Physicochemical Characterization and Oxidative Potential of Iron-Containing Particles Emitted from Xuanwei Coal Combustion
Abstract
1. Introduction
2. Materials and Methods
2.1. Coal Burning Experiment and Coal Combustion Particle Sampling
2.2. Chemical Element Analysis of the Coal Combustion Particles
2.3. Detection of Environmentally Persistent Free Radicals (EPFRs) and OH Radicals in the Coal Combustion Particles
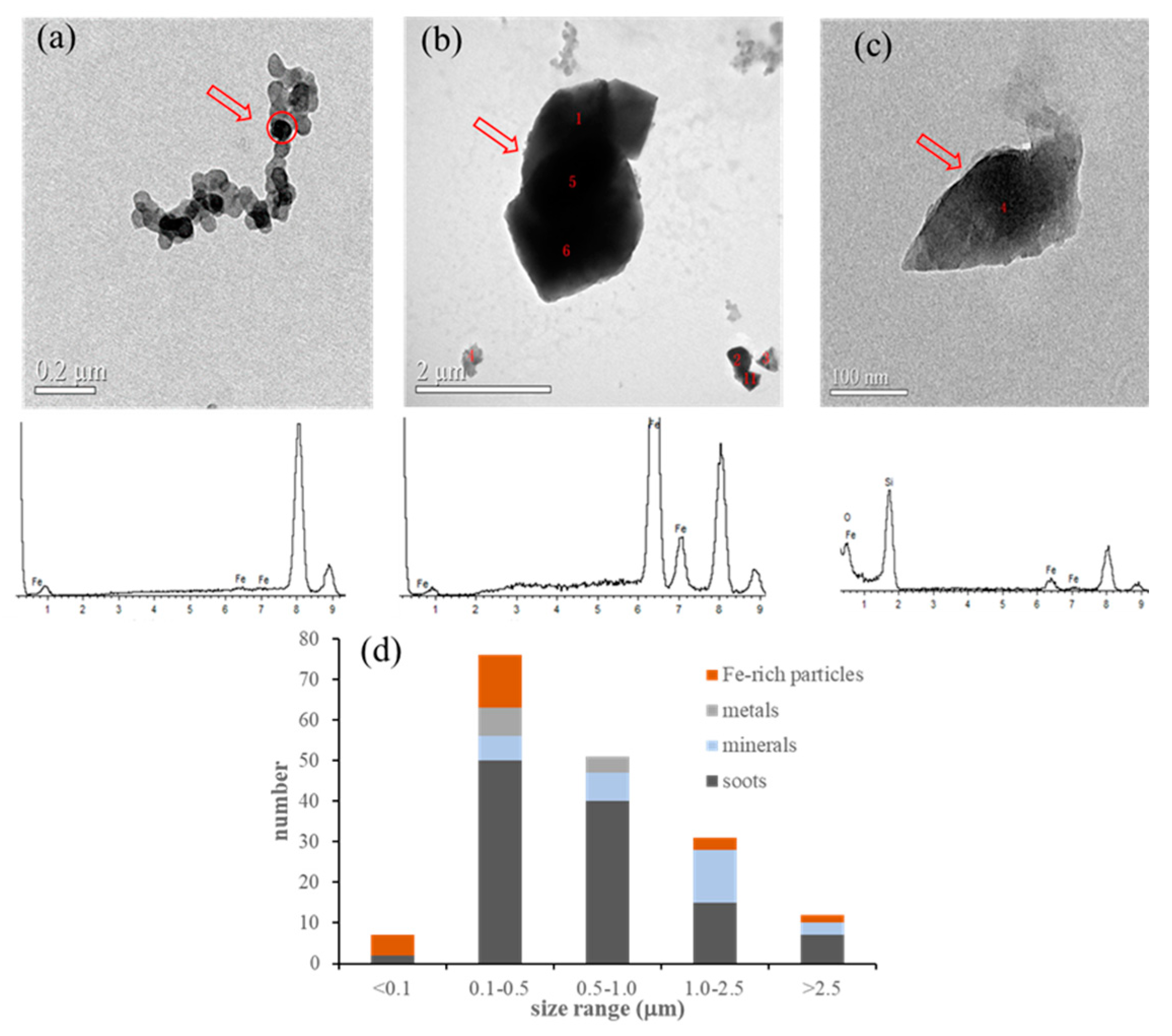
2.4. Identification of Iron-Containing Particles
2.5. Iron Dissolution from the Coal Combustion Particles
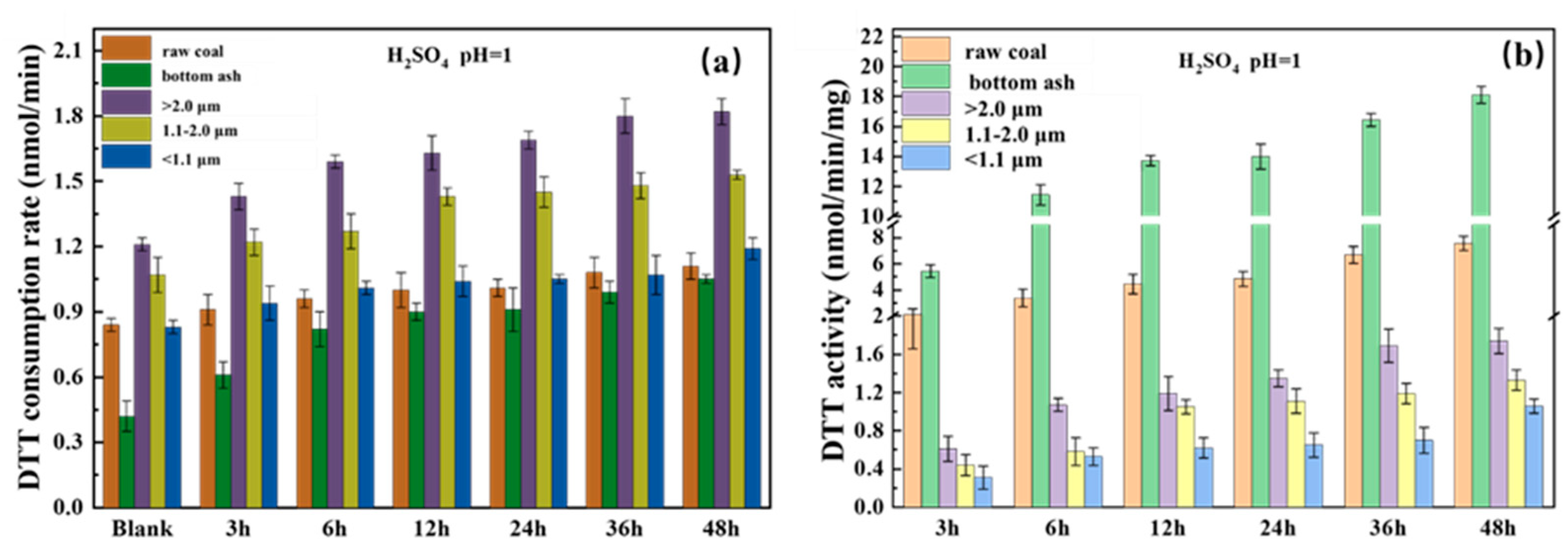
2.6. Oxidative Potential of Coal Combustion Particles
2.7. Statistical Analysis
3. Results
3.1. Percentage of Iron-Containing Particles among the Measured Particles
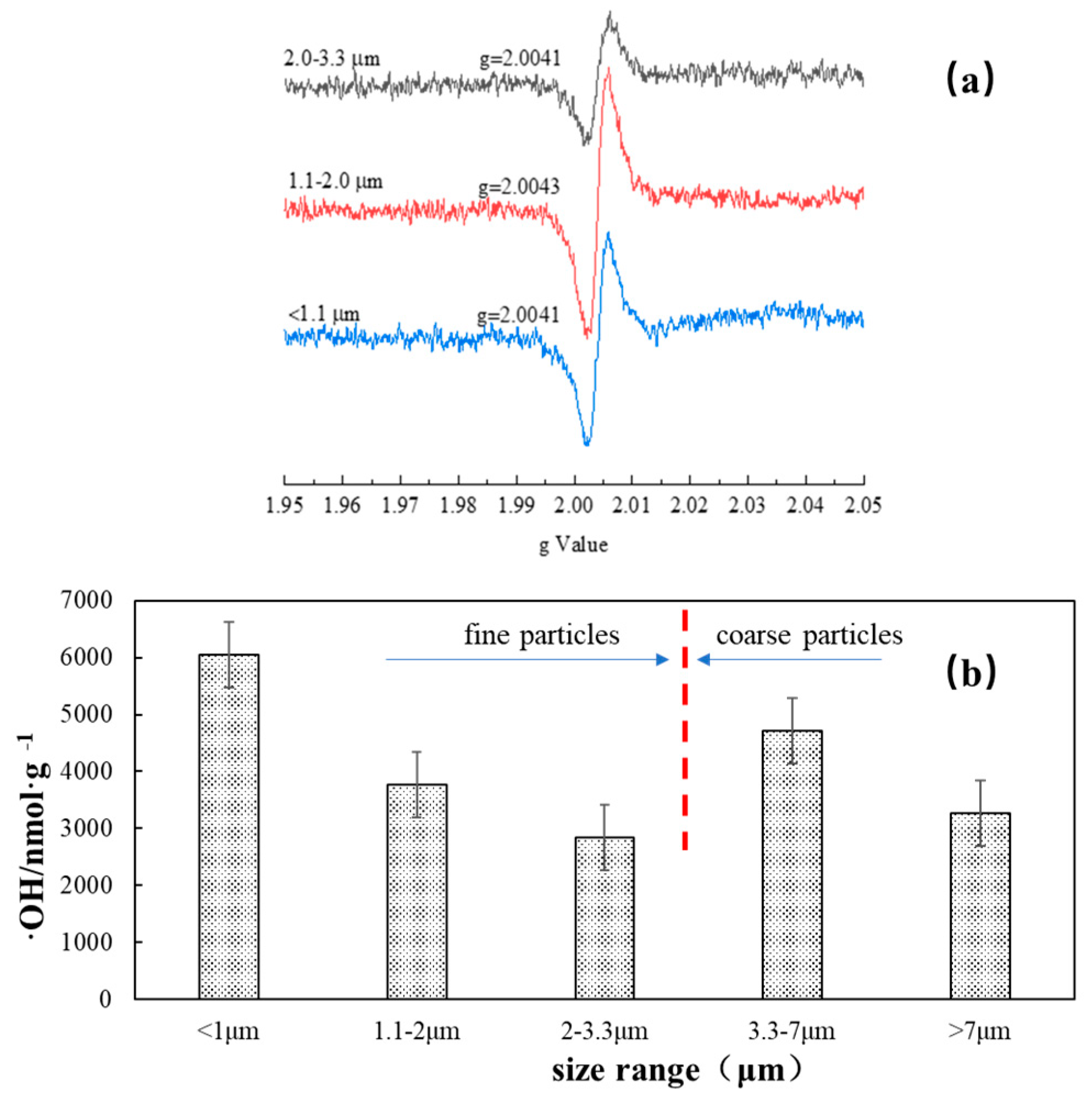
3.2. Intensity of Free Radicals Generated by Xuanwei Coal Combustion Particles
3.3. Chemical Elements in the Size-Resolved Particles from Xuanwei Coal Combustion
3.4. Dissolution Ratio of Iron-Bearing Minerals
3.5. Oxidation Potential of Iron
4. Discussion
4.1. Free Radicals Generated by Fe-Containing Particles
4.2. Solubility and Oxidative Potential of Iron-Containing Particles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, X.Z.; Chen, W.; Liu, Z.Y.; Chapman, R.S. An epidemiological study of lung cancer in Xuan Wei County, China: Current progress. Case-control study on lung cancer and cooking fuel. Environ. Health Perspect. 1991, 94, 9–13. [Google Scholar] [PubMed]
- Mumford, J.L.; He, X.Z.; Chapman, R.S.; Cao, S.R.; Harris, D.B.; Li, X.M.; Xian, Y.L.; Jiang, W.Z.; Xu, C.W.; Chuang, J.C.; et al. Lung Cancer and Indoor Air Pollution in Xuan Wei, China. Science 1987, 235, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Dai, S.; Wang, J.; Huang, Y.; Ho, S.C.; Zhou, Y.; Lucas, D.; Koshland, C.P. Nanoquartz in Late Permian C1 coal and the high incidence of female lung cancer in the Pearl River Origin area: A retrospective cohort study. BMC Public Health 2008, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yi, F.; Hao, X.; Yu, S.; Ren, J.; Wu, M.; Feng, J.; Yonemochi, S.; Wang, Q. Physicochemical properties and ability to generate free radicals of ambient coarse, fine, and ultrafine particles in the atmosphere of Xuanwei, China, an area of high lung cancer incidence. Atmos. Environ. 2014, 97, 519–528. [Google Scholar] [CrossRef]
- Xiao, K.; Lin, Y.; Wang, Q.; Lu, S.; Wang, W.; Chowdhury, T.; Enyoh, C.E.; Rabin, M.H. Characteristics and Potential Inhalation Exposure Risks of Environmentally Persistent Free Radicals in Atmospheric Particulate Matter and Solid Fuel Combustion Particles in High Lung Cancer Incidence Area, China. Atmosphere 2021, 12, 1467. [Google Scholar] [CrossRef]
- Lui, K.H.; Bandowe, B.A.M.; Tian, L.; Chan, C.-S.; Cao, J.-J.; Ning, Z.; Lee, S.C.; Ho, K.F. Cancer risk from polycyclic aromatic compounds in fine particulate matter generated from household coal combustion in Xuanwei, China. Chemosphere 2017, 169, 660–668. [Google Scholar] [CrossRef]
- Lu, S.; Hao, X.; Liu, D.; Wang, Q.; Zhang, W.; Liu, P.; Zhang, R.; Shang, Y.; Pang, R.; Wu, M.; et al. Mineralogical characterization of ambient fine/ultrafine particles emitted from Xuanwei C1 coal combustion. Atmos. Res. 2016, 169, 17–23. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Wang, T.; Tang, J.; Lin, X.; Yao, Z.; Li, K.; Liao, Y.; Zhou, L.; Geng, Z.; et al. The characteristics of lung cancer in Xuanwei County: A review of differentially expressed genes and noncoding RNAs on cell proliferation and migration. Biomed. Pharmacother. 2019, 119, 109312. [Google Scholar] [CrossRef]
- Liu, D. Value evaluation system of ecological environment damage compensation caused by air pollution. Environ. Technol. Innov. 2021, 22, 101473. [Google Scholar] [CrossRef]
- Duan, H.; Niu, H.; Gan, L.; Duan, X.; Shi, S.; Li, L. Reinterpret the heterogeneous reaction of α-Fe2O3 and NO2 with 2D-COS: The role of SDS, UV and SO2. Chin. Chem. Lett. 2023, 109038, in press. [Google Scholar] [CrossRef]
- Shao, L.; Wang, J.; Hou, X.; Zhang, M.; Wang, H.; Spiro, B.; Large, D.; Zhou, Y. Geochemistry of C1 coal of latest Permian under mass extinction in Xuanwei, Yunnan. Acta Geol. Sin. 2015, 89, 163–179. [Google Scholar]
- Shaltout, A.A.; Harfouche, M.; Ali, S.S.M.; Karydas, A.G.; Kregsamer, P.; Wobrauschek, P.; Streli, C.; Abd-Elkader, O.H.; Yassin, M.A.; El Orabi, N.F. Elemental composition and source apportionment of atmospheric aerosols collected from urban and residential areas of Jordan using multi-secondary targets energy dispersive X-ray fluorescence. Spectrochim. Acta Part B At. Spectrosc. 2020, 170, 105900. [Google Scholar] [CrossRef]
- Anastasio, C.; McGregor, K.G. Chemistry of fog waters in California’s Central Valley: 2. Photochemical transformations of amino acids and alkyl amines. Atmos. Environ. 2001, 35, 1091–1104. [Google Scholar]
- Jung, H.; Guo, B.; Anastasio, C.; Kennedy, I.M. Quantitative measurements of the generation of hydroxyl radicals by soot particles in a surrogate lung fluid. Atmos. Environ. 2006, 40, 1043–1052. [Google Scholar] [CrossRef]
- Zepp, R.G.; Faust, B.C.; Hoigne, J. Hydroxyl radical formation in aqueous reactions (pH 3–8) of iron(II) with hydrogen peroxide: The photo-Fenton reaction. Environ. Sci. Technol. 1992, 26, 313–319. [Google Scholar] [CrossRef]
- Li, W.; Shao, L. Transmission electron microscopy study of aerosol particles from the brown hazes in northern China. J. Geophys. Res. Atmos. 2009, 114, D09304. [Google Scholar] [CrossRef]
- Xie, T.; Lu, S.; Zeng, J.; Rao, L.; Wang, X.; Win, M.S.; Zhang, D.; Lu, H.; Liu, X.; Wang, Q. Soluble Fe release from iron-bearing clay mineral particles in acid environment and their oxidative potential. Sci. Total Environ. 2020, 726, 138650. [Google Scholar] [CrossRef]
- Xie, T.; Lu, S.; Rao, L.; Zhang, L.; Wang, X.; Wang, W.; Wang, Q. Dissolution factors and oxidative potential of acid soluble irons from chlorite mineral particles. Atmos. Environ. 2021, 255, 118436. [Google Scholar] [CrossRef]
- Lu, S.; Win, M.S.; Zeng, J.; Yao, C.; Zhao, M.; Xiu, G.; Lin, Y.; Xie, T.; Dai, Y.; Rao, L.; et al. A characterization of HULIS-C and the oxidative potential of HULIS and HULIS-Fe(II) mixture in PM2.5 during hazy and non-hazy days in Shanghai. Atmos. Environ. 2019, 219, 117058. [Google Scholar] [CrossRef]
- Okada, K.; Qin, Y.; Kai, K. Elemental composition and mixing properties of atmospheric mineral particles collected in Hohhot, China. Atmos. Res. 2005, 73, 45–67. [Google Scholar] [CrossRef]
- Rao, L.; Zhang, L.; Wang, X.; Xie, T.; Zhou, S.; Lu, S.; Liu, X.; Lu, H.; Xiao, K.; Wang, W.; et al. Oxidative Potential Induced by Ambient Particulate Matters with Acellular Assays: A Review. Processes 2020, 8, 1410. [Google Scholar] [CrossRef]
- Strak, M.; Janssen, N.A.H.; Godri, K.J.; Gosens, I.; Mudway, I.S.; Cassee, F.R.; Lebret, E.; Kelly, F.J.; Harrison, R.M.; Brunekreef, B.; et al. Respiratory Health Effects of Airborne Particulate Matter: The Role of Particle Size, Composition, and Oxidative Potential—The RAPTES Project. Environ. Health Perspect. 2012, 120, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, E.; Hurab, O.; Rubasinghege, G. Atmospheric processing and iron mobilization of ilmenite: Iron-containing ternary oxide in mineral dust aerosol. J. Phys. Chem. A 2018, 122, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Xu, F.; Qiu, X.; Zhu, T.; Rudich, Y. Seasonal variations in fine particle composition from Beijing prompt oxidative stress response in mouse lung and liver. Sci. Total Environ. 2018, 626, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Lu, B.; Chen, J.; Li, X. Size distributions of particle-generated hydroxyl radical (·OH) in surrogate lung fluid (SLF) solution and their potential sources. Environ. Pollut. 2021, 268, 115582. [Google Scholar] [CrossRef] [PubMed]
- Scanza, R.A.; Hamilton, D.S.; Perez Garcia-Pando, C.; Buck, C.; Baker, A.; Mahowald, N. Atmospheric Processing of Iron in Mineral and Combustion Aerosols: Development of an Intermediate-Complexity Mechanism Suitable for Earth System Models. Atmos. Chem. Phys. 2018, 18, 14175–14196. [Google Scholar] [CrossRef]
| Coarse Particles | Fine Particles | Submicron Particles | |||
|---|---|---|---|---|---|
| >7.0 μm | 3.3–7.0 μm | 2.0–3.3 μm | 1.1–2.0 μm | <1.1 μm | |
| Na | 891.45 | 480.61 | 71.16 | 27.26 | 130.77 |
| Mg | 5215.94 | 2284.15 | 234.06 | 51.26 | 36.68 |
| Al | 3926.18 | 1818.40 | 278.81 | 49.63 | 369.00 |
| P | 9.48 | 4.95 | 0.45 | 0.41 | 0.86 |
| S | 2693.32 | 629.26 | 322.67 | 244.08 | 1267.11 |
| Cl | 322.44 | 104.05 | 105.17 | 103.73 | 4190.61 |
| K | 246.57 | 123.87 | 12.08 | 16.27 | 8.20 |
| Ca | 104.32 | 89.19 | 8.50 | 4.47 | 26.76 |
| Sc | 9.48 | 4.95 | 1.34 | 1.63 | 18.13 |
| Ti | 9.48 | 4.95 | 0.45 | 0.00 | 0.43 |
| V | 9.48 | 0.00 | 0.00 | 0.00 | 1.29 |
| Cr | 9.48 | 4.95 | 2.69 | 1.63 | 0.43 |
| Mn | 9.48 | 0.00 | 0.90 | 0.41 | 0.43 |
| Fe | 2976.69 | 2045.68 | 1399.80 | 1187.81 | 263.17 |
| Co | 37.93 | 19.82 | 0.45 | 0.00 | 0.43 |
| Ni | 85.35 | 49.55 | 4.48 | 5.70 | 0.00 |
| Cu | 37.93 | 4.95 | 0.45 | 0.41 | 0.43 |
| Zn | 37.93 | 9.91 | 1.79 | 3.66 | 8.63 |
| Ga | 9.48 | 0.00 | 0.00 | 0.41 | 0.43 |
| As | 9.48 | 0.00 | 0.45 | 0.41 | 5.18 |
| Se | 18.97 | 4.95 | 2.69 | 2.44 | 58.69 |
| Br | 85.35 | 29.73 | 14.77 | 12.61 | 444.09 |
| Sr | 9.48 | 4.95 | 0.45 | 0.00 | 0.86 |
| Ba | 28.45 | 24.77 | 6.71 | 2.03 | 10.36 |
| Pb | 28.45 | 0.00 | 0.00 | 0.00 | 55.67 |
| H2SO4 pH = 1 | 0.5 h | 1 h | 3 h | 6 h | 12 h | 24 h | 36 h | 48 h | |
|---|---|---|---|---|---|---|---|---|---|
| coarse particles (>2.0 μm) | FeT | 0.51 | 0.51 | 0.52 | 0.53 | 0.54 | 0.56 | 0.57 | 0.59 |
| Fe2+ | 0.06 | 0.06 | 0.07 | 0.07 | 0.08 | 0.1 | 0.11 | 0.12 | |
| Fe3+ | 0.45 | 0.45 | 0.45 | 0.46 | 0.46 | 0.46 | 0.46 | 0.47 | |
| Fe2+/FeT | 0.12 | 0.12 | 0.13 | 0.13 | 0.15 | 0.18 | 0.19 | 0.20 | |
| fine particles (1.1–2.0 μm) | FeT | 0.39 | 0.4 | 0.42 | 0.43 | 0.44 | 0.48 | 0.53 | 0.56 |
| Fe2+ | 0.05 | 0.05 | 0.07 | 0.08 | 0.09 | 0.11 | 0.14 | 0.17 | |
| Fe3+ | 0.34 | 0.35 | 0.35 | 0.35 | 0.35 | 0.37 | 0.39 | 0.39 | |
| Fe2+/FeT | 0.13 | 0.13 | 0.17 | 0.19 | 0.20 | 0.23 | 0.26 | 0.30 | |
| submicron particles (<1.1 μm) | FeT | 0.28 | 0.33 | 0.38 | 0.41 | 0.43 | 0.44 | 0.46 | 0.48 |
| Fe2+ | 0.14 | 0.17 | 0.19 | 0.19 | 0.2 | 0.21 | 0.23 | 0.25 | |
| Fe3+ | 0.14 | 0.16 | 0.19 | 0.22 | 0.23 | 0.23 | 0.23 | 0.23 | |
| Fe2+/FeT | 0.50 | 0.52 | 0.50 | 0.46 | 0.47 | 0.48 | 0.50 | 0.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Liu, J.; Hou, G.; Zhao, J.; Liu, X.; Xie, T.; Xiao, K.; Yonemochi, S.; Ebere, E.C.; Wang, W.; et al. Physicochemical Characterization and Oxidative Potential of Iron-Containing Particles Emitted from Xuanwei Coal Combustion. Toxics 2023, 11, 921. https://doi.org/10.3390/toxics11110921
Lu S, Liu J, Hou G, Zhao J, Liu X, Xie T, Xiao K, Yonemochi S, Ebere EC, Wang W, et al. Physicochemical Characterization and Oxidative Potential of Iron-Containing Particles Emitted from Xuanwei Coal Combustion. Toxics. 2023; 11(11):921. https://doi.org/10.3390/toxics11110921
Chicago/Turabian StyleLu, Senlin, Jin Liu, Guoqing Hou, Jiumei Zhao, Xinchun Liu, Tingting Xie, Kai Xiao, Shinichi Yonemochi, Enyoh Christian Ebere, Weiqian Wang, and et al. 2023. "Physicochemical Characterization and Oxidative Potential of Iron-Containing Particles Emitted from Xuanwei Coal Combustion" Toxics 11, no. 11: 921. https://doi.org/10.3390/toxics11110921
APA StyleLu, S., Liu, J., Hou, G., Zhao, J., Liu, X., Xie, T., Xiao, K., Yonemochi, S., Ebere, E. C., Wang, W., & Wang, Q. (2023). Physicochemical Characterization and Oxidative Potential of Iron-Containing Particles Emitted from Xuanwei Coal Combustion. Toxics, 11(11), 921. https://doi.org/10.3390/toxics11110921










