Genomic Instability and Cytotoxicity Evaluation of Two Communities Exposed to Pesticides in the Mexicali Valley by the L-CBMN Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
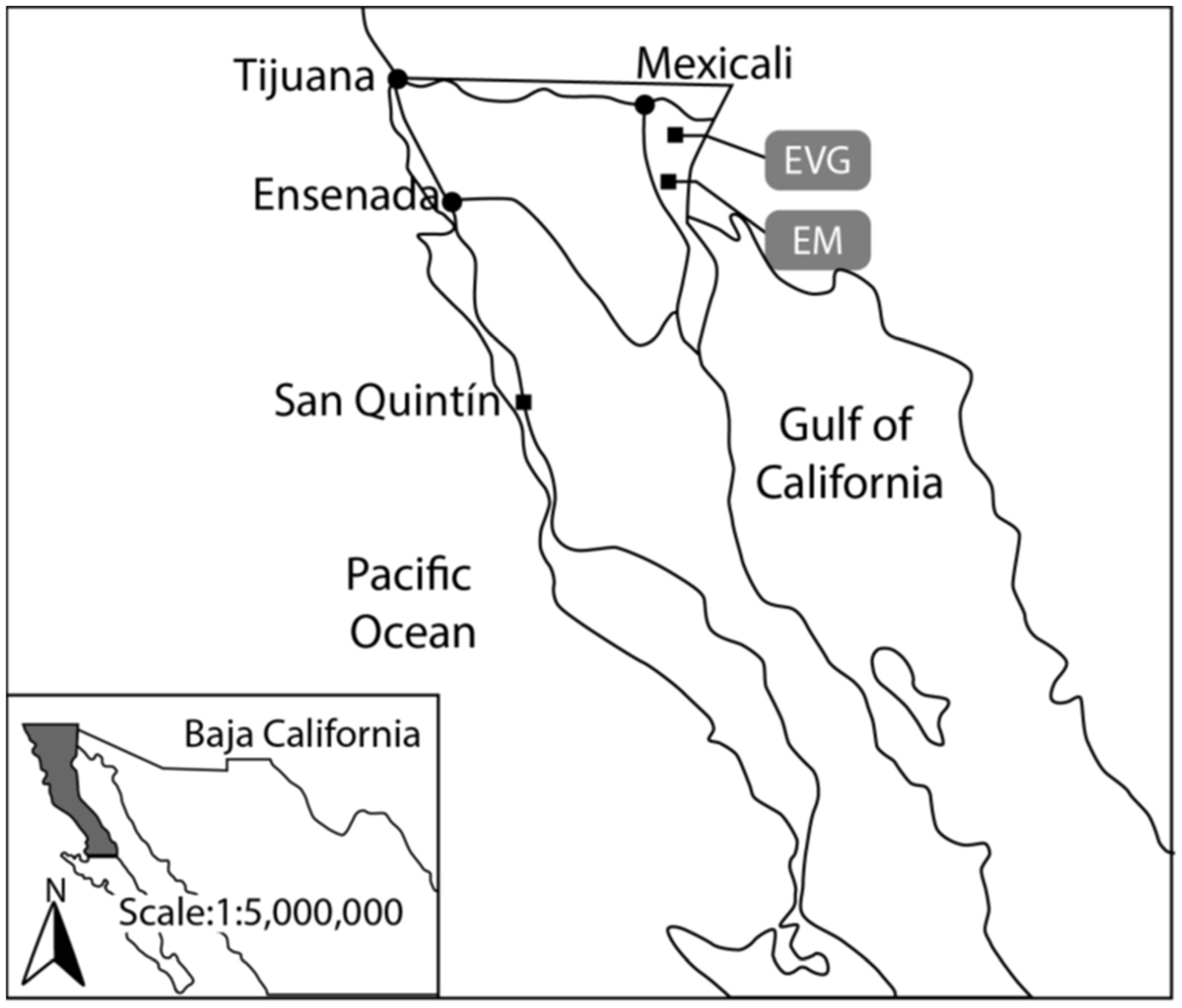
2.2. Study Area
2.3. Study Groups
2.4. Participants Inclusion
2.5. Selection Criteria
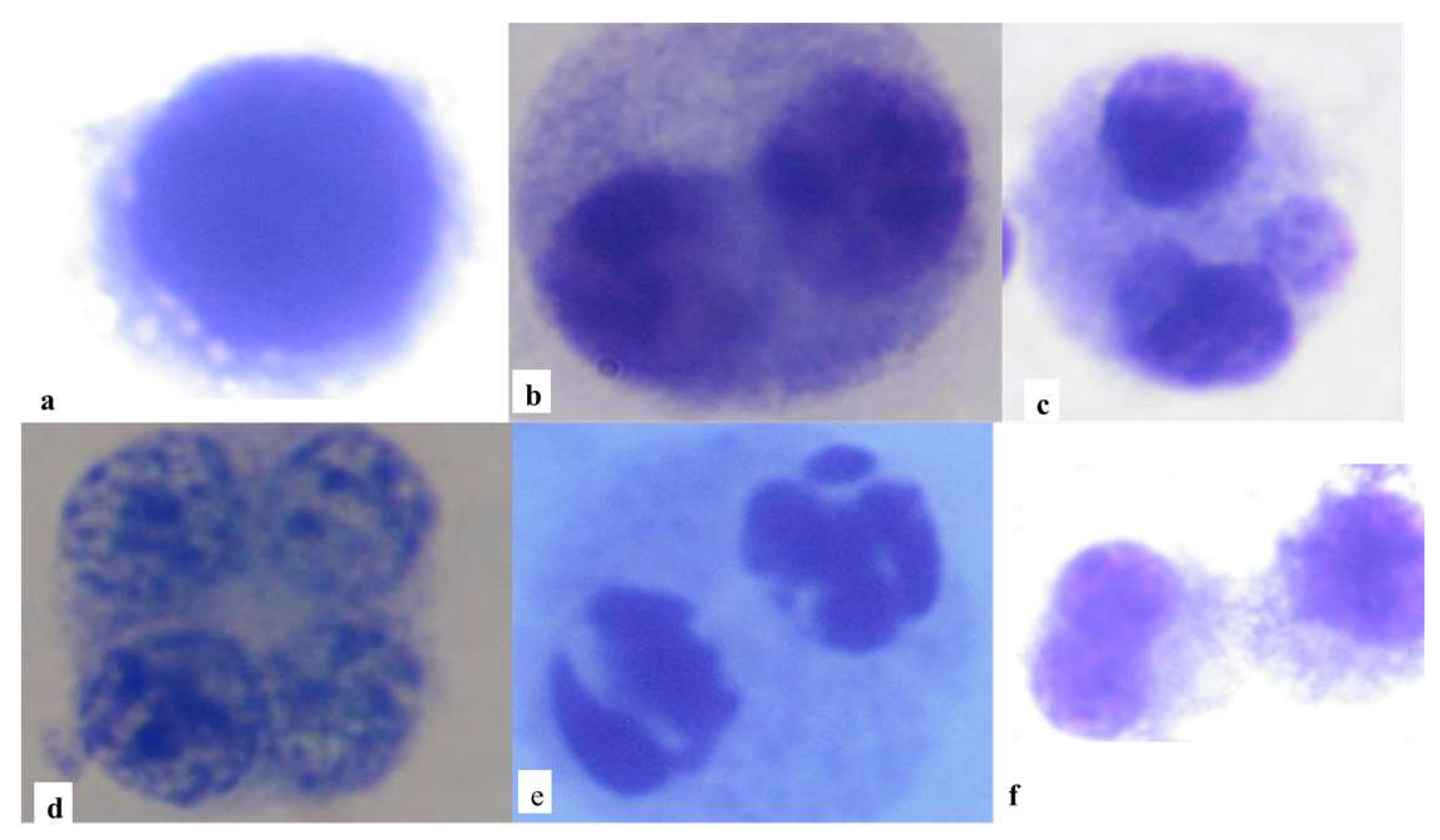
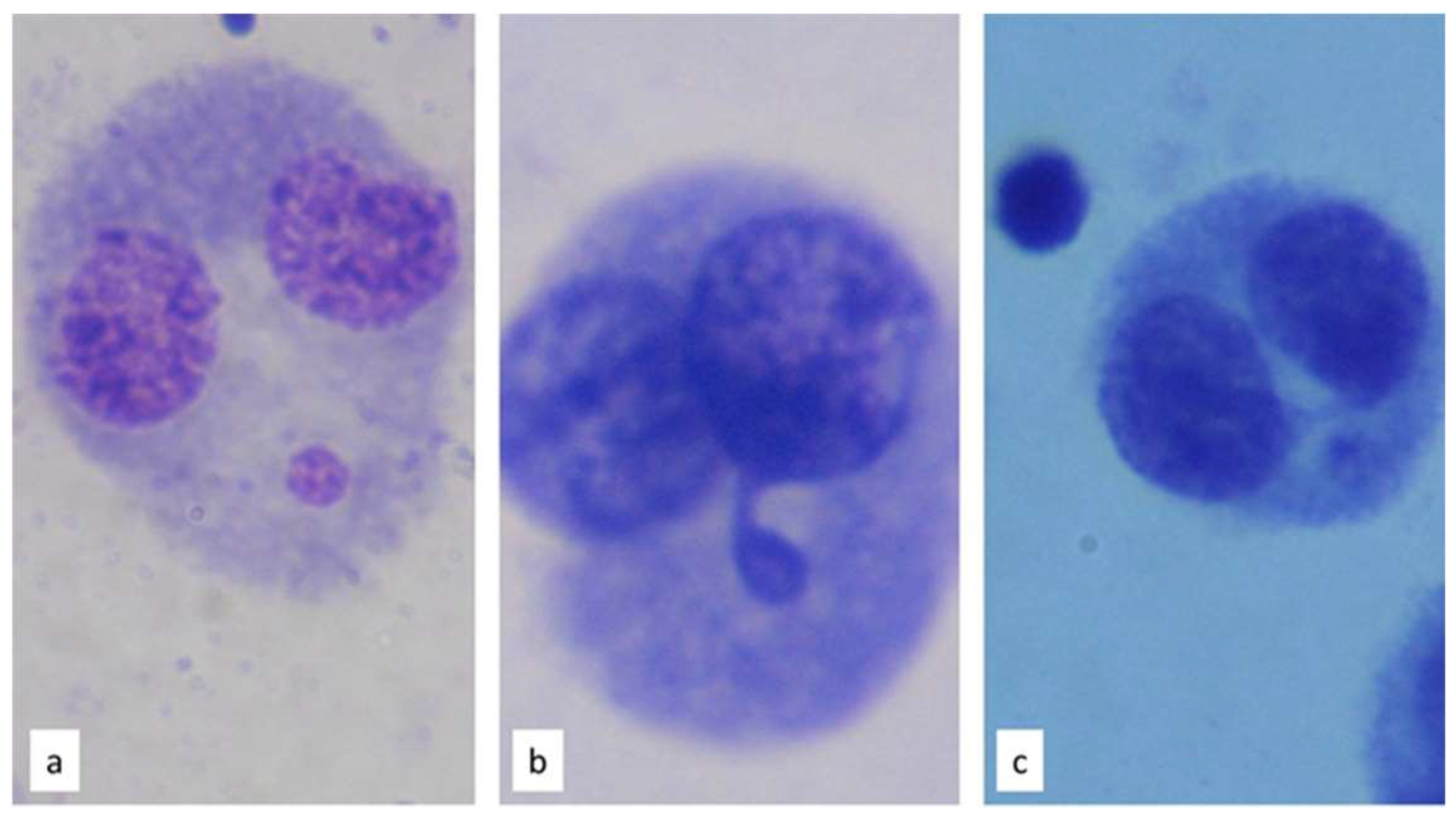
2.6. L-CBMN Assay
2.7. Slide Analysis
2.8. Statistical Analysis
3. Results
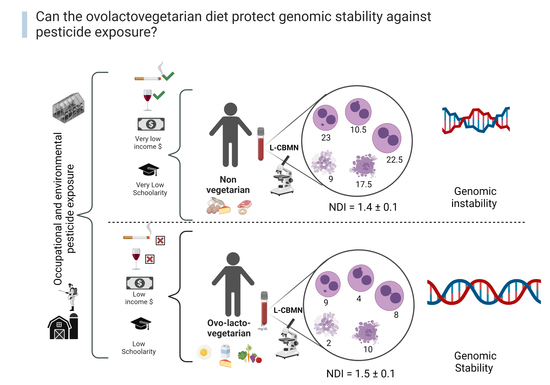
3.1. Socio-Environmental Information
3.2. Cytotoxicity and Genotoxicity
3.3. Cytogenotoxic Damage Associated to Substance Abuse
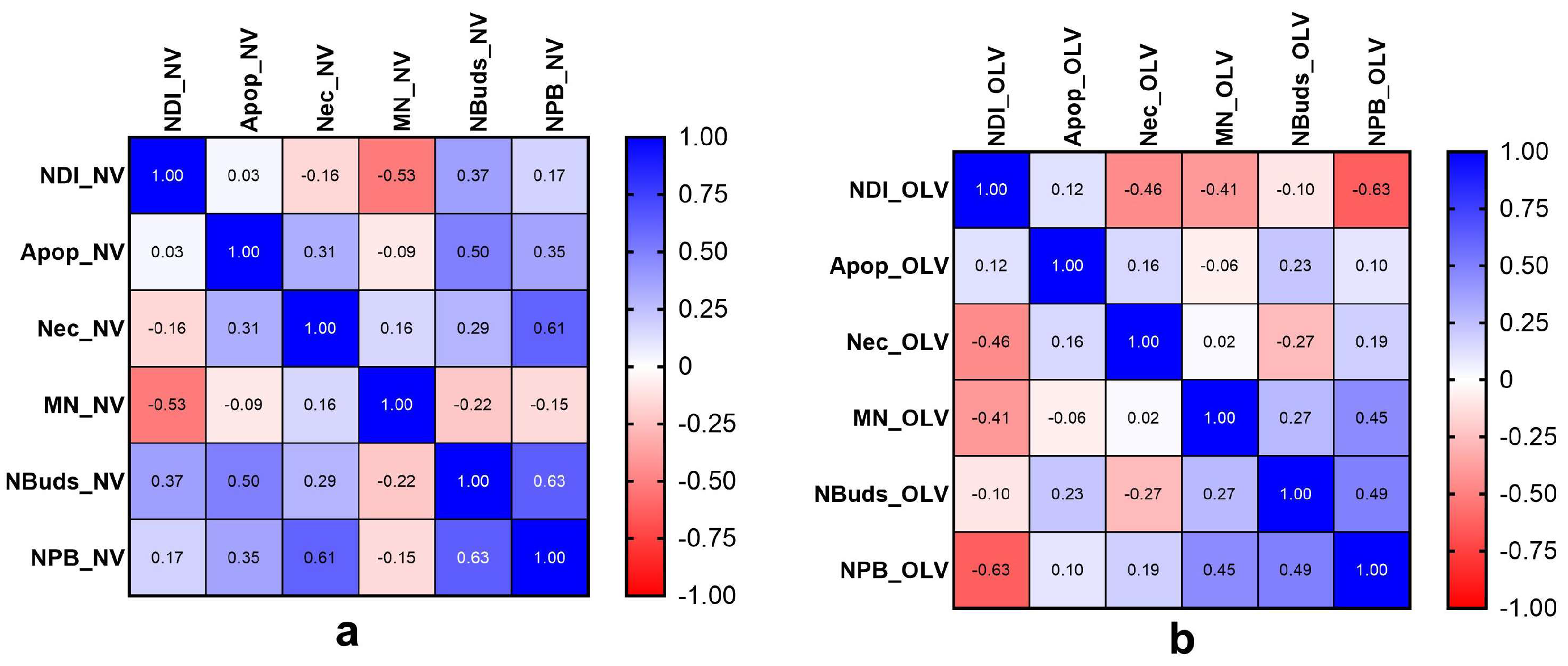
3.4. Correlation
4. Discussion
Effect of the Exposure to Pesticides on Genotoxicity Biomarkers and the CBMN Cell Death Pathway in the Study Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donley, N.; Bullard, R.D.; Economos, J.; Figueroa, I.; Lee, J.; Liebman, A.K.; Martinez, D.N.; Shafiei, F. Pesticides and Environmental Injustice in the USA: Root Causes, Current Regulatory Reinforcement and a Path Forward. BMC Public Health 2022, 22, 708. [Google Scholar] [CrossRef]
- Molina-Guzmán, L.P.; Ríos-Osorio, L.A. Occupational Health and Safety in Agriculture. A Systematic Review. Rev. Fac. Med. 2020, 68, 625–638. [Google Scholar] [CrossRef]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The Global Distribution of Acute Unintentional Pesticide Poisoning: Estimations Based on a Systematic Review. BMC Public Health 2020, 20, 1875. [Google Scholar] [CrossRef]
- Vera-Herrera, L.; Sadutto, D.; Picó, Y. Non-Occupational Exposure to Pesticides: Experimental Approaches and Analytical Techniques (from 2019). Molecules 2021, 26, 3688. [Google Scholar] [CrossRef]
- Ortiz-Uribe, N.; Salomón-Torres, R.; Krueger, R. Date Palm Status and Perspective in Mexico. Agriculture 2019, 9, 46. [Google Scholar] [CrossRef]
- Velasco López, J.L.; Soto Ortiz, R.; Ail Catzim, C.E.; Grimaldo Juárez, O.; Avilés Marín, S.M.; Lozano del Río, A.J. Biomass and Grain Yield in Triticale Varieties in the Mexicali Valley. Rev. Mex. Cienc. Agríc. 2020, 11, 1097–1109. [Google Scholar] [CrossRef]
- Castañeda-Yslas, I.J.; Arellano-García, M.E.; García-Zarate, M.A.; Ruíz-Ruíz, B.; Zavala-Cerna, M.G.; Torres-Bugarín, O. Biomonitoring with Micronuclei Test in Buccal Cells of Female Farmers and Children Exposed to Pesticides of Maneadero Agricultural Valley, Baja California, Mexico. J. Toxicol. 2016, 2016, 7934257. [Google Scholar] [CrossRef] [PubMed]
- Lazalde-Ramos, B.P.; Zamora-Pérez, A.L.; Sosa-Macías, M.; Galaviz-Hernández, C.; Zúñiga-González, G.M. Micronuclei and Nuclear Anomalies in Mexico’s Indigenous Population. Salud Publ. Mex. 2017, 59, 532–539. [Google Scholar] [CrossRef]
- Instituto Nacional de los Pueblos Indigenas. Atlas de Los Pueblos Indígenas de México. Available online: https://atlas.inpi.gob.mx/cucapa-etnografia/ (accessed on 2 August 2023).
- Linares, A. “Dying of Thirst”: The Cucapá in Mexico Fight against Climate Change and Oblivion. Available online: https://www.nbcnews.com/news/latino/dying-thirst-cucap-mexico-fight-against-climate-change-oblivion-n1270806 (accessed on 4 July 2023).
- Melton, J.G.; Smylie, J.H. Adventist Encyclopedia Britannica 2022. Available online: https://www.britannica.com/topic/Adventism (accessed on 31 August 2023).
- Gashugi, L.; Oh, J.; Mashchak, A.; Fraser, G. Lifestyle-Related Behavior and Self-Reported Health Status Among Seventh-Day Adventists. Am. J. Lifestyle Med. 2023, 1–13. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Kirwan, R.; Lamuela-Raventós, R.M.; Estruch, R. Dietary Patterns and the Risk of Obesity, Type 2 Diabetes Mellitus, Cardiovascular Diseases, Asthma, and Neurodegenerative Diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Fan, J.; Sveen, L.; Bennett, H.; Knutsen, S.F.; Beeson, W.L.; Jaceldo-Siegl, K.; Butler, T.L.; et al. Vegetarian Dietary Patterns and the Risk of Colorectal Cancers. JAMA Intern. Med. 2015, 175, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian Diets and Incidence of Diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef]
- Wulf, H.C.; Iversen, A.S.; Husum, B.; Niebuhr, E. Very Low Sister-Chromatid Exchange Rate in Seventh-Day Adventists. Mutat. Res. 1986, 162, 131–135. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Jakaša, I.; Peremin, I.; Domijan, A.M.; Vučić Lovrenčić, M.; Kežić, S.; Bituh, M.; Moraes de Andrade, V. Inflammatory, Oxidative and DNA Damage Status in Vegetarians: Is the Future of Human Diet Green? Crit. Rev. Food Sci. Nutr. 2021, 63, 3189–3221. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health Effects of Vegan Diets. Am. J. Clin. Nutr. 2009, 89, 3–9. [Google Scholar] [CrossRef]
- Cumpstey, A.F.; Clark, A.D.; Santolini, J.; Jackson, A.A.; Feelisch, M. COVID-19: A Redox Disease—What a Stress Pandemic Can Teach Us about Resilience and What We May Learn from the Reactive Species Interactome about Its Treatment. Antioxid. Redox Signal. 2021, 35, 1226–1268. [Google Scholar] [CrossRef]
- Molenaar, R.J.; Van Noorden, C.J. Type 2 Diabetes and Cancer as Redox Diseases? Lancet 2014, 384, 853. [Google Scholar] [CrossRef]
- Gajski, G.; Matković, K.; Delić, L.; Gerić, M. Evaluation of Primary DNA Damage in Young Healthy Females Based on Their Dietary Preferences. Nutrients 2023, 15, 2218. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Vučić Lovrenčić, M.; Božičević, S.; Rubelj, I.; Nanić, L.; Škrobot Vidaček, N.; Bendix, L.; Peraica, M.; Rašić, D.; et al. Analysis of Health-Related Biomarkers between Vegetarians and Non-Vegetarians: A Multi-Biomarker Approach. J. Funct. Foods 2018, 48, 643–653. [Google Scholar] [CrossRef]
- Oussalah, A.; Levy, J.; Berthezène, C.; Alpers, D.H.; Guéant, J.L. Health Outcomes Associated with Vegetarian Diets: An Umbrella Review of Systematic Reviews and Meta-Analyses. Clin. Nutr. 2020, 39, 3283–3307. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Rinaldi, J. A Comparison of Lymphocyte Micronuclei and Plasma Micronutrients in Vegetarians and Non-Vegetarians. Carcinogenesis 1995, 16, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kažimírová, A.; Barančoková, M.; Krajčovičová-Kudláčková, M.; Volkovová, K.; Staruchová, M.; Valachovičová, M.; Pauková, V.; Blažíček, P.; Wsólová, L.; Dušinská, M. The Relationship between Micronuclei in Human Lymphocytes and Selected Micronutrients in Vegetarians and Non-Vegetarians. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 611, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kotova, N.; Frostne, C.; Abramsson-Zetterberg, L.; Tareke, E.; Bergman, R.; Haghdoost, S.; Paulsson, B.; Törnqvist, M.; Segerbäck, D.; Jenssen, D.; et al. Differences in Micronucleus Frequency and Acrylamide Adduct Levels with Hemoglobin between Vegetarians and Non-Vegetarians. Eur. J. Nutr. 2015, 54, 1181–1190. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Oreščanin, V.; Garaj-Vrhovac, V. Cytokinesis-Block Micronucleus Cytome Assay Parameters in Peripheral Blood Lymphocytes of the General Population: Contribution of Age, Sex, Seasonal Variations and Lifestyle Factors. Ecotoxicol. Environ. Saf. 2018, 148, 561–570. [Google Scholar] [CrossRef]
- Zúñiga-Violante, E.; Walter Daesslé, L.; Lourdes Camarena-Ojinaga, M.; Gutiérrez-Galindo, E.A.; Evarista Arellano-García, M.; Valle Dorado, C.C. Distribution of Organic and Inorganic Pollutants in the Agricultural Valley of Maneadero, Baja California, Mexico. Investig. Ambient. 2015, 7, 13–24. [Google Scholar]
- Nersesyan, A.; Kundi, M.; Fenech, M.; Stopper, H.; da Silva, J.; Bolognesi, C.; Mišík, M.; Knasmueller, S. Recommendations and Quality Criteria for Micronucleus Studies with Humans. Mutat. Res. Rev. Mutat. Res. 2022, 789, 108410. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, B.; Arellano-García, M.E.; Radilla-Chávez, P.; Salas-Vargas, D.S.; Toledano-Magaña, Y.; Casillas-Figueroa, F.; Luna Vazquez-Gomez, R.; Pestryakov, A.; García-Ramos, J.C.; Bogdanchikova, N. Cytokinesis-Block Micronucleus Assay Using Human Lymphocytes as a Sensitive Tool for Cytotoxicity/Genotoxicity Evaluation of AgNPs. ACS Omega 2020, 5, 12005–12015. [Google Scholar] [CrossRef]
- Torres-Bugarín, O.; Arias-Ruiz, L.F. Micronúcleos: Actualización Del Papel En La Inestabilidad Genética, Inflamación, Envejecimiento y Cáncer. Revisión Panorámica. Rev. Bioméd. 2023, 34, 208–223. [Google Scholar] [CrossRef]
- Bolognesi, C. Genotoxicity of Pesticides: A Review of Human Biomonitoring Studies. Mutat. Res. Rev. Mutat. Res. 2003, 543, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, I.C.; da Silva, J.T.; Rohr, P.; van Lengert, A.H.; de Lima, M.A.; Kahl, V.F.S.; da Silva, J.; Reis, R.M.; Silveira, H.C.S. Genomic Instability Evaluation by BMCyt and Telomere Length in Brazilian Family Farmers Exposed to Pesticides. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 878, 503479. [Google Scholar] [CrossRef]
- Rangel Claudio, S.; Marques Simas, J.M.; Flygare Souza, A.C.; Baracho De Alencar, M.D.C.; Yuri Yamauchi, L.; Araki Ribeiro, D. Genomic Instability and Cytotoxicity in Buccal Mucosal Cells of Workers in Banana Farming Evaluated by Micronucleus Test. Anticancer Res. 2019, 39, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Baudry, J.; Rebouillat, P.; Allès, B.; Cravedi, J.P.; Touvier, M.; Hercberg, S.; Lairon, D.; Vidal, R.; Kesse-Guyot, E. Estimated Dietary Exposure to Pesticide Residues Based on Organic and Conventional Data in Omnivores, Pesco-Vegetarians, Vegetarians and Vegans. Food Chem. Toxicol. 2021, 153, 112179. [Google Scholar] [CrossRef] [PubMed]
- Berman, T.; Göen, T.; Novack, L.; Beacher, L.; Grinshpan, L.; Segev, D.; Tordjman, K. Urinary Concentrations of Organophosphate and Carbamate Pesticides in Residents of a Vegetarian Community. Environ. Int. 2016, 96, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kathpal, T.S.; Kumari, B. Monitoring of Pesticide Residues in Vegetarian Diet. Environ. Monit. Assess. 2009, 151, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Van Audenhaege, M.; Héraud, F.; Menard, C.; Bouyrie, J.; Morois, S.; Calamassi-Tran, G.; Lesterle, S.; Volatier, J.L.; Leblanc, J.C. Impact of Food Consumption Habits on the Pesticide Dietary Intake: Comparison between a French Vegetarian and the General Population. Food Addit. Contam.—Part A 2009, 26, 1372–1388. [Google Scholar] [CrossRef]
- Phillips, F. Vegetarian Nutrition. Nutr. Bull. 2005, 30, 132–167. [Google Scholar] [CrossRef]
- Pribis, P.; Pencak, R.C.; Grajales, T. Beliefs and Attitudes toward Vegetarian Lifestyle across Generations. Nutrients 2010, 2, 523–531. [Google Scholar] [CrossRef]
- Fraser, G.; Katuli, S.; Anousheh, R.; Knutsen, S.; Herring, P.; Fan, J. Vegetarian Diets and Cardiovascular Risk Factors in Black Members of the Adventist Health Study-2. Public Health Nutr. 2015, 18, 537–545. [Google Scholar] [CrossRef]
- Le, L.T.; Sabaté, J. Beyond Meatless, the Health Effects of Vegan Diets: Findings from the Adventist Cohorts. Nutrients 2014, 6, 2131–2147. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian Dietary Patterns and Mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Valdez Salas, B.; Garcia Duran, E.; Cobo Rivera, J.M.; López Badilla, G. Impact of Plaguicides on the Health of the Inhabitants of the Mexicali Valley, Mexico. Rev. Ecol. Lat. Am. 2000, 6, 15–21. [Google Scholar]
- Von Glascoe, C.A.; de Camarena Ojinaga, M.L.; Arellano-García, M.E. Riesgos Sociambientales y Salud de La Población Cucapá Del Valle de Mexicali. In El Análisis del Riesgo y Riesgos de Forntera. Aportes Desde las Ciencias Sociales; Pérez Floriano, L.R., Rodríguez Esteves, J.M., Eds.; Colmex: Mexico City, Mexico, 2022; pp. 17–42. [Google Scholar]
- Delgado Ramírez, C.E. Una Aproximación a La Cultura Cucapá. Cuicuilco Rev. Cienc. Antropol. 2017, 69, 265–269. [Google Scholar]
- Fenech, M.; Crott, J.W. Micronuclei, Nucleoplasmic Bridges and Nuclear Buds Induced in Folic Acid Deficient Human Lymphocytes—Evidence for Breakage-Fusion-Bridge Cycles in the Cytokinesis-Block Micronucleus Assay. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2002, 504, 131–136. [Google Scholar] [CrossRef]
- Carrillo-Gómez, C.S.; Molina-Noyola, L.D.; Torres-Bugarín, O. Ácido Fólico: Económico Modulador De La Estabilidad Genómica, Epigenética Y El Cáncer; Deficiencias, Fuentes, Efectos Adversos Por Exceso Y Recomendaciones Gubernamentales. El Resid. 2017, 12, 89–103. [Google Scholar]
- Russo, A.; Degrassi, F. Molecular Cytogenetics of the Micronucleus: Still Surprising. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 836, 36–40. [Google Scholar] [CrossRef]
- Wang, X.; Thomas, P.; Xue, J.; Fenech, M. Folate Deficiency Induces Aneuploidy in Human Lymphocytes in Vitro—Evidence Using Cytokinesis-Blocked Cells and Probes Specific for Chromosomes 17 and 21. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 551, 167–180. [Google Scholar] [CrossRef]
- Ahrendt, S.A.; Chow, J.T.; Yang, S.C.; Wu, L.; Zhang, M.J.; Jen, J.; Sidransky, D. Alcohol Consumption and Cigarette Smoking Increase the Frequency of P53 Mutations in Non-Small Cell Lung Cancer. Cancer Res. 2000, 60, 3155–3159. [Google Scholar]
- Vassoler, T.; Dogenski, L.C.; Sartori, V.K.; Presotto, J.S.; Cardoso, M.Z.; Zandoná, J.; Trentin, M.S.; Linden, M.S.; Palhano, H.S.; Vargas, J.E.; et al. Evaluation of the Genotoxicity of Tobacco and Alcohol in Oral Mucosa Cells: A Pilot Study. J. Contemp. Dent. Pract. 2021, 22, 745–750. [Google Scholar] [CrossRef]
- Zięba, S.; Maciejczyk, M.; Zalewska, A. Ethanol- and Cigarette Smoke-Related Alternations in Oral Redox Homeostasis. Front. Physiol. 2022, 12, 793028. [Google Scholar] [CrossRef]
- Fenech, M.; Crott, J.; Turner, J.; Brown, S. Necrosis, Apoptosis, Cytostasis and DNA Damage in Human Lymphocytes Measured Simultaneously within the Cytokinesis-Block Micronucleus Assay: Description of the Method and Results for Hydrogen Peroxide. Mutagenesis 1999, 14, 605–612. [Google Scholar] [CrossRef]
- Cheong, H.S.J.; Seth, I.; Joiner, M.C.; Tucker, J.D. Relationships among Micronuclei, Nucleoplasmic Bridges and Nuclear Buds within Individual Cells in the Cytokinesis-Block Micronucleus Assay. Mutagenesis 2013, 28, 433–440. [Google Scholar] [CrossRef]
- Lindberg, H.K.; Wang, X.; Järventaus, H.; Falck, G.C.M.; Norppa, H.; Fenech, M. Origin of Nuclear Buds and Micronuclei in Normal and Folate-Deprived Human Lymphocytes. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007, 617, 33–45. [Google Scholar] [CrossRef]
- Zúniga Violante, E.; Arellano García, E.; Camarena Ojinaga, L.; Daesslé Heusser, W.; Von-Glascoe, C.; Leyva Aguilera, J.R.R.B. Daño Genético y Exposición a Plaguicidas En Trabajadores Agrícolas Del Valle de San Quintín, Baja California, México. Rev. Salud Ambient. 2012, 12, 93–101. [Google Scholar]
- Montaño-Soto, T.; Arellano-García, E.; Camarena-Ojinaga, L.; von Glascoe, C.B.R.R. Genotoxic Biomonitoring and Exposure to Pesticides in Women Laborers at Maneadero Valley in Baja California, Mexico. Int. J. Appl. Nat. Sci. 2014, 3, 89–96. [Google Scholar]
- Vazquez Boucard, C.; Lee-Cruz, L.; Mercier, L.; Ramírez Orozco, M.; Serrano Pinto, V.; Anguiano, G.; Cazares, L.; Díaz, D. A Study of DNA Damage in Buccal Cells of Consumers of Well- and/or Tap-Water Using the Comet Assay: Assessment of Occupational Exposure to Genotoxicants. Environ. Mol. Mutagen. 2017, 58, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Anguiano-Vega, G.A.; Cazares-Ramirez, L.H.; Rendon-Von Osten, J.; Santillan-Sidon, A.P.; Vazquez-Boucard, C.G. Risk of Genotoxic Damage in Schoolchildren Exposed to Organochloride Pesticides. Sci. Rep. 2020, 10, 17584. [Google Scholar] [CrossRef] [PubMed]
| Variables | NV n (%) | LOV n (%) |
|---|---|---|
| Total | 14 | 21 |
| Men | 4 (28.5) | 5 (23.8) |
| Woman | 10 (71.5) | 16 (76.1) |
| Average Body Mass Index | ||
| Men | 28.0 (28.5) | 23.9 (23.8) |
| Woman | 29.9 (71.5) | 27.3 (76.1) |
| Scholar status | ||
| Non | 0 | 3 (14.3) |
| Elementary | 6 (42.8) | 2 (9.5) |
| Middle | 6 (42.8) | 3 (14.3) |
| High School | 2 (14.3) | 10 (47.6) |
| University | 0 | 2 (9.5) |
| Average Income * | 110 | 350 |
| No tobacco, no alcohol | 5 (29) | 15 (71.4) |
| Tobacco | 2 (12) | 0 |
| Tobacco and alcohol | 10 (59) | 0 |
| Diabetics | 0 | 1 (4.7) |
| Familiar antecedents | 14 (100) | 12 (57.1) |
| Fish and seafood intake | ||
| Little or never | 5 (35) | 19 (90.5) |
| Regularly/almost daily | 9 (65) | 2 (9.5) |
| Fruits and vegetable intake | ||
| Little or never | 6 (42.8) | 3 (14.3) |
| Regularly/almost daily | 8 (57.1) | 18 (85.7) |
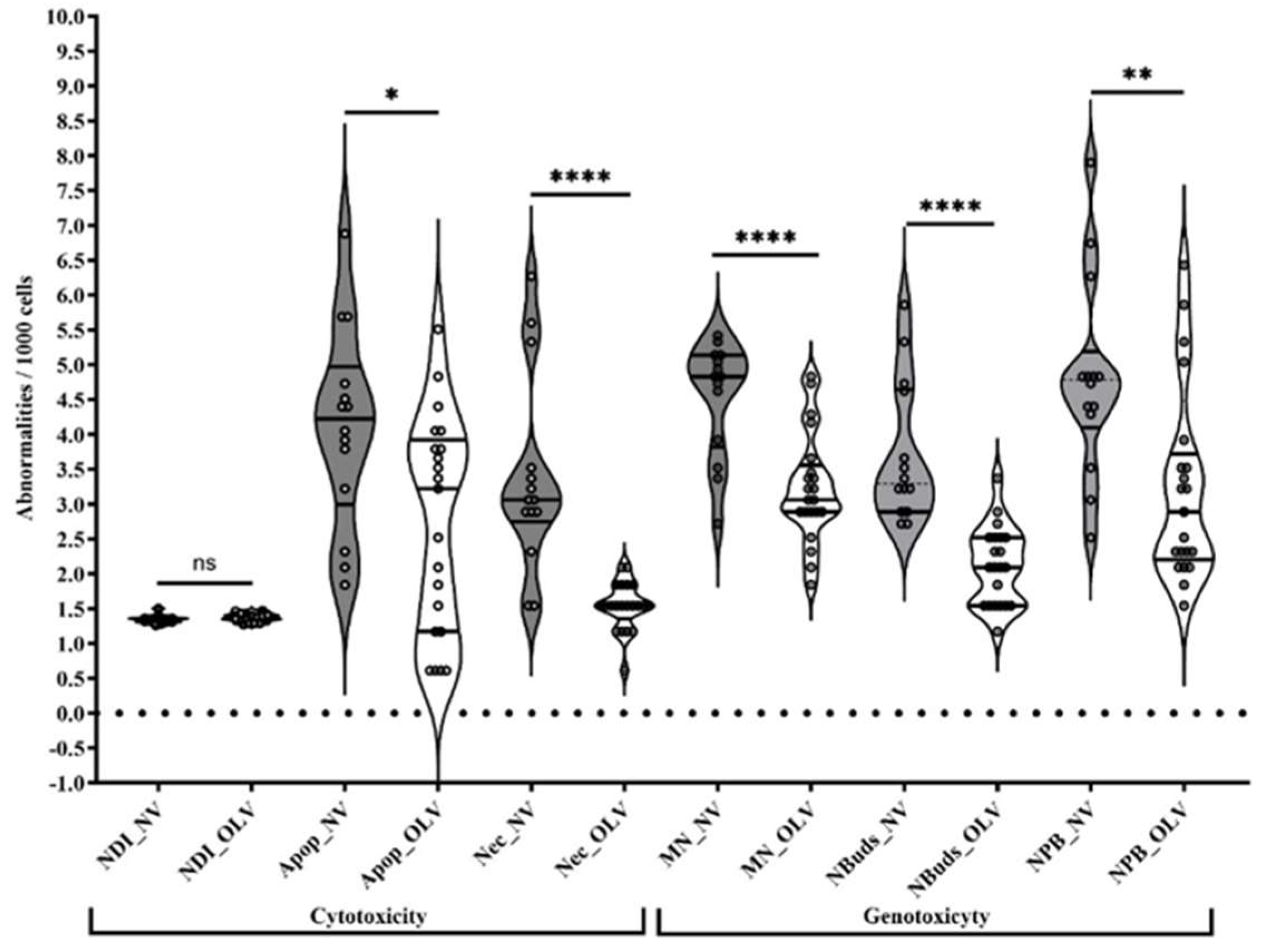
| Biomarker | Group | Median | Mean ± s | p Value | Significance | ||
|---|---|---|---|---|---|---|---|
| Cytotoxicity | NDI | NV | 1.4 | 1.4 ± 0.1 | 0.1112 | NS | |
| LOV | 1.5 | 1.5 ± 0.1 | |||||
| Reference value | 1.5 ± 0.003 | ||||||
| Cellular dead | Apoptosis | NV | 17.5 | 18.4 ± 12.2 | 0.019 | * | |
| LOV | 11 | 9.2 ± 8.62 | |||||
| Reference value | 5.5 ± 2.1 | ||||||
| Necrosis | NV | 9 | 13.0 ± 11.2 | <0.0001 | **** | ||
| LOV | 2 | 2.1 ± 1.0 | |||||
| Reference value | 0 | ||||||
| Genotoxicity | MN | NV | 23 | 20.8 ± 6.8 | <0.0001 | **** | |
| LOV | 9 | 10.6 ± 5.3 | |||||
| Reference value | 4.5 ± 0.7 | ||||||
| NBUDs | NV | 10.5 | 14.3 ± 8.5 | <0.0001 | **** | ||
| LOV | 4 | 4.3 ± 2.4 | |||||
| Reference value | 7 ± 0 | ||||||
| NPBs | NV | 22.5 | 24.5 ± 14.9 | 0.0027 | ** | ||
| LOV | 8 | 11.8 ± 10.9 | |||||
| Reference value | 2.5 ± 0.7 | ||||||
| Biomarker | No Smoke, No Alcohol (n = 5) | Smoke but Not Alcohol (n = 2) | Smoke and Alcohol (n = 6) |
|---|---|---|---|
| NDI | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 |
| Apoptosis | 15 ± 18 | 51 ± 44 *** | 18 ± 6 ** |
| Necrosis | 6.9 ± 8.3 | 20 ± 3 | 15 ± 11 |
| MN | 21 ± 5 | 16 ± 13 | 21 ± 6 |
| NBUDs | 12 ± 9 | 21 ± 18 | 12 ± 4 |
| NPBs | 13 ± 7 | 41 ± 30 ** | 23 ± 7 |
| Town | Tissue | Group | n | MN | NBUDs | NPB | Ref |
|---|---|---|---|---|---|---|---|
| San Quintin | Lymphocytes | NE | 15 | 11 | 8 | 4 | [59] |
| E | 25 | 18 | 11 | 8 | |||
| p | <0.05 | NS | <0.05 | ||||
| Maneadero | Lymphocytes | NE | 22 | 7.86 | 6.45 | 2.80 | [60,61] |
| E | 26 | 10.15 | 7.94 | 2.38 | |||
| p | <0.001 | 0.03 | NS | ||||
| Buccal mucosa | NE | 73 | 0.7 | [7] | |||
| E | 71 | 4.5 | |||||
| p | <0.0001 | - | - | ||||
| Todos Santos | Comet assay (buccal cells) | NE | 24 | 24.51 | - | - | [61] |
| E | 57 | 46.92 | - | - | |||
| p | <0.0001 | ||||||
| Buccal mucosa | NE | 24 | 58.3 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Ruiz, B.; Torres-Bugarin, O.; Zúñiga-Violante, E.; Casillas-Figueroa, F.; Luna-Vázquez-Gómez, R.; Campos Gallegos, V.; Ruiz-Arellano, A.E.; Arellano-García, M.E. Genomic Instability and Cytotoxicity Evaluation of Two Communities Exposed to Pesticides in the Mexicali Valley by the L-CBMN Assay. Toxics 2023, 11, 807. https://doi.org/10.3390/toxics11100807
Ruiz-Ruiz B, Torres-Bugarin O, Zúñiga-Violante E, Casillas-Figueroa F, Luna-Vázquez-Gómez R, Campos Gallegos V, Ruiz-Arellano AE, Arellano-García ME. Genomic Instability and Cytotoxicity Evaluation of Two Communities Exposed to Pesticides in the Mexicali Valley by the L-CBMN Assay. Toxics. 2023; 11(10):807. https://doi.org/10.3390/toxics11100807
Chicago/Turabian StyleRuiz-Ruiz, Balam, Olivia Torres-Bugarin, Erika Zúñiga-Violante, Francisco Casillas-Figueroa, Roberto Luna-Vázquez-Gómez, Verónica Campos Gallegos, Ana Erika Ruiz-Arellano, and María Evarista Arellano-García. 2023. "Genomic Instability and Cytotoxicity Evaluation of Two Communities Exposed to Pesticides in the Mexicali Valley by the L-CBMN Assay" Toxics 11, no. 10: 807. https://doi.org/10.3390/toxics11100807
APA StyleRuiz-Ruiz, B., Torres-Bugarin, O., Zúñiga-Violante, E., Casillas-Figueroa, F., Luna-Vázquez-Gómez, R., Campos Gallegos, V., Ruiz-Arellano, A. E., & Arellano-García, M. E. (2023). Genomic Instability and Cytotoxicity Evaluation of Two Communities Exposed to Pesticides in the Mexicali Valley by the L-CBMN Assay. Toxics, 11(10), 807. https://doi.org/10.3390/toxics11100807