,000 mg·L−1 | There are some bacterial cells around, but no obvious morphological changes. It adhered to the cell surface without obvious cell damage. | [51] |
| Properties of nZVI | Microbial Properties | Influence | References |
|---|---|---|---|
| Particle size | Gram− | When <50 nm, the toxicity decreased. | [41] |
| Gram+ | The smaller the particle size, the stronger the toxicity. | [40] | |
| Dosage | Gram− | The duration of low concentration toxicity effect was stronger than that of high concentration. | [52] |
| Gram+ | |||
| Aging | Gram− | The toxicity decreased significantly after oxidation. | [42,43,53,54] |
| Gram+ | |||
| Modification method | Gram− | CMC/nZVI effectively alleviated the damage of cell membranes. | [55] |
| Gram+ | Negatively charged polymer coatings reduce toxicity | [56] | |
| The toxicity was significantly enhanced after modification of active metals. | [47] |
5. Factors Affecting the Microbial Toxicity of nZVI
5.1. Properties of nZVI
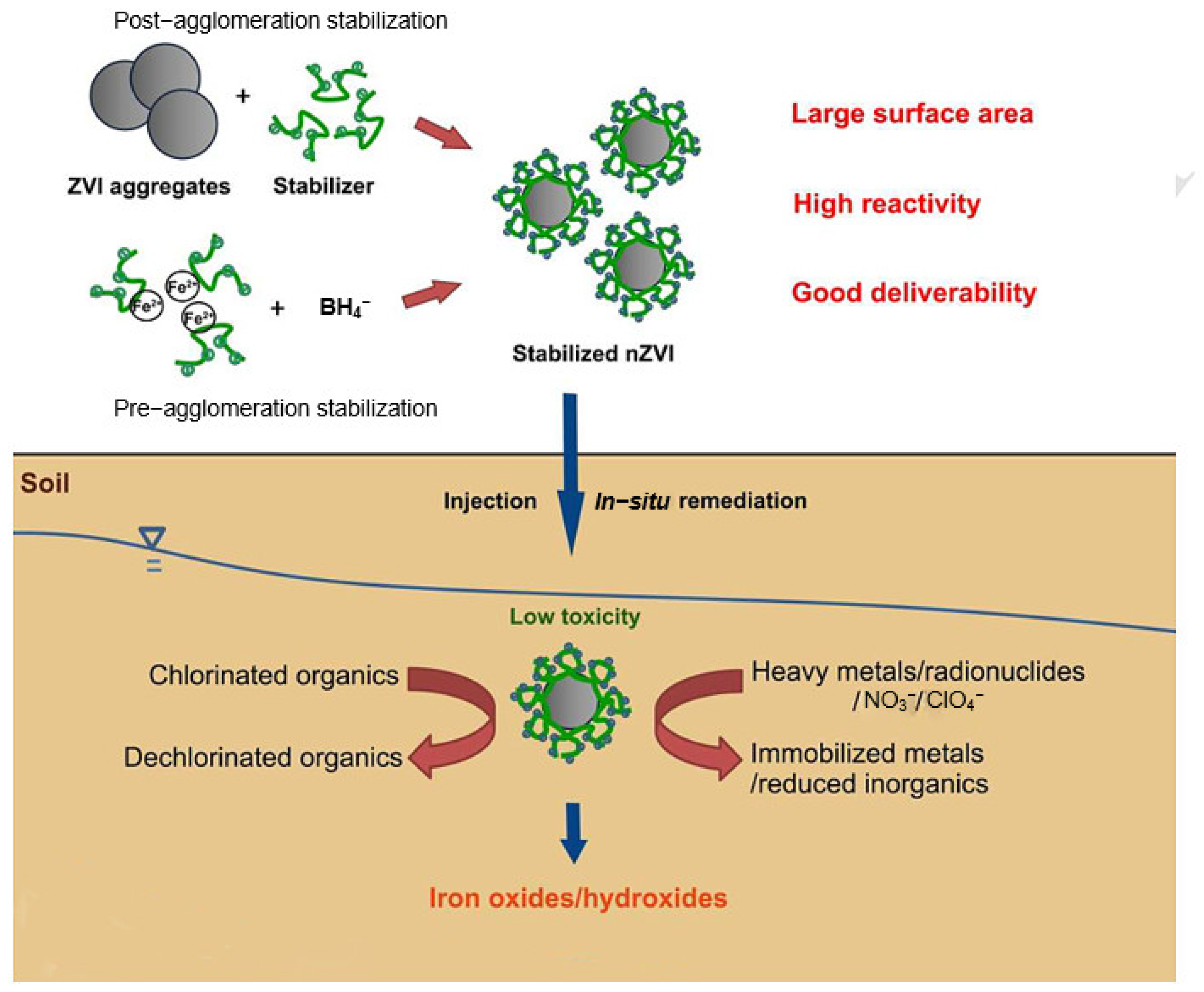
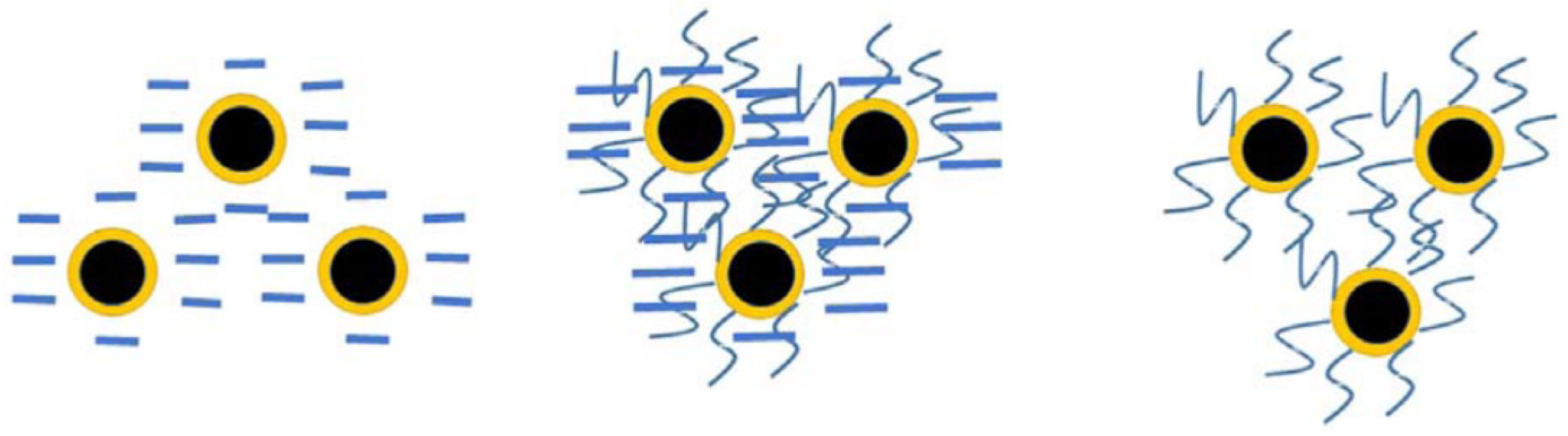
5.2. Surface Modification and Soil Properties of nZVI
6. The Toxic Mechanism of nZVI on Microorganisms
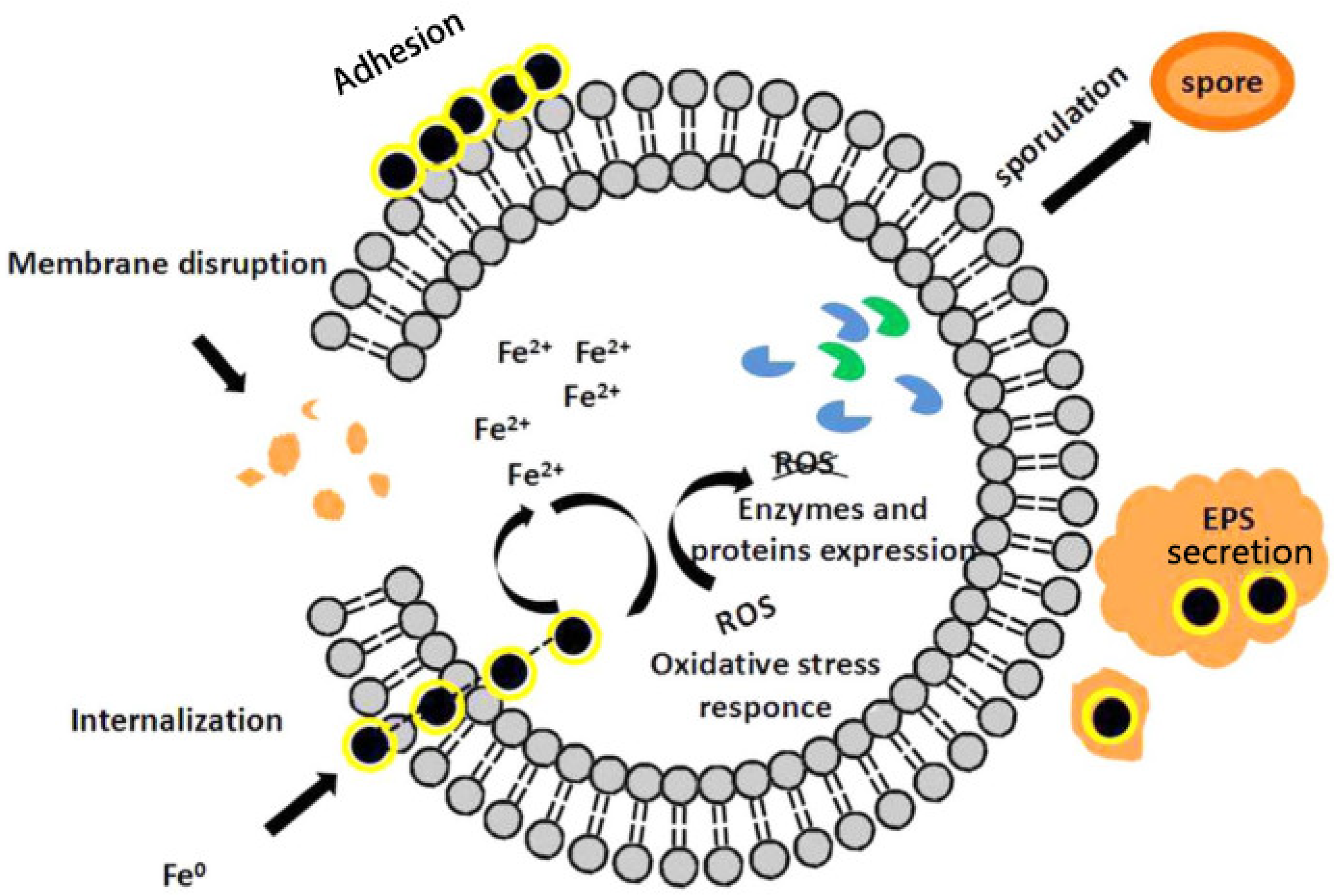
6.1. Microbial Cell Membrane Morphology or Function Damage
6.2. Oxidative Damage
6.3. Release of Iron Ions
6.4. Genetic Damage
7. Cell Defense Behavior
8. Methods to Alleviate the Toxicity of Nano-Zero-Valent Iron
9. Conclusions and Perspectives
- (1)
- The factors affecting the toxicity of nZVI to soil microorganisms are complex and are influenced by the nature of nZVI itself, whether it is modified, the amount used, and even in practical applications, the soil properties and the linkage with other substances in the environment. In addition, the result of the interaction between nZVI and other substances in the soil in practical application may enhance the toxicity of nZVI or weaken it, and strengthening the research in this aspect is beneficial to determine the performance of nZVI in the actual environment.
- (2)
- The mechanisms of nZVI toxicity are currently agreed upon by academics as morphological or functional damage to microbial cell membranes, oxidative damage, release of iron ions and genetic damage. The first three are toxicological studies at the cellular level, while gene damage is a study at the molecular level and is still in the research stage. Although there are many hypotheses about the mechanism of toxicity, the exact mechanism is still unclear, and the existence of other mechanisms needs to be further investigated, taking into account environmental factors, test organisms and the properties of nZVI itself.
- (3)
- The defenses of microbial cells in soil against nZVI toxicity are currently more clearly elucidated at the cellular level, but studies at the genetic level are still lacking. Meanwhile, the commonalities and characteristics of cellular defense behaviors need to be further investigated and summarized.
- (4)
- Toxicity studies on the temporal effects of nZVI need to be strengthened. nZVI toxicity may be a long-term and chronic process after injection into the environment, and under certain conditions, the short-term toxic effects may not be obvious, so there is a need to strengthen the long-term and large-scale studies on the toxic effects of nZVI on the ecological environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef]
- Asha, A.B.; Narain, R. Nanomaterials properties. In Polymer Science and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–359. [Google Scholar]
- Mdlovu, N.V.; Lin, K.-S.; Chen, Z.-W.; Liu, Y.-J.; Mdlovu, N.B. Treatment of simulated chromium-contaminated wastewater using polyethylenimine-modified zero-valent iron nanoparticles. J. Taiwan Inst. Chem. Eng. 2020, 108, 92–101. [Google Scholar] [CrossRef]
- Sun, H.; Li, G.; An, T.; Zhao, H.; Wong, P.K. Unveiling the photoelectrocatalytic inactivation mechanism of Escherichia coli: Convincing evidence from responses of parent and anti-oxidation single gene knockout mutants. Water Res. 2016, 88, 135–143. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Li, C.; Yamauchi, Y. Nanostructured nonprecious metal catalysts for electrochemical reduction of carbon dioxide. Nano Today 2016, 11, 373–391. [Google Scholar] [CrossRef]
- Gajanan, K.; Tijare, S.N. Applications of nanomaterials. Mater. Today Proc. 2018, 5, 1093–1096. [Google Scholar] [CrossRef]
- Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ. Sci. Nano 2017, 4, 27–45. [Google Scholar] [CrossRef]
- Azzam, A.M.; El-Wakeel, S.T.; Mostafa, B.B.; El-Shahat, M.F. Removal of Pb, Cd, Cu and Ni from aqueous solution using nano scale zero valent iron particles. J. Environ. Chem. Eng. 2016, 4, 2196–2206. [Google Scholar] [CrossRef]
- Gueye, M.T.; Di Palma, L.; Allahverdiyeva, G.; Bavasso, I.; Petrucci, E.; Stoller, M. The influence of heavy metals and organic matter on hexavalent chromium reduction by nano zero valent iron in soil. Chem. Eng. Trans. 2016, 47, 289–294. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Lei, C.; Zhang, L.; Yang, K.; Zhu, L.; Lin, D. Toxicity of iron-based nanoparticles to green algae: Effects of particle size, crystal phase, oxidation state and environmental aging. Environ. Pollut. 2016, 218, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Yirsaw, B.D.; Megharaj, M.; Chen, Z.; Naidu, R. Environmental application and ecological significance of nano-zero valent iron. J. Environ. Sci. (China) 2016, 44, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Abudayyak, M.; Oztas, E.; Arici, M.; Ozhan, G. Investigation of the toxicity of bismuth oxide nanoparticles in various cell lines. Chemosphere 2017, 169, 117–123. [Google Scholar] [CrossRef]
- Yang, M.; Wu, X.; He, C.; Zhang, J.; Hou, J.; Lin, D. nZVI-induced iron poisoning aggravated the toxicity of TCEP to earthworm in soil. Environ. Pollut. 2023, 317, 120785. [Google Scholar] [CrossRef]
- Yoon, H.; Pangging, M.; Jang, M.H.; Hwang, Y.S.; Chang, Y.S. Impact of surface modification on the toxicity of zerovalent iron nanoparticles in aquatic and terrestrial organisms. Ecotoxicol. Environ. Saf. 2018, 163, 436–443. [Google Scholar] [CrossRef]
- Chaithawiwat, K.; Vangnai, A.; McEvoy, J.M.; Pruess, B.; Krajangpan, S.; Khan, E. Impact of nanoscale zero valent iron on bacteria is growth phase dependent. Chemosphere 2016, 144, 352–359. [Google Scholar] [CrossRef]
- Le, T.T.; Murugesan, K.; Kim, E.J.; Chang, Y.S. Effects of inorganic nanoparticles on viability and catabolic activities of Agrobacterium sp. PH-08 during biodegradation of dibenzofuran. Biodegradation 2014, 25, 655–668. [Google Scholar] [CrossRef]
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Environ. 2016, 565, 889–901. [Google Scholar] [CrossRef]
- Pan, X.; Lv, N.; Li, C.; Ning, J.; Wang, T.; Wang, R.; Zhou, M.; Zhu, G. Impact of nano zero valent iron on tetracycline degradation and microbial community succession during anaerobic digestion. Chem. Eng. J. 2019, 359, 662–671. [Google Scholar] [CrossRef]
- Xu, C.; Qi, J.; Yang, W.; Chen, Y.; Yang, C.; He, Y.; Wang, J.; Lin, A. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci. Total Environ. 2019, 686, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, M.; Kumar, D.; Roy, R.; Chakraborty, S.; Parashar, A.; Mukherjee, A.; Chandrasekaran, N.; Mukherjee, A. Toxicity, accumulation, and trophic transfer of chemically and biologically synthesized nano zero valent iron in a two species freshwater food chain. Aquat. Toxicol. 2017, 183, 63–75. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, J.; Chen, B.; Song, Y.; Xu, X.; You, S.; Song, F.; Wang, X.; Zou, J. Need a balance? relationship between removal reactivity of cationic dye and bacterial cytotoxicity by iron carbide-stabilized nano zero-valent iron. Chem. Eng. J. 2023, 452, 139150. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Wang, Y.; Fang, G.; Zhu, X.; Zhou, D. Review of chemical and electrokinetic remediation of PCBs contaminated soils and sediments. Environ. Sci. Process Impacts 2016, 18, 1140–1156. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.; Dias-Ferreira, C.; Ottosen, L.M.; Ribeiro, A.B. Electroremediation of PCB contaminated soil combined with iron nanoparticles: Effect of the soil type. Chemosphere 2015, 131, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Feng, L.; Yang, L. Degradation of PCB67 in soil using the heterogenous Fenton process induced by montmorillonite supported nanoscale zero-valent iron. J. Hazard. Mater. 2021, 406, 124305. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Lu, C.; Peng, C.; Zhang, W.; Lin, K.; Zhou, B. Characteristics of legacy and novel brominated flame retardants in water and sediment surrounding two e-waste dismantling regions in Taizhou, eastern China. Sci. Total Environ. 2021, 794, 148744. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wan, J.; Chen, X.; Zhang, W.; Lin, K.; Peng, C. Removal of decabromodiphenyl ethane (DBDPE) by BC/nZVI in the soil: Kinetics, pathways and mechanisms. J. Environ. Chem. Eng. 2022, 10, 107004. [Google Scholar] [CrossRef]
- Zeng, Y.; Walker, H.; Zhu, Q. Reduction of nitrate by NaY zeolite supported Fe, Cu/Fe and Mn/Fe nanoparticles. J. Hazard. Mater. 2017, 324, 605–616. [Google Scholar] [CrossRef]
- Zhang, S.; Kong, Z.; Wang, H.; Yan, Q.; Vayenas, D.V. Enhanced nitrate removal by biochar supported nano zero-valent iron (nZVI) at biocathode in bioelectrochemical system (BES). Chem. Eng. J. 2022, 433, 133535. [Google Scholar] [CrossRef]
- Yan, C.; Yang, Y.; Zhou, J.; Nie, M.; Liu, M.; Hochella, M.F., Jr. Selected emerging organic contaminants in the Yangtze Estuary, China: A comprehensive treatment of their association with aquatic colloids. J. Hazard. Mater. 2015, 283, 14–23. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Z.; Yuan, Z.; Zhang, J.; Guo, X.; Yang, Y.; He, F.; Zhao, Y.; Xu, J. Insight into the kinetics and mechanism of removal of aqueous chlorinated nitroaromatic antibiotic chloramphenicol by nanoscale zero-valent iron. Chem. Eng. J. 2018, 334, 508–518. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Zeng, G.; Wan, J.; Cheng, M.; Zhang, C.; Hu, C. Performance and toxicity assessment of nanoscale zero valent iron particles in the remediation of contaminated soil: A review. Chemosphere 2018, 210, 1145–1156. [Google Scholar] [CrossRef]
- Fajardo, C.; Sacca, M.L.; Costa, G.; Nande, M.; Martin, M. Impact of Ag and Al(2)O(3) nanoparticles on soil organisms: In vitro and soil experiments. Sci. Total Environ. 2014, 473–474, 254–261. [Google Scholar] [CrossRef]
- Fajardo, C.; Gil-Diaz, M.; Costa, G.; Alonso, J.; Guerrero, A.M.; Nande, M.; Lobo, M.C.; Martin, M. Residual impact of aged nZVI on heavy metal-polluted soils. Sci. Total Environ. 2015, 535, 79–84. [Google Scholar] [CrossRef]
- Vanzetto, G.V.; Thome, A. Toxicity of nZVI in the growth of bacteria present in contaminated soil. Chemosphere 2022, 303, 135002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef]
- Binh, N.D.; Imsapsangworn, C.; Kim Oanh, N.T.; Parkpian, P.; Karstensen, K.; Giao, P.H.; DeLaune, R.D. Sequential anaerobic-aerobic biodegradation of 2,3,7,8-TCDD contaminated soil in the presence of CMC-coated nZVI and surfactant. Environ. Technol. 2016, 37, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y. Impact of biochar supported nano zero-valent iron on anaerobic co-digestion of sewage sludge and food waste: Methane production, performance stability and microbial community structure. Bioresour. Technol. 2021, 340, 125715. [Google Scholar] [CrossRef]
- Hsueh, Y.H.; Tsai, P.H.; Lin, K.S.; Ke, W.J.; Chiang, C.L. Antimicrobial effects of zero-valent iron nanoparticles on gram-positive Bacillus strains and gram-negative Escherichia coli strains. J. Nanobiotechnol. 2017, 15, 77. [Google Scholar] [CrossRef]
- Dong, H.; Xie, Y.; Zeng, G.; Tang, L.; Liang, J.; He, Q.; Zhao, F.; Zeng, Y.; Wu, Y. The dual effects of carboxymethyl cellulose on the colloidal stability and toxicity of nanoscale zero-valent iron. Chemosphere 2016, 144, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xiong, Z.; Lai, B. Effect of initial pH on the tetracycline (TC) removal by zero-valent iron: Adsorption, oxidation and reduction. Chem. Eng. J. 2018, 343, 492–499. [Google Scholar] [CrossRef]
- Li, L.; Dong, H.; Lu, Y.; Zhang, H.; Li, Y.; Xiao, J.; Xiao, S. In-depth exploration of toxicity mechanism of nanoscale zero-valent iron and its aging products toward Escherichia coli under aerobic and anaerobic conditions. Environ. Pollut. 2022, 313, 120118. [Google Scholar] [CrossRef]
- Crampon, M.; Joulian, C.; Ollivier, P.; Charron, M. Shift in natural groundwater bacterial community structure due to zero-valent iron nanoparticles (nZVI). Front. Microbiol. 2019, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Mahmoud, R.K.; Gadelhak, Y.; Abo El-Ela, F.I. Gamma irradiated green synthesized zero valent iron nanoparticles as promising antibacterial agents and heavy metal nano-adsorbents. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100461. [Google Scholar] [CrossRef]
- Kim, H.E.; Lee, H.J.; Kim, M.S.; Kim, T.; Lee, H.; Kim, H.H.; Cho, M.; Hong, S.W.; Lee, C. Differential Microbicidal Effects of Bimetallic Iron-Copper Nanoparticles on Escherichia coli and MS2 Coliphage. Environ. Sci. Technol. 2019, 53, 2679–2687. [Google Scholar] [CrossRef]
- Jian, L.; Da, A.; Yue, W.; Juan, L.; Beidou, X.; Minghong, W.; Lian, Z.; Ting, T. Research Advances on Microbial Toxic Effects of Nanoscale Zero-Valent Iron. Asian J. Ecotoxicol. 2017, 129–137. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Sevcu, A.; Bobcikova, K.; Cernik, M.; Joner, E.J. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere 2016, 144, 2221–2228. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Jimenez-Sanchez, C.; Pratarolo, P.; Pullin, H.; Scott, T.B.; Thompson, I.P. Tactic response of bacteria to zero-valent iron nanoparticles. Environ. Pollut. 2016, 213, 438–445. [Google Scholar] [CrossRef]
- Sacca, M.L.; Fajardo, C.; Nande, M.; Martin, M. Effects of nano zero-valent iron on Klebsiella oxytoca and stress response. Microb. Ecol. 2013, 66, 806–812. [Google Scholar] [CrossRef]
- Fajardo, C.; Sacca, M.L.; Martinez-Gomariz, M.; Costa, G.; Nande, M.; Martin, M. Transcriptional and proteomic stress responses of a soil bacterium Bacillus cereus to nanosized zero-valent iron (nZVI) particles. Chemosphere 2013, 93, 1077–1083. [Google Scholar] [CrossRef]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ Pollut 2018, 232, 10–30. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, T.; Jin, Z.; Dong, M.; Xia, H.; Wang, X. Effect of bimetallic and polymer-coated Fe nanoparticles on biological denitrification. Bioresour. Technol. 2010, 101, 9825–9828. [Google Scholar] [CrossRef]
- Zhou, L.; Thanh, T.L.; Gong, J.; Kim, J.H.; Kim, E.J.; Chang, Y.S. Carboxymethyl cellulose coating decreases toxicity and oxidizing capacity of nanoscale zerovalent iron. Chemosphere 2014, 104, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dong, H.; Zeng, G.; Tang, L.; Jiang, Z.; Zhang, C.; Deng, J.; Zhang, L.; Zhang, Y. The interactions between nanoscale zero-valent iron and microbes in the subsurface environment: A review. J. Hazard. Mater. 2017, 321, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Sacca, M.L.; Fajardo, C.; Costa, G.; Lobo, C.; Nande, M.; Martin, M. Integrating classical and molecular approaches to evaluate the impact of nanosized zero-valent iron (nZVI) on soil organisms. Chemosphere 2014, 104, 184–189. [Google Scholar] [CrossRef]
- Gonzalo, S.; Llaneza, V.; Pulido-Reyes, G.; Fernandez-Pinas, F.; Bonzongo, J.C.; Leganes, F.; Rosal, R.; Garcia-Calvo, E.; Rodea-Palomares, I. A colloidal singularity reveals the crucial role of colloidal stability for nanomaterials in-vitro toxicity testing: nZVI-microalgae colloidal system as a case study. PLoS ONE 2014, 9, e109645. [Google Scholar] [CrossRef]
- Pawlett, M.; Ritz, K.; Dorey, R.A.; Rocks, S.; Ramsden, J.; Harris, J.A. The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ. Sci. Pollut. Res. Int. 2013, 20, 1041–1049. [Google Scholar] [CrossRef]
- Ken, D.S.; Sinha, A. Recent developments in surface modification of nano zero-valent iron (nZVI): Remediation, toxicity and environmental impacts. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100344. [Google Scholar] [CrossRef]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Lv, Y.; Niu, Z.; Chen, Y.; Hu, Y. Bacterial effects and interfacial inactivation mechanism of nZVI/Pd on Pseudomonas putida strain. Water Res. 2017, 115, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, H.; Lu, Y.; Hou, K.; Wang, Y.; Ning, Q.; Li, L.; Wang, B.; Zhang, L.; Zeng, G. Toxicity of sulfide-modified nanoscale zero-valent iron to Escherichia coli in aqueous solutions. Chemosphere 2019, 220, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Chaithawiwat, K.; Vangnai, A.; McEvoy, J.M.; Pruess, B.; Krajangpan, S.; Khan, E. Role of oxidative stress in inactivation of Escherichia coli BW25113 by nanoscale zero-valent iron. Sci. Total Environ. 2016, 565, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Semerad, J.; Pacheco, N.I.N.; Grasserova, A.; Prochazkova, P.; Pivokonsky, M.; Pivokonska, L.; Cajthaml, T. In Vitro Study of the Toxicity Mechanisms of Nanoscale Zero-Valent Iron (nZVI) and Released Iron Ions Using Earthworm Cells. Nanomaterials 2020, 10, 2189. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Zhou, J.; You, X.; Niu, B.; Yang, X.; Gong, L.; Zhou, Y.; Wang, J.; Zhang, H. Enhancement of methanogenic activity in anaerobic digestion of high solids sludge by nano zero-valent iron. Sci. Total Environ. 2020, 703, 135532. [Google Scholar] [CrossRef]
- Qiu, X.; Fang, Z.; Yan, X.; Cheng, W.; Lin, K. Chemical stability and toxicity of nanoscale zero-valent iron in the remediation of chromium-contaminated watershed. Chem. Eng. J. 2013, 220, 61–66. [Google Scholar] [CrossRef]
- Gomes, T.; Araujo, O.; Pereira, R.; Almeida, A.C.; Cravo, A.; Bebianno, M.J. Genotoxicity of copper oxide and silver nanoparticles in the mussel Mytilus galloprovincialis. Mar. Environ. Res. 2013, 84, 51–59. [Google Scholar] [CrossRef]
- Demir, E. A review on nanotoxicity and nanogenotoxicity of different shapes of nanomaterials. J. Appl. Toxicol. 2021, 41, 118–147. [Google Scholar] [CrossRef]
- Peipei, L.; Hanjin, L. As (III) removal by nanoscale zero valent iron-graphene/silica nanocomposites. Chin. J. Environ. Eng. 2016, 10, 3607–3615. [Google Scholar] [CrossRef]
- Neramittagapong, A.; Tanangteerpong, D.; Lin, C.; Neramittagapong, S.; Wantala, K.; Pojananukij, N. Improvement of As(III) removal with diatomite overlay nanoscale zero-valent iron (nZVI-D): Adsorption isotherm and adsorption kinetic studies. Water Supply 2017, 17, 212–220. [Google Scholar] [CrossRef]
- Sacca, M.L.; Fajardo, C.; Martinez-Gomariz, M.; Costa, G.; Nande, M. Molecular stress responses to nano-sized zero-valent iron (nZVI) particles in the soil bacterium Pseudomonas stutzeri. PLoS ONE 2014, 9, e89677. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; He, Y.; Wang, F.; Luo, H.; Liang, D.; Wang, J.; Huang, J.; Yu, C.; Jin, L.; Sun, D. Toxicity of Nanoscale Zero-Valent Iron to Soil Microorganisms and Related Defense Mechanisms: A Review. Toxics 2023, 11, 514. https://doi.org/10.3390/toxics11060514
Zeng G, He Y, Wang F, Luo H, Liang D, Wang J, Huang J, Yu C, Jin L, Sun D. Toxicity of Nanoscale Zero-Valent Iron to Soil Microorganisms and Related Defense Mechanisms: A Review. Toxics. 2023; 11(6):514. https://doi.org/10.3390/toxics11060514
Chicago/Turabian StyleZeng, Guoming, Yu He, Fei Wang, Heng Luo, Dong Liang, Jian Wang, Jiansheng Huang, Chunyi Yu, Libo Jin, and Da Sun. 2023. "Toxicity of Nanoscale Zero-Valent Iron to Soil Microorganisms and Related Defense Mechanisms: A Review" Toxics 11, no. 6: 514. https://doi.org/10.3390/toxics11060514
APA StyleZeng, G., He, Y., Wang, F., Luo, H., Liang, D., Wang, J., Huang, J., Yu, C., Jin, L., & Sun, D. (2023). Toxicity of Nanoscale Zero-Valent Iron to Soil Microorganisms and Related Defense Mechanisms: A Review. Toxics, 11(6), 514. https://doi.org/10.3390/toxics11060514








