Punica granatum (Pomegranate) Peel Extract Pre-Treatment Alleviates Fenpropathrin-Induced Testicular Injury via Suppression of Oxidative Stress and Inflammation in Adult Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Punica granatum Peel Extract Preparation
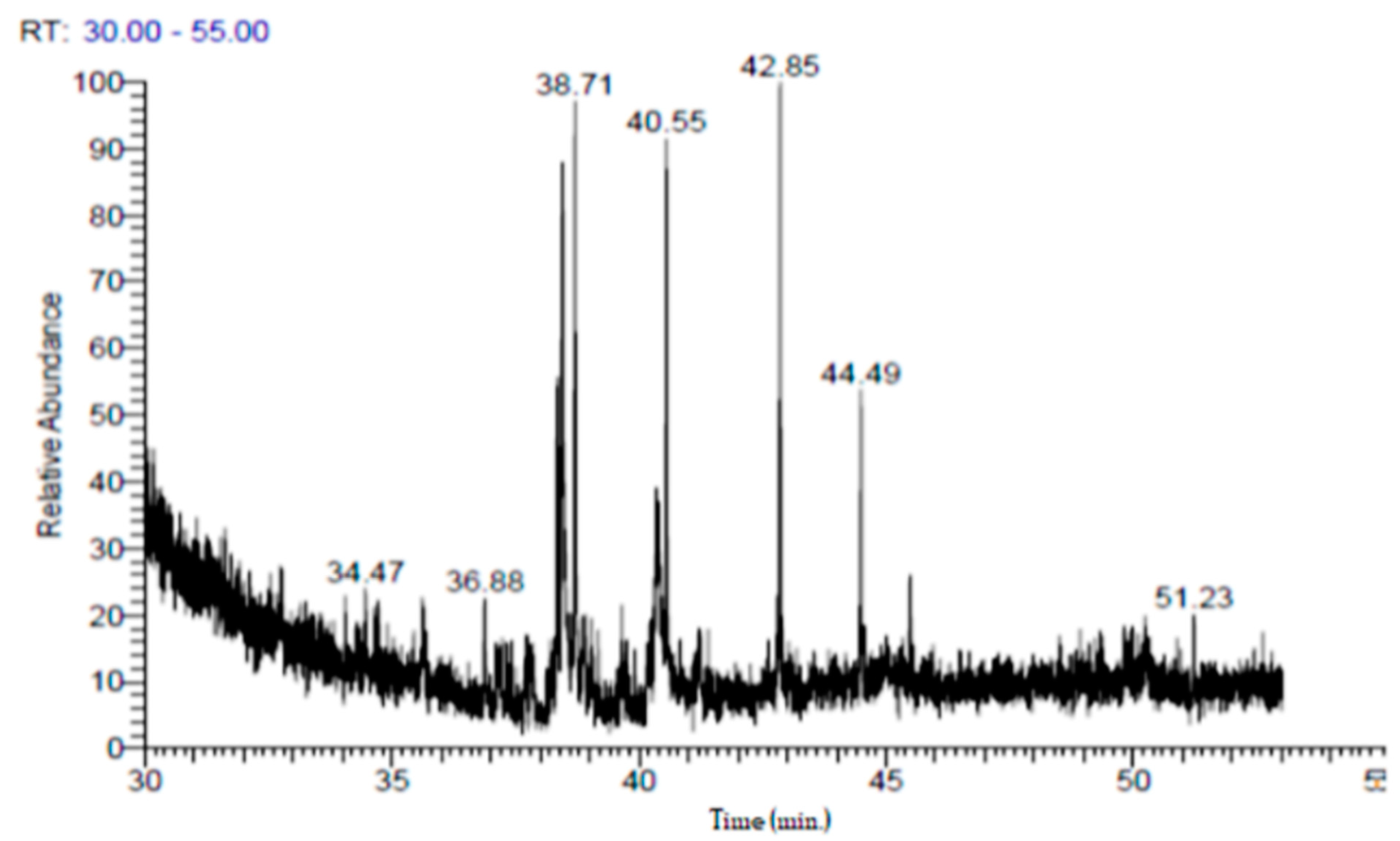
2.3. Gas Chromatography/Mass Spectrometry(GC/MS) Analysis of Punica granatum Peel Extract
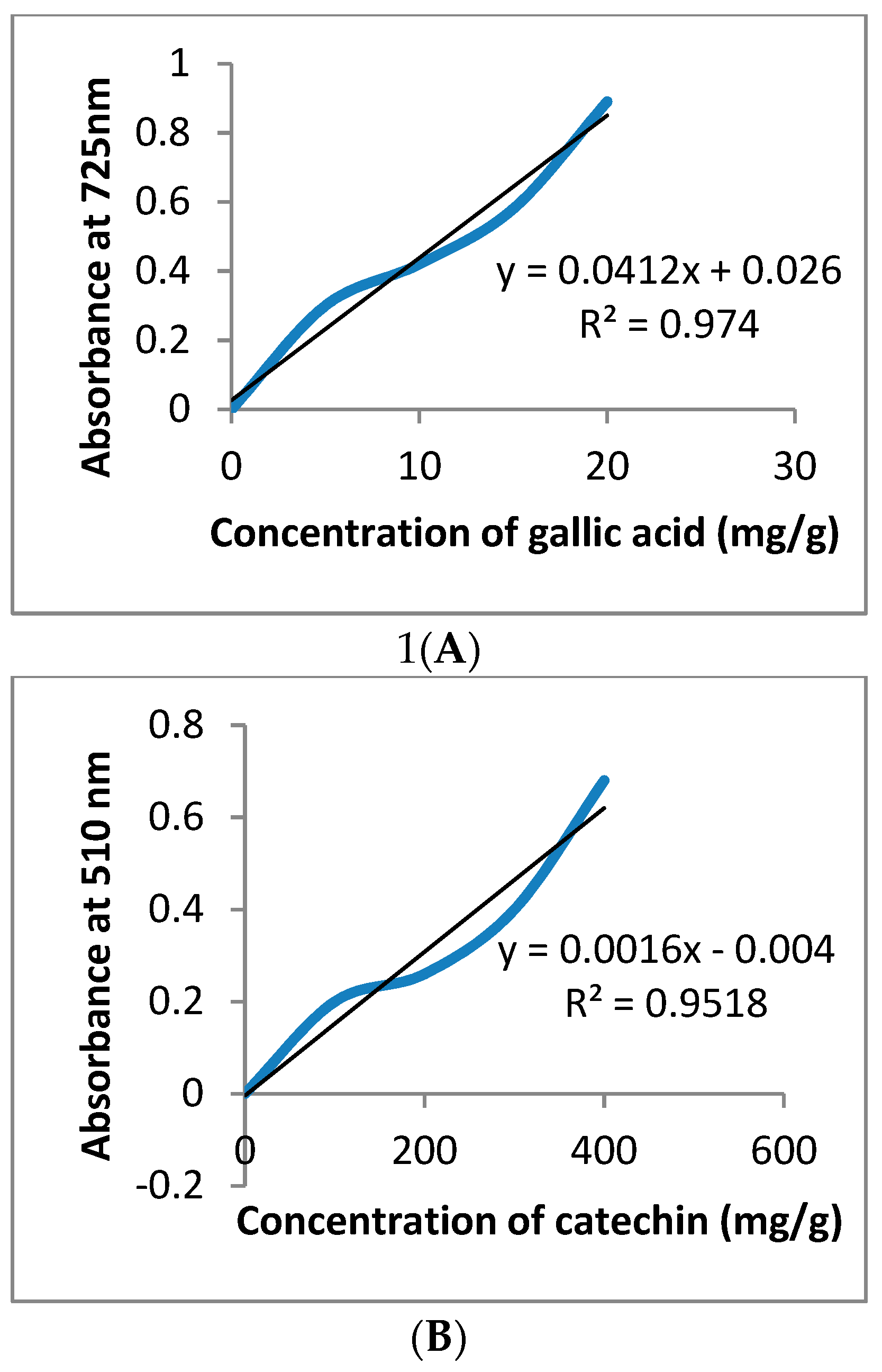
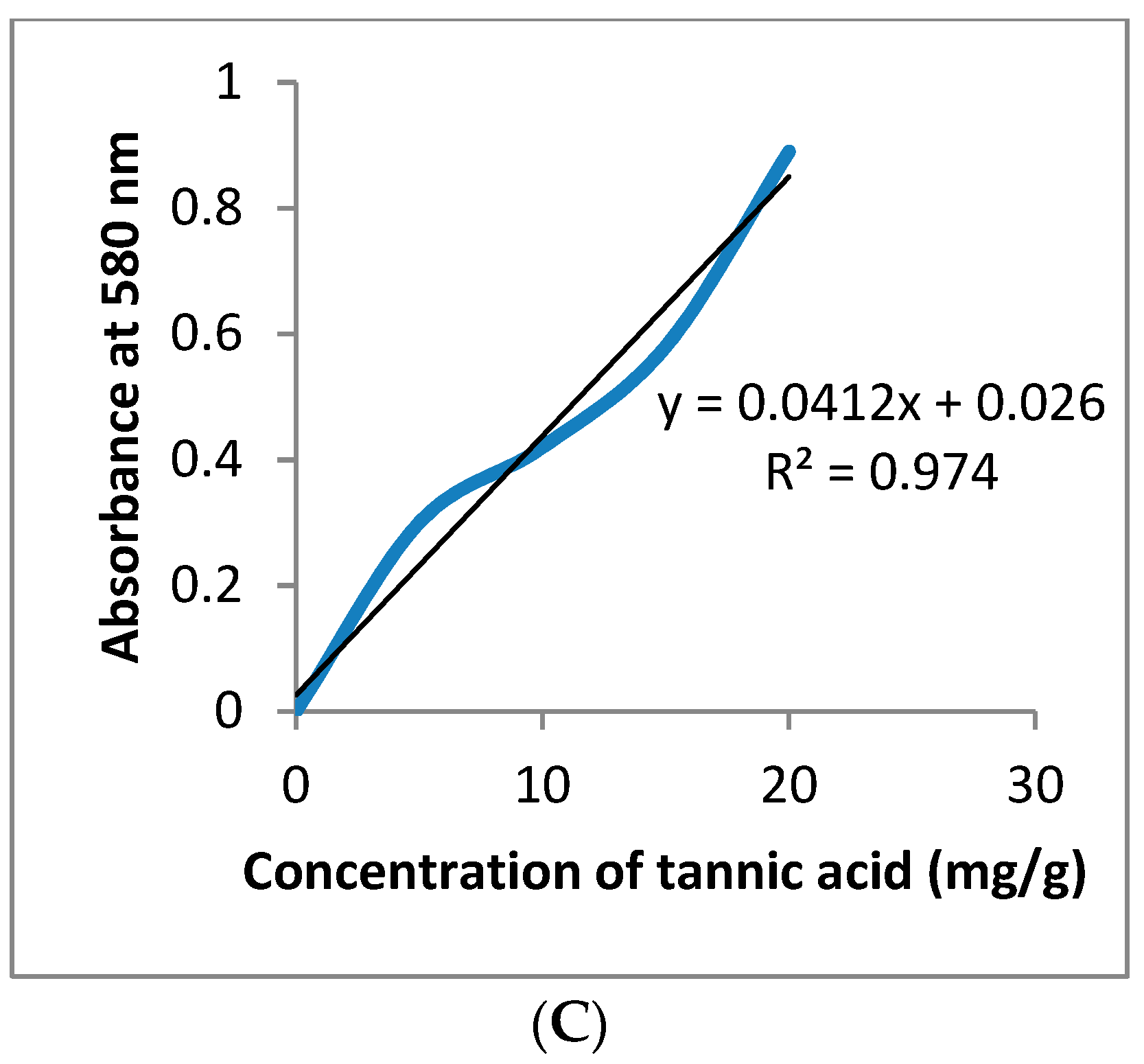
2.4. In Vitro Measurement of Total Phenolic, Flavonoids, and Tannins Content
2.5. Experimental Design
2.6. Blood Collection and Serum Sample Preparation
2.7. Evaluation of Sperm Quality
2.8. Hormone Analyses
2.9. Testis Homogenate Preparation
2.10. Determination of Oxidative Stress, Non-Enzymatic and Enzymatic Antioxidants
2.11. Determination of Testicular Enzymes, Aminotransferases, Phosphatase Activity, and Protein Content
2.12. Quantitative Real-Time PCR
2.13. Measurement of Inflammatory and Anti-Inflammatory Cytokines
2.14. Histological Investigation
2.15. Statistical Analysis
3. Results
3.1. GC-MS and Phytochemical Analysis
3.2. Body and Organ Weights
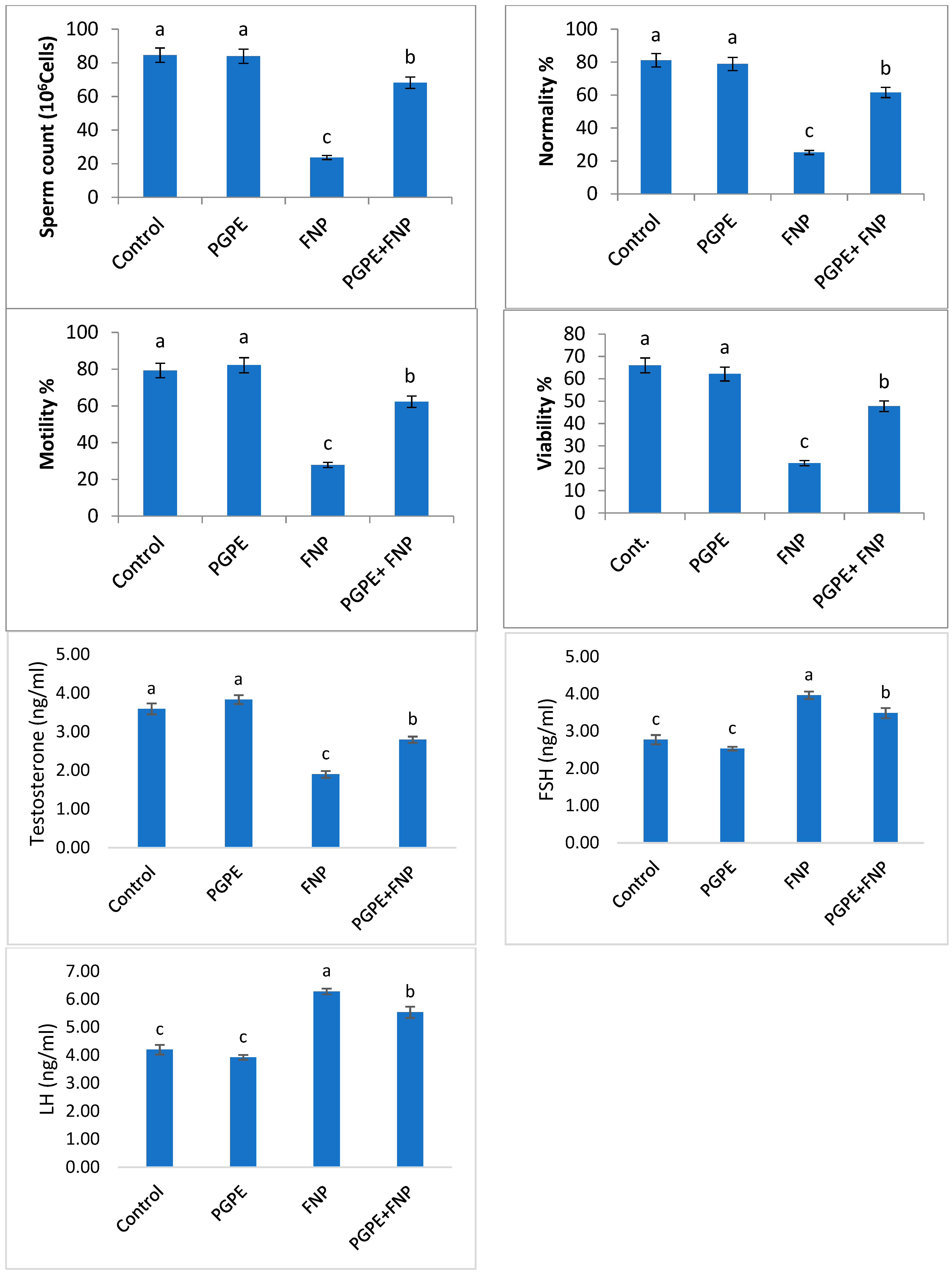
3.3. Sperm Parameters and Hormone Levels
3.4. Oxidant Stress and Antioxidant Biomarkers
3.5. Aminotransferases, Phosphatases, 3 β, and 17 β Hydroxysteroid Dehydrogenase Activities and Protein Content
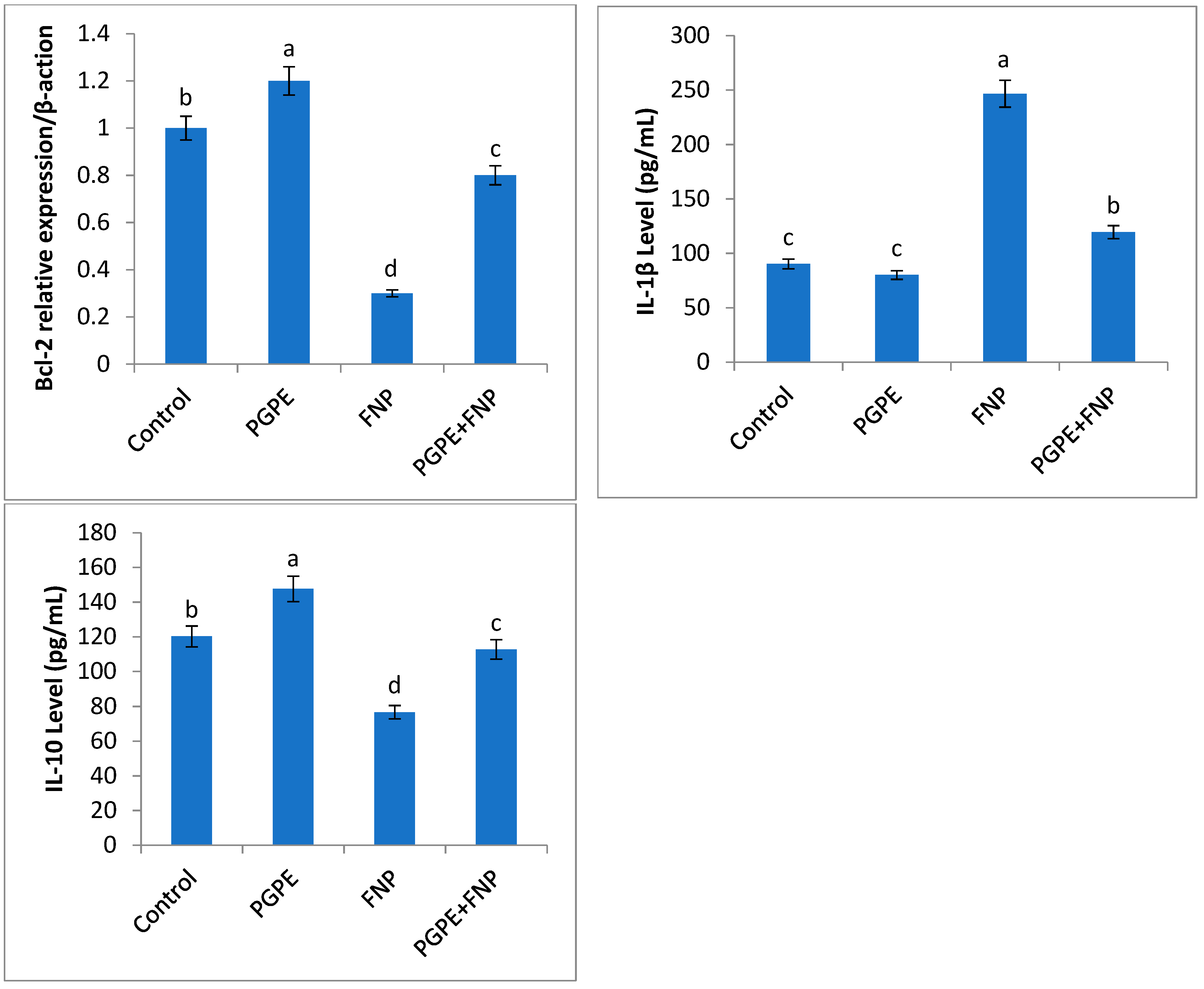
3.6. Gene Expression, Inflammatory and Anti-Inflammatory Cytokines
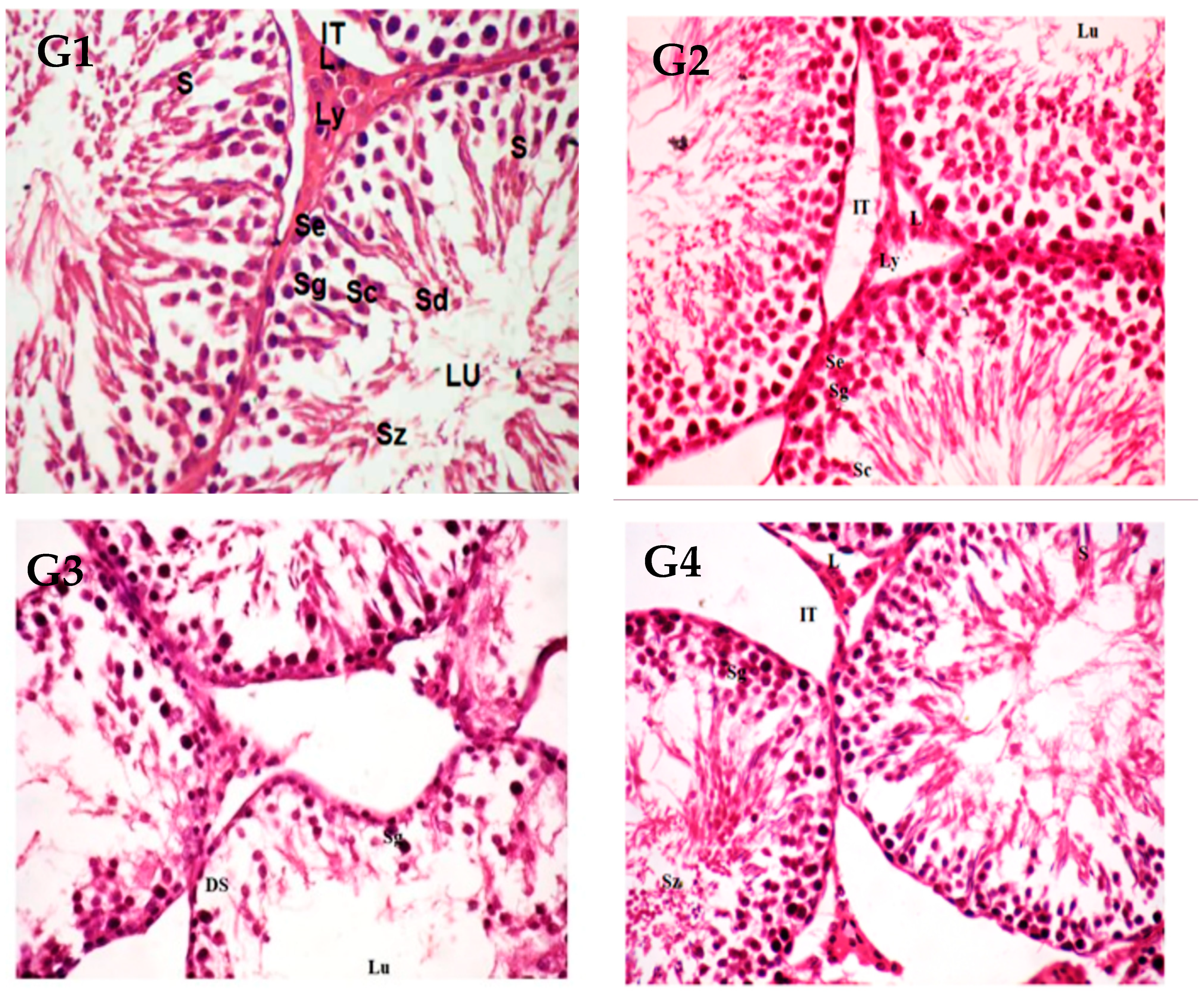
3.7. Histological Observation of Testis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, T.M.; Stratton, G.W. Effects of synthetic pyrethroid insecticides on nontarget organisms. Res. Rev. 1986, 97, 93–120. [Google Scholar] [CrossRef]
- Grov, T.; Beer, P.; Joubert, M. Developing a systems approach for Thaumatotibia leucotreta (Lepidoptera: Tortricidae) on ‘Hass’ avocado in South. Afr. J. Econ. Entomol. 2010, 103, 1112–1128. [Google Scholar] [CrossRef] [PubMed]
- Pazini, J.; Padilha, A.; Cagliari, D.; Bueno, F.; Rakes, M.; Zotti, M.; Martins, J.; Grützmacher, A. Differential impacts of pesticides on Euschistus heros (Hem.: Pentatomidae) and its parasitoid Telenomus podisi (Hym.: Platygastridae). Sci. Rep. 2019, 9, 6544. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L.; Nemec, M.; Sheets, L.; Sargent, D.; Breckenridge, C. Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. Neurotoxicology 2009, 30 (Suppl. 1), S1–S16. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, Z.; Wan, Z.; Qu, S. Role of autophagy and oxidative stress to astrocytes in fenpropathrin-induced Parkinson-like damage. Neurochem. Int. 2021, 145, 105000. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D. Contaminant-stimulated Reactive Oxygen Species Production and Oxidative Damage in Aquatic Organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- El-Demerdash, F.M. Lambda-cyhalothrin-induced changes in oxidative stress biomarkers in rabbit erythrocytes and alleviation effect of some antioxidants. Toxicol. Vitr. 2007, 21, 392–397. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; Abdellatief, S.A.; Khater, S.I.; Ali, H.; Al-Gabri, N.A. Fenpropathrin induces testicular damage, apoptosis, and genomic DNA damage in adult rats: Protective role of camel milk. Ecotoxicol. Environ. Saf. 2019, 181, 548–558. [Google Scholar] [CrossRef]
- Weselak, M.; Arbuckle, T.E.; Wigle, D.T.; Walker, M.C.; Krewski, D. Pre- and post-conception pesticide exposure and the risk of birth defects in an Ontario farm population. Reprod. Toxicol. 2008, 25, 472–480. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B.; Borzęcki, A. Effect of 28-day exposure to fenpropathrin on the activities of serum alanine transaminase and liver antioxidant enzymes in mice. Bull. Vet. Inst. Pulawy 2015, 59, 165–169. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef] [PubMed]
- Türk, G.; Sönmez, M.; Aydin, M.; Yüce, A.; Gür, S.; Yüksel, M.; Aksu, E.H.; Aksoy, H. Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin. Nutr. 2008, 27, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Fawole, A.O.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Gouveia, D.N.; Guimarães, A.G.; da Rocha Santos, W.B.; Quintans-Júnior, L.J. Antidiabetic Effects of Punica Granatum L (Pomegranate): A Review. In Bioactive Food as Dietary Interventions for Diabetes; Watson, R.R., Preedy, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 355–369. [Google Scholar]
- Gouveia, D.; Guimarães, A.; Santos, W.; Quintans-Júnior, L. Natural products as a perspective for cancer pain management: A systematic review. Phytomedicine 2019, 58, 152766. [Google Scholar] [CrossRef]
- Kaur, G.; Jabbar, Z.; Athar, M.; Alam, M.S. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. 2006, 44, 984–993. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef] [PubMed]
- Leiva, K.P.; Rubio, J.; Peralta, F.; Gonzales, G. Effect of Punica granatum (pomegranate) on sperm production in male rats treated with lead acetate. Toxicol. Mech. Methods 2011, 21, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Türk, G.; Çeribaşı, S.; Sönmez, M.; Çiftçi, M.; Yüce, A.; Güvenç, M.; Kaya, S.Ö.; Çay, M.; Aksakal, M. Ameliorating effect of pomegranate juice consumption on carbon tetrachloride-induced sperm damages, lipid peroxidation, and testicular apoptosis. Toxicol. Ind. Health 2013, 32, 126–137. [Google Scholar] [CrossRef]
- Shaban, N.Z.; El-Kersh, M.A.; El-Rashidy, F.H.; Habashy, N.H. Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem. 2013, 141, 1587–1596. [Google Scholar] [CrossRef]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate Juice, Total Pomegranate Ellagitannins, and Punicalagin Suppress Inflammatory Cell Signaling in Colon Cancer Cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef]
- Parsaei, P.; Karimi, M.; Asadi, S.Y.; Rafieian-Kopaei, M. Bioactive components and preventive effect of green tea (Camellia sinensis) extract on post-laparotomy intra-abdominal adhesion in rats. Int. J. Surg. 2013, 11, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martinson, T.; Liu, R.H. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009, 116, 332–339. [Google Scholar] [CrossRef]
- Willis, R.; Allen, P. Improved method for measuring hydrolyzable tannins using potassium iodate. Analyst 1998, 123, 435–439. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Zaki, N.Z. Assessment the ameliorative effect of pomegranate and rutin on chlorpyrifos-ethyl-induced oxidative stress in rats. Nat. Sci. 2009, 7, 49–61. [Google Scholar]
- Xiong, J.; Zhang, X.; Huang, J.; Chen, C.; Chen, Z.; Liu, L.; Zhang, G.; Yang, J.; Zhang, Z.; Zhang, Z.; et al. Fenpropathrin, a Widely Used Pesticide, Causes Dopaminergic Degeneration. Mol. Neurobiol. 2016, 53, 995–1008. [Google Scholar] [CrossRef]
- Adamkovicova, M.; Toman, R.; Martiniakova, M.; Omelka, R.; Babosova, R.; Krajcovicova, V.; Grosskopf, B.; Massanyi, P. Sperm motility and morphology changes in rats exposed to cadmium and diazinon. Reprod. Biol. Endocrinol. 2016, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology, 3rd ed.; Packer, L., Ed.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1984; pp. 121–126. [Google Scholar]
- Hafeman, D.G.; Sunde, R.A.; Hoekstra, W.G. Effect of Dietary Selenium on Erythrocyte and Liver Glutathione Peroxidase in the Rat. J. Nutr. 1974, 104, 580–587. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Talalay, P. Hydroxysteroid Dehydrogenase; Methods in enzymology, Volume V; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press Inc.: New York, NY, USA, 1962; pp. 512–516. [Google Scholar]
- De Heuvel, E.; Wallace, L.; Sharkey, K.A.; Sigalet, D.L. Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phophatidylinositol 3-kinase-γ signaling. Am. J. Physiol. Metab. 2012, 303, E994–E1005. [Google Scholar] [CrossRef]
- Bancroft, J.; Stevens, A. Theory and Practice of Histological Techniques, 3rd ed.; Churchill Livingstone: Edinburgh, UK, 1990. [Google Scholar]
- Manfo, F.P.T.; Nantia, E.A.; Mathur, P.P. Effect of Environmental Contaminants on Mammalian Testis. Curr. Mol. Pharmacol. 2014, 7, 119–135. [Google Scholar] [CrossRef]
- Mathur, P.P.; D’Cruz, S. The effect of environmental contaminants on testicular function. Asian J. Androl. 2011, 13, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Uzunhisarcikli, M.; Kalender, Y.; Dirican, K.; Kalender, S.; Ogutcu, A.; Buyukkomurcu, F. Acute, subacute and subchronic administration of methyl parathion-induced testicular damage in male rats and protective role of vitamins C and E. Pestic. Biochem. Physiol. 2006, 87, 115–122. [Google Scholar] [CrossRef]
- Meeker, J.D.; Godfrey-Bailey, L.; Hauser, R. Relationships Between Serum Hormone Levels and Semen Quality Among Men From an Infertility Clinic. J. Androl. 2006, 28, 397–406. [Google Scholar] [CrossRef] [PubMed]
- DiBiasio, K.W.; Silva, M.H.; Shull, L.R.; Overstreet, J.W.; Hammock, B.D.; Miller, M.G. Xenobiotic metabolizing enzyme activities in rat, mouse, monkey, and human testes. Drug Metab. Dispos. 1991, 19, 227–232. [Google Scholar] [PubMed]
- Abdou, R.; Sasaki, K.; Khalil, W.; Shah, S.; Murasawa, Y.; Shimoda, M. Effects of several pyrethroids on hepatic cytochrome P450 activities in rats. J. Vet. Med. Sci. 2010, 72, 425. [Google Scholar] [CrossRef] [PubMed]
- Giray, B.; Gürbay, A.; Hincal, F. Cypermethrin-induced oxidative stress in rat brain and liver is prevented by Vitamin E or allopurinol. Toxicol. Lett. 2001, 118, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gradowska-Olszewska, I.; Brzezinski, J.; Rusiecki, W. Excretion and peripheral metabolism of 1,2-3H- Testosterone and androgens in rats following intoxication with organophosphorous insecticides. J. Appl. Toxicol. 1984, 4, 261–264. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Jebur, A.B.; Nasr, H.M.; Hamid, H.M. Modulatory effect of Turnera diffusa against testicular toxicity induced by fenitrothion and/or hexavalent chromium in rats. Environ. Toxicol. 2019, 34, 330–339. [Google Scholar] [CrossRef]
- Gupta, R.S.; Kim, J.; Gomes, C.; Oh, S.; Park, J.; Im, W.-B.; Seong, J.Y.; Ahn, R.S.; Kwon, H.-B.; Soh, J. Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol. Cell. Endocrinol. 2004, 221, 57–66. [Google Scholar] [CrossRef]
- Jebur, A.; El-Sayed, R.; El-Demerdash, F. Ocimum basilicum Essential Oil Modulates Hematotoxicity, Oxidative Stress, DNA Damage, and Cell Cycle Arrest Induced by β-cyfluthrin in Rat Liver. Front. Pharmacol. 2022, 12, 784281. [Google Scholar] [CrossRef]
- Aitken, R.J.; Roman, S.D. Antioxidant Systems and Oxidative Stress in the Testes. Oxidative Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Cantalamessa, F.; Falcioni, G.; Gabbianelli, R. Different effects of Type I and Type II pyrethroids on erythrocyte plasma membrane properties and enzymatic activity in rats. Toxicology 2003, 191, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.E. Anderson. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Halliwell, J.M.C. Gutteridge. In Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Farag, A.G.; Elhalwagy, M.E.; Farid, H.E. Effect of ginger supplementation on developmental toxicity induced by fenitrothion insecticide and/or lead in albino rats. Pestic. Biochem. Physiol. 2010, 97, 267–274. [Google Scholar] [CrossRef]
- Elhalwagy, M.E.; Darwish, N.S.; Zaher, E.M. Prophylactic effect of green tea polyphenols against liver and kidney injury induced by fenitrothion insecticide. Pestic. Biochem. Physiol. 2008, 91, 81–89. [Google Scholar] [CrossRef]
- Rahman, M.F.; Siddiqui, M.K.J.; Jamil, K. Acid and alkaline phosphatase activities in a novel phosphorothionate (rpr-11) treated male and female rats. evidence of dose and time-dependent response. Drug Chem. Toxicol. 2000, 23, 497–509. [Google Scholar] [CrossRef]
- Sugár, J.; Tóth, K.; Csuka, O.; Gáti, É.; Somfai-Relle, S. Role of pesticides in hepatocarcinogenesis. J. Toxicol. Environ. Health 1979, 5, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.-C.; Wang, J.-J. Curcumin Attenuates Liver Warm Ischemia and Reperfusion–Induced Combined Restrictive and Obstructive Lung Disease by Reducing Matrix Metalloprotease 9 Activity. Transplant. Proc. 2014, 46, 1135–1138. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Wang, C.; Fu, Z.; Liu, W.; Gan, J. Induction of Macrophage Apoptosis by an Organochlorine Insecticide Acetofenate. Chem. Res. Toxicol. 2009, 22, 504–510. [Google Scholar] [CrossRef]
- Wang, T.; Chen, F.; Chen, Z.; Wu, Y.-F.; Xu, X.-L.; Zheng, S.; Hu, X. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J. Gastroenterol. 2004, 10, 2205–2208. [Google Scholar] [CrossRef]
- Abu-Qare, A.W.; Abou-Donia, M.B. Biomarkers of apoptosis: Release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2′-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J. Toxicol. Environ. Health Part B Crit. Rev. 2001, 4, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Osama, E.; Galal, A.A.A.; Abdalla, H.; El-Sheikh, S.M.A. Chlorella vulgaris ameliorates testicular toxicity induced by deltamethrin in male rats via modulating oxidative stress. Andrologia 2019, 51, e13214. [Google Scholar] [CrossRef] [PubMed]
- Maphetu, N.; Unuofin, J.; Masuku, N.; Olisah, C.; Lebelo, S. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef]
- Manna, P.; Sinha, M.; Sil, P.C. Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Complement. Altern. Med. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, R.; Ahamed, K.N.; Kumar, V.; Mukherjee, K.; Bandyopadhyay, A.; Mukherjee, P.K. Antioxidant effect of Cytisus scoparius against carbon tetrachloride treated liver injury in rats. J. Ethnopharmacol. 2007, 109, 41–47. [Google Scholar] [CrossRef]
- Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Huerta-Gertrudis, B.; García-Cruz, M.E.; Ramírez-Morales, A.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Camacho-Carranza, R.; Espinosa-Aguirre, J.J. Hepatoprotective effect of acetonic and methanolic extracts of Heterotheca inuloides against CCl4-induced toxicity in rats. Exp. Toxicol. Pathol. 2011, 63, 363–370. [Google Scholar] [CrossRef]
- Moneim, A.E.A. Antioxidant activities of Punica granatum (pomegranate) peel extract on brain of rats. J. Med. Plants Res. 2011, 6, 195–199. [Google Scholar] [CrossRef]
- Qnais, E.Y.; Elokda, A.S.; Abu Ghalyun, Y.Y.; Abdulla, F.A. Antidiarrheal Activity of the Aqueous Extract of Punica granatum. (Pomegranate) Peels. Pharm. Biol. 2007, 45, 715–720. [Google Scholar] [CrossRef]
- Singh, R.P.; Murthy, K.N.C.; Jayaprakasha, G.K. Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using in Vitro Models. J. Agric. Food Chem. 2001, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant Activities of Pomegranate Fruit Extract and Its Anthocyanidins: Delphinidin, Cyanidin, and Pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica granatum L. Planta Medica 2007, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ashoush, I.; El-Batawy, O.; El-Shourbagy, G.A. Antioxidant activity and hepatoprotective effect of pomegranate peel and whey powders in rats. Ann. Agric. Sci. 2013, 58, 27–32. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Mayasankaravalli, C.; Deepika, K.; Lydia, D.E.; Agada, R.; Thagriki, D.; Govindasamy, C.; Chinnadurai, V.; Gatar, O.M.O.; Khusro, A.; Kim, Y.O.; et al. Profiling the phyto-constituents of Punica granatum fruits peel extract and accessing its in-vitro antioxidant, anti-diabetic, anti-obesity, and angiotensin-converting enzyme inhibitory properties. Saudi J. Biol. Sci. 2020, 27, 3228–3234. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsi’ pomegranate peel extracts. Ind. Crops Prod. 2018, 111, 345–352. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 1–29. [Google Scholar] [CrossRef]
- Hoshmand, M.; Jafari, B.; Dehghan, M.; Vahdati, A.; Zargar, H.; Mahmoudi, R. Protective effects of lycopene and Ellagic acid on gonadal tissue, Matern Newborn Rats Induced by Cadmiumchloride. Armaghane Danesh 2015, 20, 369–380. [Google Scholar]
- Utomo, B.; Daningtia, N.; Yuliani, G.; Yuniarti, W. Efects of a standardized 40% ellagic acid pomegranate (Punica granatum L.) extract on seminiferous tubule histopathology, diameter, and epithelium thickness in albino Wistar rats after heat exposure. Vet. World 2019, 12, 1261. [Google Scholar] [CrossRef]
- Karimi, M.; Sadeghi, R.; Kokini, J. Pomegranate as a promising opportunity in medicine and nanotechnology. Trends Food Sci. Technol. 2017, 69, 59–73. [Google Scholar] [CrossRef]
- Fouad, A.A.; Qutub, H.O.; Al-Melhim, W.N. Nephroprotection of punicalagin in rat model of endotoxemic acute kidney injury. Toxicol. Mech. Methods 2016, 26, 538–543. [Google Scholar] [CrossRef]
- Sayed, S.; Alotaibi, S.S.; El-Shehawi, A.M.; Hassan, M.M.; Shukry, M.; Alkafafy, M.; Soliman, M.M. The Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Effects of a Pomegranate-Peel Extract against Acrylamide-Induced Hepatotoxicity in Rats. Life 2022, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Volkova, N.; Borochov-Neori, H.; Judeinstein, S.; Aviram, M. Anti-atherogenic properties of date vs. pomegranate polyphenols: The benefits of the combination. Food Funct. 2015, 6, 1496–1509. [Google Scholar] [CrossRef]
- Gouda, M.; Moustafa, A.; Hussein, L.; Hamza, M. Three week dietary intervention using apricots, pomegranate juice or/and fermented sour sobya and impact on biomarkers of antioxidative activity, oxidative stress and erythrocytic glutathione transferase activity among adults. Nutr. J. 2016, 15, 52. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| P53 | AACTGGAAGAATTCGCG GCCGCAGGAAT | GCTACCCGAAGACCA AGAAGG |
| Caspase3 | GGTATTGAGACAGAC AGTGG | CATGGGATCTGTTTC TTTGC |
| Bcl-2 | ATCGCTCTGTG GATGACTGAGTAC | AGAGACAGCCAGGAGA AATCAAAC |
| β-actin | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC |
| Parameter | PGPE |
|---|---|
| Total phenolic contents | 211.2 ± 5.61 (mg GAE/g DW) |
| Total flavonoid | 59.84 ± 6.41 (mg/g CAE) |
| Total tannins | 106 ± 11.35 (mg/g TAE) |
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | PGPE | FNP | PGPE + FNP | |
| Initial body weight (g) | 164.71 ± 0.86 a | 166.68 ± 0.60 a | 167.00 ± 2.31 a | 163.58 ± 1.89 a |
| Final body weight (g) | 190.33 ± 0.33 a | 188.33 ± 0.33 a | 144.20 ± 1.53 c | 174.89 ± 1.62 b |
| Body weight gain (g) | 25.62 ± 0.49 a | 21.65 ± 0.31 a | −22.8 ± 0.87 c | 11.3 ± 0.31 b |
| Testes weight (g) | 1.63 ± 0.05 a | 1.70 ± 0.06 a | 0.82 ± 0.07 c | 1.17 ± 0.08 b |
| Gonadosomatic index | 0.85 ± 0.03 a | 0.90 ± 0.06 a | 0.56 ± 0.05 c | 0.66 ± 0.051 b |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control | PGPE | FNP | PGPE + FNP | |
| TBARS (nmol/g tissue) | 18.20 ± 0.725 c | 14.18 ± 0.591 d | 26.08 ± 0.616 a | 22.63 ± 0.434 b |
| H2O2 (μmol/g tissue) | 40.99 ± 0.663 c | 32.34 ± 0.707 d | 59.11 ± 1.639 a | 50.62 ± 1.982 b |
| PCC (nmol carbonyl/mg protein) | 0.41 ± 0.02 c | 0.42 ± 0.01 c | 1.36 ± 0.07 a | 0.68 ± 0.04 b |
| SOD (U/mg protein) | 69.07 ± 2.71 b | 84.50 ± 2.11 a | 35.49 ± 1.23 d | 48.14 ± 1.56 c |
| CAT (U/mg protein) | 7.13 ± 0.21 b | 8.47 ± 0.23 a | 3.74 ± 0.108 d | 5.17 ± 0.17 c |
| GPx (U/mg protein) | 7.59 ± 0.172 b | 8.94 ± 0.297 a | 4.226 ± 0.181 d | 5.774 ± 0.177 c |
| GR (U/mg protein) | 20.33 ± 0.492 b | 24.38 ± 0.591 a | 11.37 ± 0.338 d | 15.14 ± 0.543 c |
| GST (µmol/hr/mg protein) | 0.611 ± 0.024 b | 0.741 ± 0.022 a | 0.310 ± 0.013 d | 0.452 ± 0.019 c |
| GSH (mmol/mg protein) | 2.44 ± 0.067 b | 2.92 ± 0.084 a | 1.19 ± 0.054 d | 1.76 ± 0.067 c |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| Control | PGPE | FNP | PGPE + FNP | |
| Homogenate | ||||
| 3β-HSD (μmol/min/mg protein) | 0.28 ± 0.02 a | 0.27 ± 0.01 a | 0.14 ± 0.01 c | 0.20 ± 0.02 b |
| 17β-HSD (μmol/min/mg protein) | 0.22 ± 0.005 a | 0.23 ± 0.008 a | 0.11 ± 0.005 c | 0.18 ± 0.02 b |
| Protein content (mg/g tissue) | 73.19 ± 2.10 a | 79.90 ± 2.27 a | 42.52 ± 1.98 c | 53.68 ± 2.35 b |
| Serum | ||||
| AST (U/l) | 53.65 ± 1.58 c | 50.57 ± 1.68 c | 74.01 ± 2.47 a | 63.75 ± 1.85 b |
| ALT (U/l) | 56.77 ± 1.69 c | 53.90 ± 2.04 c | 78.80 ± 2.82 a | 69.13 ± 2.37 b |
| ALP (U/l) | 63.93 ± 2.21 c | 59.27 ± 2.00 c | 88.90 ± 3.19 a | 76.70 ± 2.29 b |
| ACP (U/l) | 10.42 ± 0.430 c | 9.45 ± 0.297 c | 14.14 ± 0.466 a | 12.89 ± 0.392 b |
| Parameters | Control | PGPE | FNP | PGPE + FNP |
|---|---|---|---|---|
| Disorganized seminiferous tubules | + | ++ | ++++ | ++ |
Characterize spermatogenic cells
| +++ + + | +++ ++ + | + +++ +++ | +++ ++ ++ |
| Necrotic spermatocytes | + | + | +++ | ++ |
| Dilated lumen | − | + | +++ | ++ |
| Dilated interstitial tissue | + | ++ | ++++ | +++ |
| Infiltrating lymphocytes | - | + | +++ | ++ |
| Spermatid | ++ | ++ | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebur, A.B.; El-Sayed, R.A.; Abdel-Daim, M.M.; El-Demerdash, F.M. Punica granatum (Pomegranate) Peel Extract Pre-Treatment Alleviates Fenpropathrin-Induced Testicular Injury via Suppression of Oxidative Stress and Inflammation in Adult Male Rats. Toxics 2023, 11, 504. https://doi.org/10.3390/toxics11060504
Jebur AB, El-Sayed RA, Abdel-Daim MM, El-Demerdash FM. Punica granatum (Pomegranate) Peel Extract Pre-Treatment Alleviates Fenpropathrin-Induced Testicular Injury via Suppression of Oxidative Stress and Inflammation in Adult Male Rats. Toxics. 2023; 11(6):504. https://doi.org/10.3390/toxics11060504
Chicago/Turabian StyleJebur, Ali B., Raghda A. El-Sayed, Mohamed M. Abdel-Daim, and Fatma M. El-Demerdash. 2023. "Punica granatum (Pomegranate) Peel Extract Pre-Treatment Alleviates Fenpropathrin-Induced Testicular Injury via Suppression of Oxidative Stress and Inflammation in Adult Male Rats" Toxics 11, no. 6: 504. https://doi.org/10.3390/toxics11060504
APA StyleJebur, A. B., El-Sayed, R. A., Abdel-Daim, M. M., & El-Demerdash, F. M. (2023). Punica granatum (Pomegranate) Peel Extract Pre-Treatment Alleviates Fenpropathrin-Induced Testicular Injury via Suppression of Oxidative Stress and Inflammation in Adult Male Rats. Toxics, 11(6), 504. https://doi.org/10.3390/toxics11060504








