Mechanism of Interaction between hsa_circ_0002854 and MAPK1 Protein in PM2.5-Induced Apoptosis of Human Bronchial Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell and Cell Culture
2.2. Collection and Treatment of Particulate Matter
2.3. Cell Counting Kit-8 Assay
2.4. Flow Cytometry Experiment
2.5. RT-qPCR Analysis
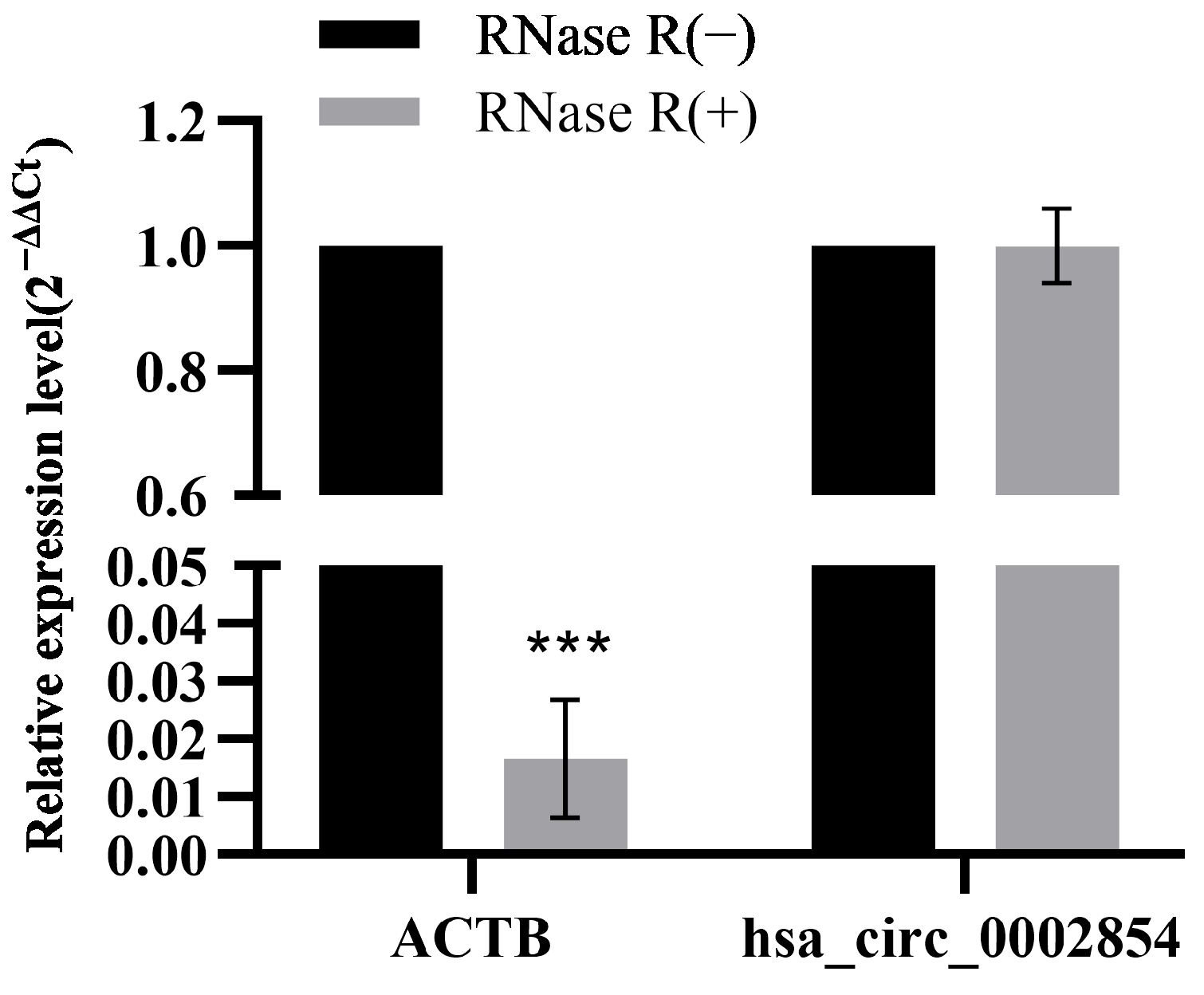
2.6. RNase R Digestive Experiment
2.7. Nucleocytoplasmic Separation Experiment
2.8. Fluorescence In Situ Hybridization Experiment (FISH)
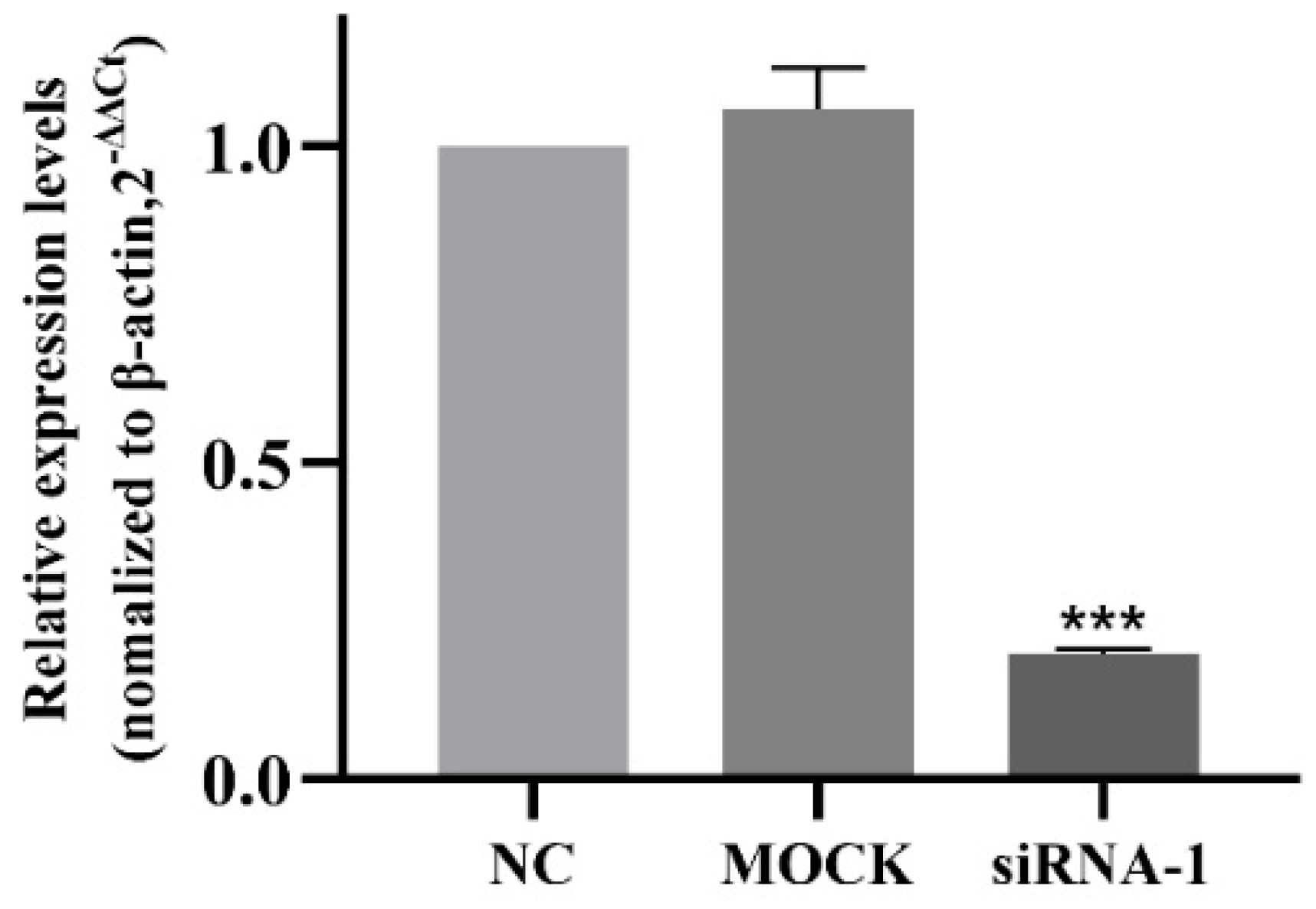
2.9. Small Interfering RNA (siRNA) Transfection
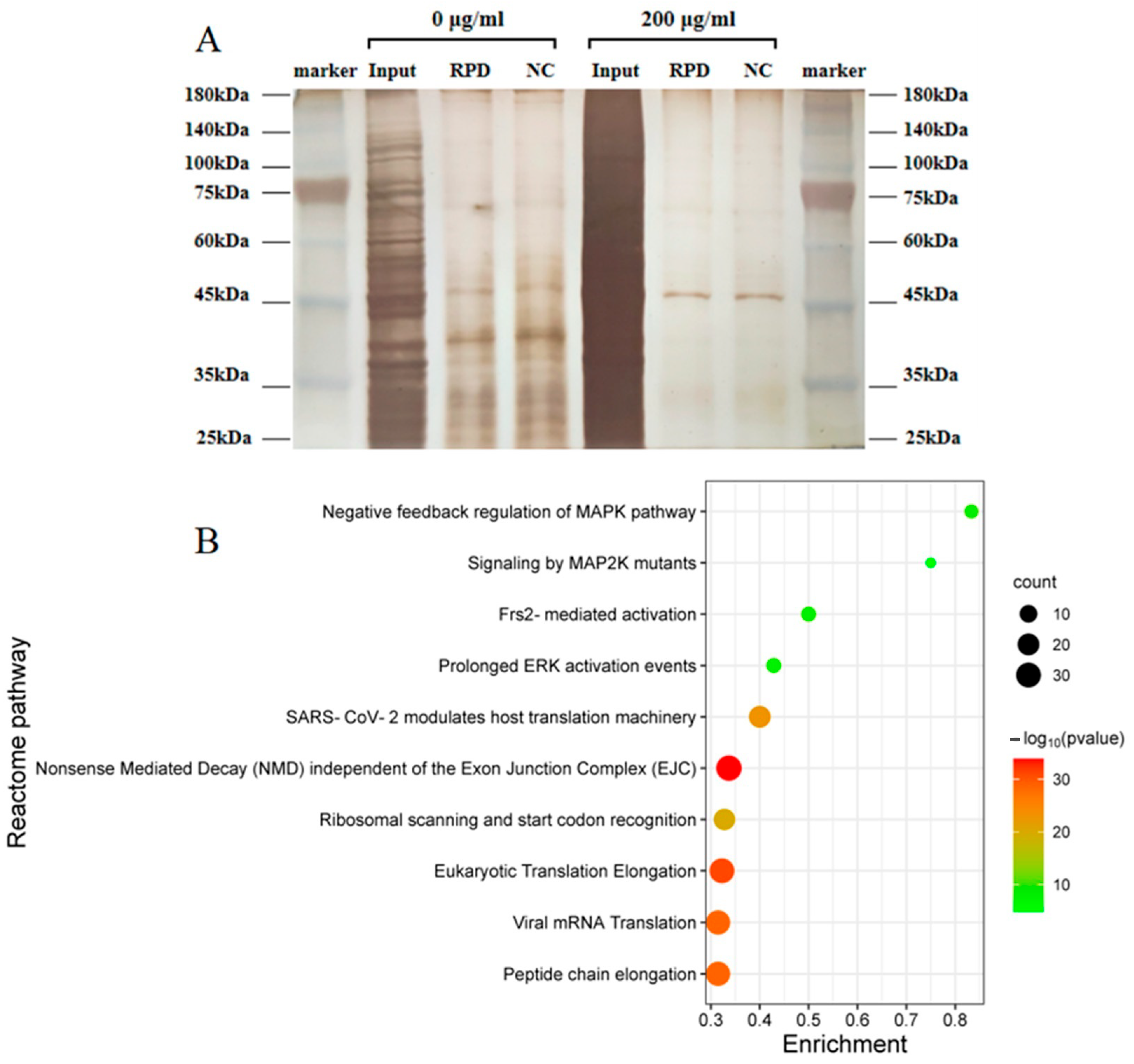
2.10. RNA Pull-Down Experiment
2.11. Protein Extraction and Western Blot Analysis
2.12. Statistical Analysis
3. Result
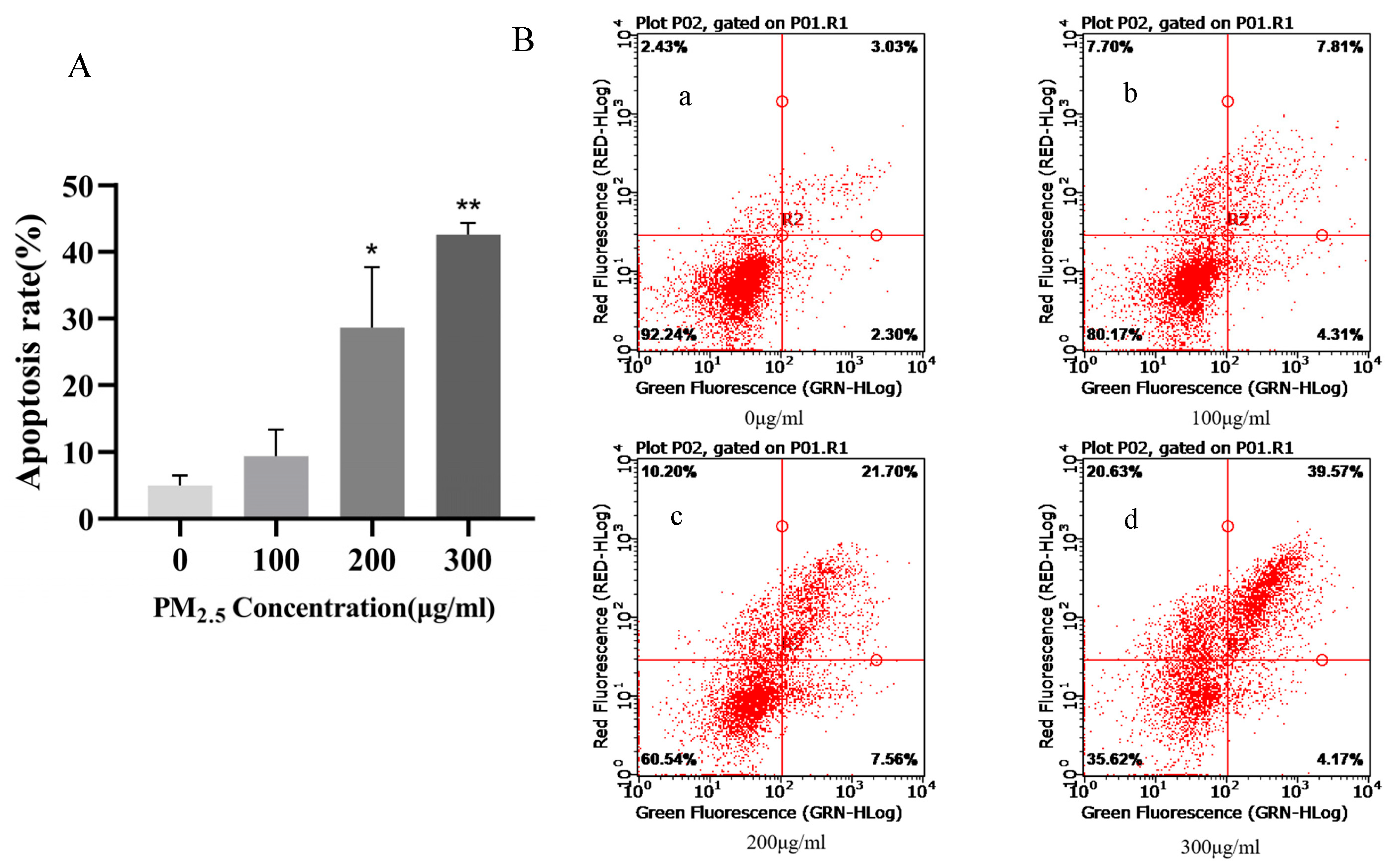
3.1. Survival Rate of 16HBE Cells after 24 h of Exposure to PM2.5
3.2. Apoptosis Rate of 16HBE Cells after 24 h of Exposure to PM2.5
3.3. qRT-PCR-Validated circRNAs Associated with Apoptosis Pathways
3.4. RNase R Digestive Test
3.5. Nucleocytoplasmic Separation Experiment
3.6. Fluorescence In Situ Hybridization Experiment
3.7. Interference Efficiency of siRNA
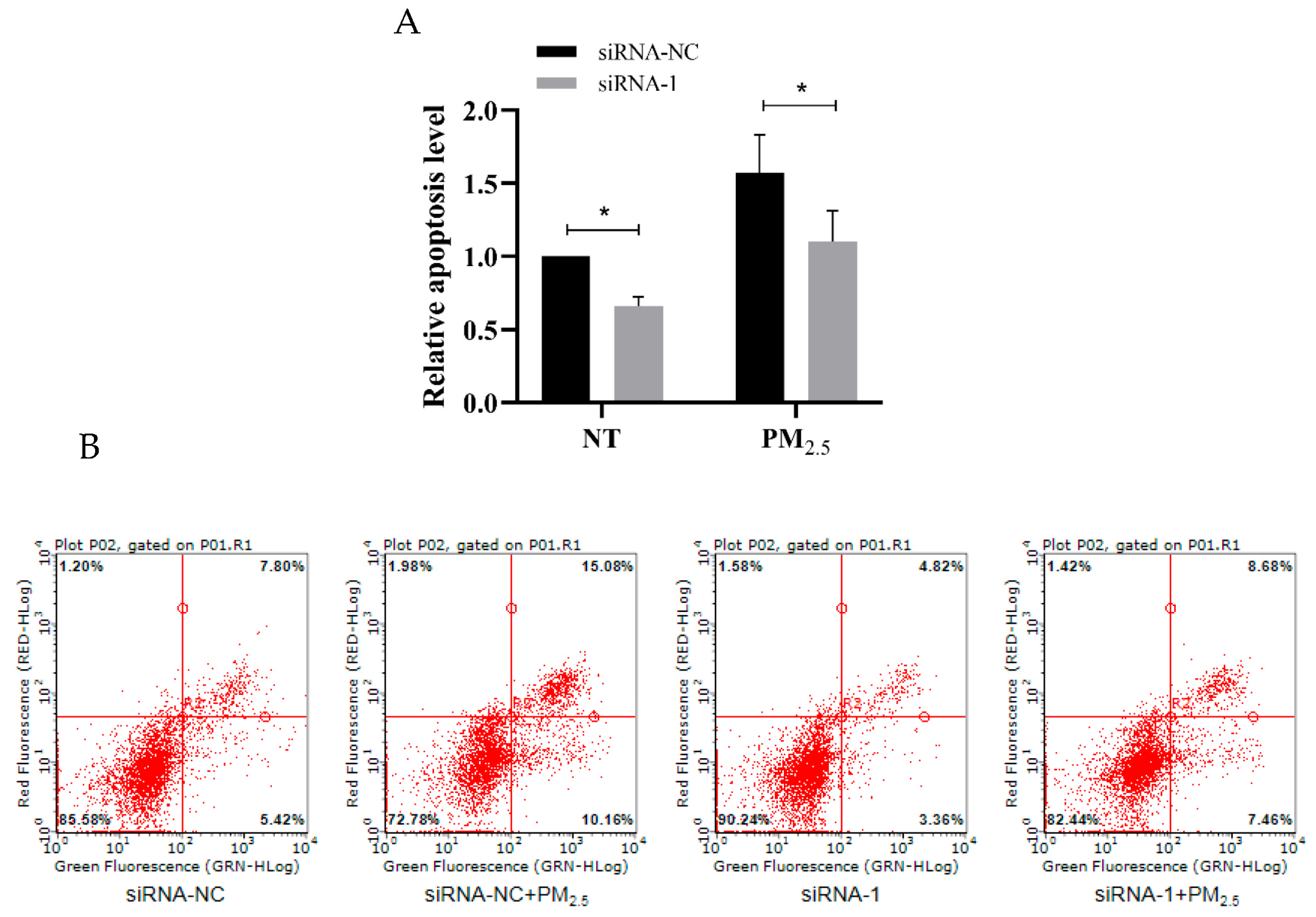
3.8. hsa_circ_0002854 Functional Verification Experiment
3.9. Study on the Mechanism of hsa_circ_0002854
3.10. Western Blot Assay Verified the Regulatory Effect of hsa_circ_0002854 on MAPK1 Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO’s Global Air-Quality Guidelines. Lancet 2006, 368, 1302. [CrossRef] [PubMed]
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Moller, W.; Felten, K.; Sommerer, K.; Scheuch, G.; Meyer, G.; Meyer, P.; Haussinger, K.; Kreyling, W.G. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit. Care Med. 2008, 177, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Pun, V.C.; Kazemiparkouhi, F.; Manjourides, J.; Suh, H.H. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am. J. Epidemiol. 2017, 186, 961–969. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Bao, H. Revalue associations of short-term exposure to air pollution with respiratory hospital admissions in Lanzhou, China after the control and treatment of current pollution. Int. J. Hyg. Environ. Health 2021, 231, 113658. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Li, X.; Lv, S.; Ma, R.; Qi, Y.; Abulikemu, A.; Duan, H.; Guo, C.; Li, Y.; et al. PM(2.5) triggered apoptosis in lung epithelial cells through the mitochondrial apoptotic way mediated by a ROS-DRP1-mitochondrial fission axis. J. Hazard. Mater. 2020, 397, 122608. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest 2019, 155, 417–426. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- The Lancet. Air pollution: A major threat to lung health. Lancet 2019, 393, 1774. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef]
- Yin, J.; Xia, W.; Li, Y.; Guo, C.; Zhang, Y.; Huang, S.; Jia, Z.; Zhang, A. COX-2 mediates PM2.5-induced apoptosis and inflammation in vascular endothelial cells. Am. J. Transl. Res. 2017, 9, 3967–3976. [Google Scholar] [PubMed]
- Wang, Y.; Tang, M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci. Total Environ. 2020, 710, 136397. [Google Scholar] [CrossRef] [PubMed]
- Taraseviciene-Stewart, L.; Scerbavicius, R.; Choe, K.H.; Moore, M.; Sullivan, A.; Nicolls, M.R.; Fontenot, A.P.; Tuder, R.M.; Voelkel, N.F. An animal model of autoimmune emphysema. Am. J. Respir. Crit. Care Med. 2005, 171, 734–742. [Google Scholar] [CrossRef]
- Cohen, L.E.X.; Tarsi, J.; Ramkumar, T.; Horiuchi, T.K.; Cochran, R.; DeMartino, S.; Schechtman, K.B.; Hussain, I.; Holtzman, M.J.; Castro, M.; et al. Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 138–145. [Google Scholar] [CrossRef]
- Sisson, T.H.; Mendez, M.; Choi, K.; Subbotina, N.; Courey, A.; Cunningham, A.; Dave, A.; Engelhardt, J.F.; Liu, X.; White, E.S.; et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 254–263. [Google Scholar] [CrossRef]
- Mauad, T.; Hajjar, L.A.; Callegari, G.D.; da Silva, L.F.; Schout, D.; Galas, F.R.; Alves, V.A.; Malheiros, D.M.; Auler, J.O., Jr.; Ferreira, A.F.; et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am. J. Respir. Crit. Care Med. 2010, 181, 72–79. [Google Scholar] [CrossRef]
- Alastalo, T.P.; Li, M.; Perez Vde, J.; Pham, D.; Sawada, H.; Wang, J.K.; Koskenvuo, M.; Wang, L.; Freeman, B.A.; Chang, H.Y.; et al. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J. Clin. Investig. 2011, 121, 3735–3746. [Google Scholar] [CrossRef]
- Sharma, A.R.; Bhattacharya, M.; Bhakta, S.; Saha, A.; Lee, S.S.; Chakraborty, C. Recent research progress on circular RNAs: Biogenesis, properties, functions, and therapeutic potential. Mol. Ther. Nucleic Acids 2021, 25, 355–371. [Google Scholar] [CrossRef]
- Chen, L.L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016, 17, 205–211. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Sauler, M.; Bazan, I.S.; Lee, P.J. Cell Death in the Lung: The Apoptosis-Necroptosis Axis. Annu. Rev. Physiol. 2019, 81, 375–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, Y.; Liao, J.; Wang, G. PM2.5-related cell death patterns. Int. J. Med. Sci. 2021, 18, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Y.; Zou, Y.; Zhou, C.; Yi, Y.; Ling, Y.; Liao, F.; Jiang, Y.; Peng, X. LncRNA LOC101927514 regulates PM(2.5)-driven inflammation in human bronchial epithelial cells through binding p-STAT3 protein. Toxicol. Lett. 2020, 319, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yuan, X.; Zhang, L.; Su, B.; Tian, D.; Li, Y.; Zhao, J.; Wang, Y.; Peng, S. Overexpression of HO-1 assisted PM2.5-induced apoptosis failure and autophagy-related cell necrosis. Ecotoxicol. Environ. Saf. 2017, 145, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, Y.; Liu, L.; Guan, L.; Wang, T.; Zhang, L.; Wang, Y.; Cao, J.; Ding, W.; Zhang, F.; et al. DDAH1 plays dual roles in PM2.5 induced cell death in A549 cells. Biochim. Biophys. Acta 2016, 1860, 2793–2801. [Google Scholar] [CrossRef]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, H.D.; Gorenc, T.; Steller, H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev. Cell 2004, 7, 491–501. [Google Scholar] [CrossRef]
- Fan, Y.; Bergmann, A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev. Cell 2008, 14, 399–410. [Google Scholar] [CrossRef]
- Chera, S.; Ghila, L.; Dobretz, K.; Wenger, Y.; Bauer, C.; Buzgariu, W.; Martinou, J.C.; Galliot, B. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev. Cell 2009, 17, 279–289. [Google Scholar] [CrossRef]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, X.; Nan, A.; Zhang, N.; Chen, L.; Zhou, H.; Zhang, H.; Qiu, M.; Zhu, J.; Ling, Y.; et al. Circular RNA 406961 interacts with ILF2 to regulate PM(2.5)-induced inflammatory responses in human bronchial epithelial cells via activation of STAT3/JNK pathways. Environ. Int. 2020, 141, 105755. [Google Scholar] [CrossRef]
- Dou, C.; Zhang, J.; Qi, C. Cooking oil fume-derived PM(2.5) induces apoptosis in A549 cells and MAPK/NF-small ka, CyrillicB/STAT1 pathway activation. Environ. Sci. Pollut. Res. Int. 2018, 25, 9940–9948. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef] [PubMed]






| Primer | Primer Sequence (5′ to 3′) |
|---|---|
| ACTB-F | TCTGGCGGCACCACCATGTAC |
| ACTB-R | CTGCTTGCTGATCCACATCTGCTG |
| U6_F1 | CTCGCTTCGGCAGCACA |
| U6_R1 | AACGCTTCACGAATTTGCGT |
| hsa_circ_0002854_F | GTCAGCTCAAACCACTAGTTATACAGGTAC |
| hsa_circ_0002854_R | CAGTCATCGTCATCATCATTTTCGTCC |
| hsa_circ_0003036_F | GGAGAAATTAACTGAATGCCAAGGG |
| hsa_circ_0003036_R | TGATTTCGTATGATCCGGTCGC |
| hsa_circ_0034296_F | TTCCAGCCAGCCTGCTAAAC |
| hsa_circ_0034296_R | AGCTGTTTCCTCCATTGCTGT |
| hsa_circ_0000780_F | TTAGCACACTGGTGGAAGATCA |
| hsa_circ_0000780_R | CCAAGATACAACCAAAAGCCAT |
| hsa_circ_0003352_F | ACTGTTGGATGCAGATGGATGAG |
| hsa_circ_0003352_R | GAAATAGCCCAGGGGAAGGAGAT |
| hsa_circ_0001571_F | CGTGCTGAAAGCCGAGGG |
| hsa_circ_0001571_R | CCAAAGCCTCCGCTGTCC |
| hsa_circ_0005791_F | AGAACAGTGAACAAGGTGG |
| hsa_circ_0005791_R | AAACAGTTAATATCATCCC |
| hsa_circ_0071106_F | GGTTTTGTTTTGACATAGAAGCTGCTG |
| hsa_circ_0071106_R | CTGCCTCTTGTAAATGTGAGTCTTTCT |
| Probe Name | Probe Sequence | |
|---|---|---|
| full-length sequence | sense | GTACGAGTGGCTGCTTTACAGAATCTGGTGAAGATAAT GTCCTTATATTATCAGTACATGGAGACATATATGGGTCC TGCTCTTTTTGCAATCACAATCGAAGCAATGAAAAGTGA CATTGATGAGGTGGCTTTACAAGGGATAGAATTCTGGTC CAATGTCTGTGATGAGGAAATGGATTTGGCCATTGAAGC TTCAGAGGCAGCAGAACAAGGACGGCCCCCTGAGCACAC CAGCAAGTTTTATGCGAAGGGAGCACTACAGTATCTGGT TCCAATCCTCACACAGACACTAACTAAACAGGACGAAAA TGATGATGACGATGACTGGAACCCCTGCAAAGCAGCAGG GGTGTGCCTCATGCTTCTGGCCACCTGCTGTGAAGATGA CATTGTCCCACATGTCCTCCCCTTCATTAAAGAACACAT CAAGAACCCAGATTGGCGGTACCGGGATGCAGCAGTGAT GGCTTTTGGTTGTATCTTGGAAGGACCAGAGCCCAGTCA GCTCAAACCACTAGTTATACAG |
| anti-sense | CTGTATAACTAGTGGTTTGAGCTGACTGGGCTCTGGTC CTTCCAAGATACAACCAAAAGCCATCACTGCTGCATCC CGGTACCGCCAATCTGGGTTCTTGATGTGTTCTTTAAT GAAGGGGAGGACATGTGGGACAATGTCATCTTCACAGC AGGTGGCCAGAAGCATGAGGCACACCCCTGCTGCTTTG CAGGGGTTCCAGTCATCGTCATCATCATTTTCGTCCTG TTTAGTTAGTGTCTGTGTGAGGATTGGAACCAGATACT GTAGTGCTCCCTTCGCATAAAACTTGCTGGTGTGCTCA GGGGGCCGTCCTTGTTCTGCTGCCTCTGAAGCTTCAAT GGCCAAATCCATTTCCTCATCACAGACATTGGACCAGA ATTCTATCCCTTGTAAAGCCACCTCATCAATGTCACTTT TCATTGCTTCGATTGTGATTGCAAAAAGAGCAGGACCC ATATATGTCTCCATGTACTGATAATATAAGGACATTAT CTTCACCAGATTCTGTAAAGCAGCCACTCGTAC | |
| joint sequence | sense | CCACTAGTTATACAGGTACGAGTGGCTGCT |
| anti-sense | AGCAGCCACTCGTACCTGTATAACTAGTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Tan, Y.; Wang, Y.; Wang, H.; Li, C.; Jin, W.; Wu, Y.; Ni, D.; Peng, X. Mechanism of Interaction between hsa_circ_0002854 and MAPK1 Protein in PM2.5-Induced Apoptosis of Human Bronchial Epithelial Cells. Toxics 2023, 11, 906. https://doi.org/10.3390/toxics11110906
Hong J, Tan Y, Wang Y, Wang H, Li C, Jin W, Wu Y, Ni D, Peng X. Mechanism of Interaction between hsa_circ_0002854 and MAPK1 Protein in PM2.5-Induced Apoptosis of Human Bronchial Epithelial Cells. Toxics. 2023; 11(11):906. https://doi.org/10.3390/toxics11110906
Chicago/Turabian StyleHong, Jinchang, Yi Tan, Yuyu Wang, Hongjie Wang, Caixia Li, Wenjia Jin, Yi Wu, Dechun Ni, and Xiaowu Peng. 2023. "Mechanism of Interaction between hsa_circ_0002854 and MAPK1 Protein in PM2.5-Induced Apoptosis of Human Bronchial Epithelial Cells" Toxics 11, no. 11: 906. https://doi.org/10.3390/toxics11110906
APA StyleHong, J., Tan, Y., Wang, Y., Wang, H., Li, C., Jin, W., Wu, Y., Ni, D., & Peng, X. (2023). Mechanism of Interaction between hsa_circ_0002854 and MAPK1 Protein in PM2.5-Induced Apoptosis of Human Bronchial Epithelial Cells. Toxics, 11(11), 906. https://doi.org/10.3390/toxics11110906






