Determination of the Oxidative Stress Biomarkers of 8-Hydroxydeoxyguanosine and Dityrosine in the Gills, Skin, Dorsal Fin, and Liver Tissue of Atlantic Salmon (Salmo salar) Parr
Abstract
:1. Introduction
2. Methods and Materials
2.1. Chemicals and Materials
2.2. Sample Collection
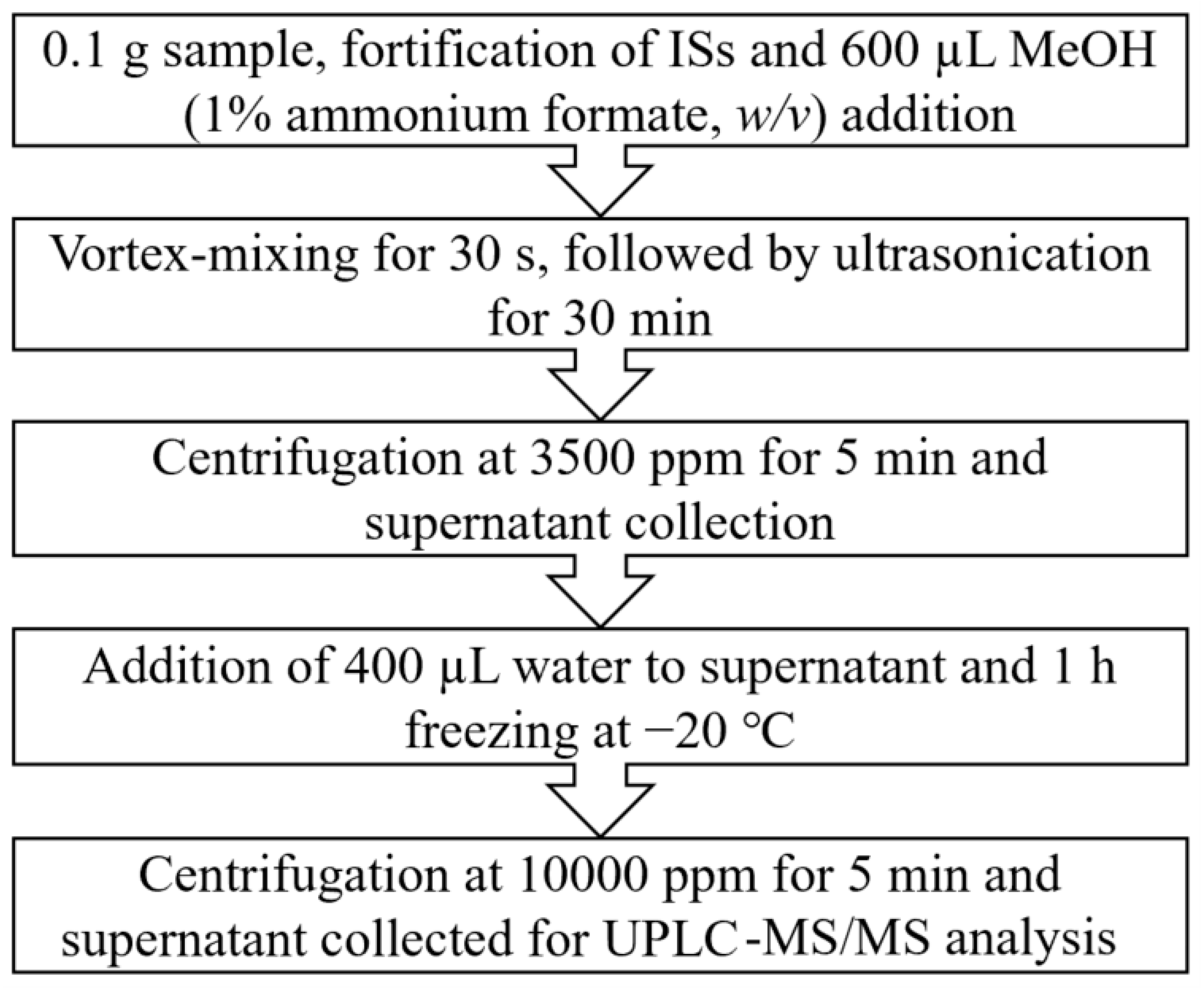
2.3. Sample Preparation
2.4. Instrumental Analysis
2.5. Method Validation
2.6. Data Analysis
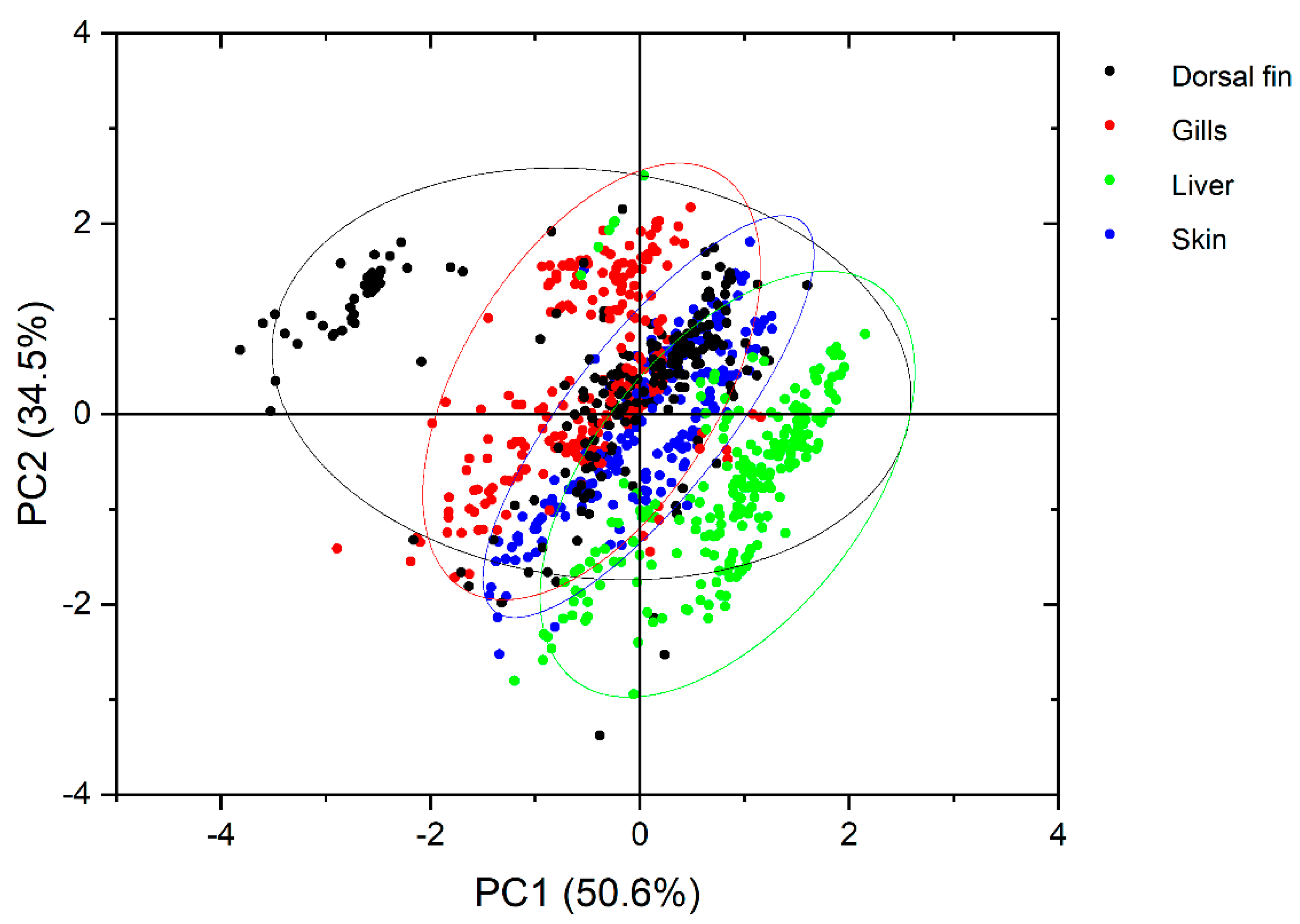
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Malencik, D.A.; Anderson, S.R. Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids 2003, 25, 233–247. [Google Scholar] [CrossRef] [PubMed]
- García-Caparrós, P.; de Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2020, 87, 421–466. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Saikia, S.K.; Chowdhury, S. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar]
- Donaghy, L.; Hong, H.K.; Jauzein, C.; Choi, K.S. The known and unknown sources of reactive oxygen and nitrogen species in haemocytes of marine bivalve molluscs. Fish Shellfish Immunol. 2015, 42, 91–97. [Google Scholar] [CrossRef]
- Tacchi, L.; Secombes, C.J.; Bickerdike, R.; Adler, M.A.; Venegas, C.; Takle, H.; Martin, S.A.M. Transcriptomic and physiological responses to fishmeal substitution with plant proteins in formulated feed in farmed Atlantic salmon (Salmo salar). BMC Genom. 2012, 13, 363. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar]
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals 2020, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, B.; Witeska, M. Blood biomarkers of herbicide, insecticide, and fungicide toxicity to fish—A review. Environ. Sci. Pollut. Res. 2020, 27, 19236–19250. [Google Scholar] [CrossRef] [PubMed]
- Oğuz, E.K.; Arihan, O.; Oğuz, A.R. Oxidative and genotoxic effects of bisphenol A on primary gill cell culture of Lake Van Fish (Alburnus tarichi Güldenstädt, 1814). Chem. Ecol. 2018, 34, 914–924. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Immune, inflammatory, autophagic and DNA damage responses to long-term H2O2 exposure in different tissues of common carp (Cyprinus carpio). Sci. Total Environ. 2021, 757, 143831. [Google Scholar] [CrossRef]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. REVIEWS Oxidative stress in fish induced by pesticides. Neuroendocrinol. Lett. 2009, 30, 2. [Google Scholar]
- Martinez, M.P.; Kannan, K. Simultaneous Analysis of Seven Biomarkers of Oxidative Damage to Lipids, Proteins, and DNA in Urine. Environ. Sci. Technol. 2018, 52, 6647–6655. [Google Scholar] [CrossRef]
- Giulivi, C.; Traaseth, N.; Davies, K. Tyrosine oxidation products: Analysis and biological relevance. Amino Acids 2003, 25, 227–232. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Perez, K.A.; Pike, K.E.; Kok, W.M.; Rowe, C.C.; White, A.R.; Bourgeat, P.; Salvado, O.; Bedo, J.; Hutton, C.A. Blood-borne amyloid-β dimer correlates with clinical markers of Alzheimer’s disease. J. Neurosci. 2010, 30, 6315–6322. [Google Scholar] [CrossRef]
- Al-Hilaly, Y.K.; Williams, T.L.; Stewart-Parker, M.; Ford, L.; Skaria, E.; Cole, M.; Bucher, W.G.; Morris, K.L.; Sada, A.A.; Thorpe, J.R. A central role for dityrosine crosslinking of Amyloid-β in Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 83. [Google Scholar] [CrossRef]
- Andries, A.; Rozenski, J.; Vermeersch, P.; Mekahli, D.; van Schepdael, A. Recent progress in the LC-MS/MS analysis of oxidative stress biomarkers. Electrophoresis 2021, 42, 402–428. [Google Scholar] [CrossRef]
- Wu, D.; Liu, B.; Yin, J.; Xu, T.; Zhao, S.; Xu, Q.; Chen, X.; Wang, H. Detection of 8-hydroxydeoxyguanosine (8-OHdG) as a biomarker of oxidative damage in peripheral leukocyte DNA by UHPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1064, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gyimah, E.; Dong, X.; Qiu, W.; Zhang, Z.; Xu, H. Sublethal concentrations of triclosan elicited oxidative stress, DNA damage, and histological alterations in the liver and brain of adult zebrafish. Environ. Sci. Pollut. Res. 2020, 27, 17329–17338. [Google Scholar] [CrossRef] [PubMed]
- Alak, G.; Yeltekin, A.C.; Tas, I.H.; Ucar, A.; Parlak, V.; Topal, A.; Kocaman, E.M.; Atamanalp, M. Investigation of 8-OHdG, CYP1A, HSP70 and transcriptional analyses of antioxidant defence system in liver tissues of rainbow trout exposed to eprinomectin. Fish Shellfish Immunol. 2017, 65, 136–144. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, T.; Giulivi, C. Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrom. Rev. 2007, 26, 108–120. [Google Scholar] [CrossRef]
- Mota, V.C.; Striberny, A.; Verstege, G.C.; Difford, G.F.; Lazado, C.C. Evaluation of a recirculating aquaculture system research facility designed to address current knowledge needs in Atlantic salmon production. Front. Anim. Sci. 2022, 3, 876504. [Google Scholar] [CrossRef]
- Lazado, C.C.; Pedersen, L.-F.; Kirste, K.H.; Soleng, M.; Breiland, M.W.; Timmerhaus, G. Oxidant-induced modifications in the mucosal transcriptome and circulating metabolome of Atlantic salmon. Aquat. Toxicol. 2020, 227, 105625. [Google Scholar] [CrossRef]
- Lazado, C.C.; Sveen, L.R.; Soleng, M.; Pedersen, L.-F.; Timmerhaus, G. Crowding reshapes the mucosal but not the systemic response repertoires of Atlantic salmon to peracetic acid. Aquaculture 2021, 531, 735830. [Google Scholar] [CrossRef]
- Lazado, C.C.; Timmerhaus, G.; Breiland, M.W.; Pittman, K.; Hytterød, S. Multiomics Provide Insights into the Key Molecules and Pathways Involved in the Physiological Adaptation of Atlantic Salmon (Salmo salar) to Chemotherapeutic-Induced Oxidative Stress. Antioxidants 2021, 10, 1931. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Vikeså, V.; Lie, K.K.; Hevrøy, E.M. Transcriptional responses to temperature and low oxygen stress in Atlantic salmon studied with next-generation sequencing technology. BMC Genom. 2013, 14, 817. [Google Scholar] [CrossRef]
- Lazado, C.C.; Stiller, K.T.; Reiten, B.-K.M.; Osório, J.; Kolarevic, J.; Johansen, L.-H. Consequences of continuous ozonation on the health and welfare of Atlantic salmon post-smolts in a brackish water recirculating aquaculture system. Aquat. Toxicol. 2021, 238, 105935. [Google Scholar] [CrossRef]
- Guragain, P.; Tkachov, M.; Båtnes, A.S.; Olsen, Y.; Winge, P.; Bones, A.M. Principles and Methods of Counteracting Harmful Salmon–Arthropod Interactions in Salmon Farming: Addressing Possibilities, Limitations, and Future Options. Front. Mar. Sci. 2021, 8, 701793. [Google Scholar] [CrossRef]
- Soleng, M.; Johansen, L.H.; Johnsen, H.; Johansson, G.S.; Breiland, M.W.; Rormark, L.; Pittman, K.; Pedersen, L.F.; Lazado, C.C. Atlantic salmon (Salmo salar) mounts systemic and mucosal stress responses to peracetic acid. Fish Shellfish Immunol. 2019, 93, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.; Torstensen, B.; Hemre, G.I.; Sanden, M.; Waagbø, R. Hepatic oxidative stress in Atlantic salmon (Salmo salar L.) transferred from a diet based on marine feed ingredients to a diet based on plant ingredients. Aquac. Nutr. 2011, 17, e424–e436. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Thomaidis, N.S.; Bisphenol, A. 4-t-octylphenol, and 4-nonylphenol determination in serum by Hybrid Solid Phase Extraction-Precipitation Technology technique tailored to liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 986–987, 85–93. [Google Scholar] [CrossRef]
- 8-Hydroxy-Deoxyguanosine, ChemSpider. Available online: http://www.chemspider.com/Chemical-Structure.66049.html?rid=e4f3885d-112f-4a81-a733-af6fe51bae9e (accessed on 27 July 2022).
- LL-Dityrosine, ChemSpider. Available online: http://www.chemspider.com/Chemical-Structure.21865767.html?rid=0fffe845-493e-4af7-8c46-3a05ba267460&page_num=0 (accessed on 27 July 2022).
- Ahmad, S.; Kalra, H.; Gupta, A.; Raut, B.; Hussain, A.; Rahman, M.A. HybridSPE: A novel technique to reduce phospholipid-based matrix effect in LC–ESI-MS Bioanalysis. J. Pharm. Bioallied Sci. 2012, 4, 267. [Google Scholar] [PubMed]
- Trimmel, S.; Vike-Jonas, K.; Gonzalez, S.V.; Ciesielski, T.M.; Lindstrøm, U.; Jenssen, B.M.; Asimakopoulos, A.G. Rapid Determination of Per-and Polyfluoroalkyl Substances (PFAS) in Harbour Porpoise Liver Tissue by HybridSPE®–UPLC®–MS/MS. Toxics 2021, 9, 183. [Google Scholar] [CrossRef]
- Vike-Jonas, K.; Gonzalez, S.V.; Mortensen, A.K.; Ciesielski, T.M.; Farkas, J.; Venkatraman, V.; Pastukhov, M.V.; Jenssen, B.M.; Asimakopoulos, A.G. Rapid determination of thyroid hormones in blood plasma from Glaucous gulls and Baikal seals by HybridSPE(R)-LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1162, 122447. [Google Scholar] [CrossRef]
- Aurand, C.; Mi, C.; Lu, X.; Trinh, A.; Ye, M. Troubleshooting analyte recovery when using HybridSPE-Precipitation Technology. Supelco Report. 2009, 27, 17–19. [Google Scholar]
- Rubensam, G.; Barreto, F.; Hoff, R.B.; Kist, T.L.; Pizzolato, T.M. A liquid-liquid extraction procedure followed by a low temperature purification step for the analysis of macrocyclic lactones in milk by liquid chromatography-tandem mass spectrometry and fluorescence detection. Anal. Chim. Acta 2011, 705, 24–29. [Google Scholar] [CrossRef]
- Orhan, H.; van Holland, B.; Krab, B.; Moeken, J.; Vermeulen, N.P.; Hollander, P.; Meerman, J.H. Evaluation of a multi-parameter biomarker set for oxidative damage in man: Increased urinary excretion of lipid, protein and DNA oxidation products after one hour of exercise. Free Radic. Res. 2004, 38, 1269–1279. [Google Scholar] [CrossRef]
- Wu, C.; Chen, S.T.; Peng, K.H.; Cheng, T.J.; Wu, K.Y. Concurrent quantification of multiple biomarkers indicative of oxidative stress status using liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2016, 512, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Martinez-Moral, M.-P.; Al-Malki, A.L.; Al-Ghamdi, M.A.; Al-Bazi, M.M.; Kumosani, T.A.; Kannan, K. Mediation analysis for the relationship between urinary phthalate metabolites and type 2 diabetes via oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Int. 2019, 126, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moral, M.P.; Kannan, K. How stable is oxidative stress level? An observational study of intra- and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environ. Int. 2019, 123, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Jia, R.; He, Q.; Cao, L.; Du, J.; Jeney, G.; Xu, P.; Yin, G. Oxidative stress, ion concentration change and immune response in gills of common carp (Cyprinus carpio) under long-term exposure to bisphenol A. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 230, 108711. [Google Scholar] [CrossRef]
- Pfefferli, C.; Jazwinska, A. The art of fin regeneration in zebrafish. Regeneration 2015, 2, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Osório, J.; Stiller, K.T.; Reiten, B.-K.; Kolarevic, J.; Johansen, L.-H.; Afonso, F.; Lazado, C.C. Intermittent administration of peracetic acid is a mild environmental stressor that elicits mucosal and systemic adaptive responses from Atlantic salmon post-smolts. BMC Zool. 2022, 7, 1. [Google Scholar] [CrossRef]
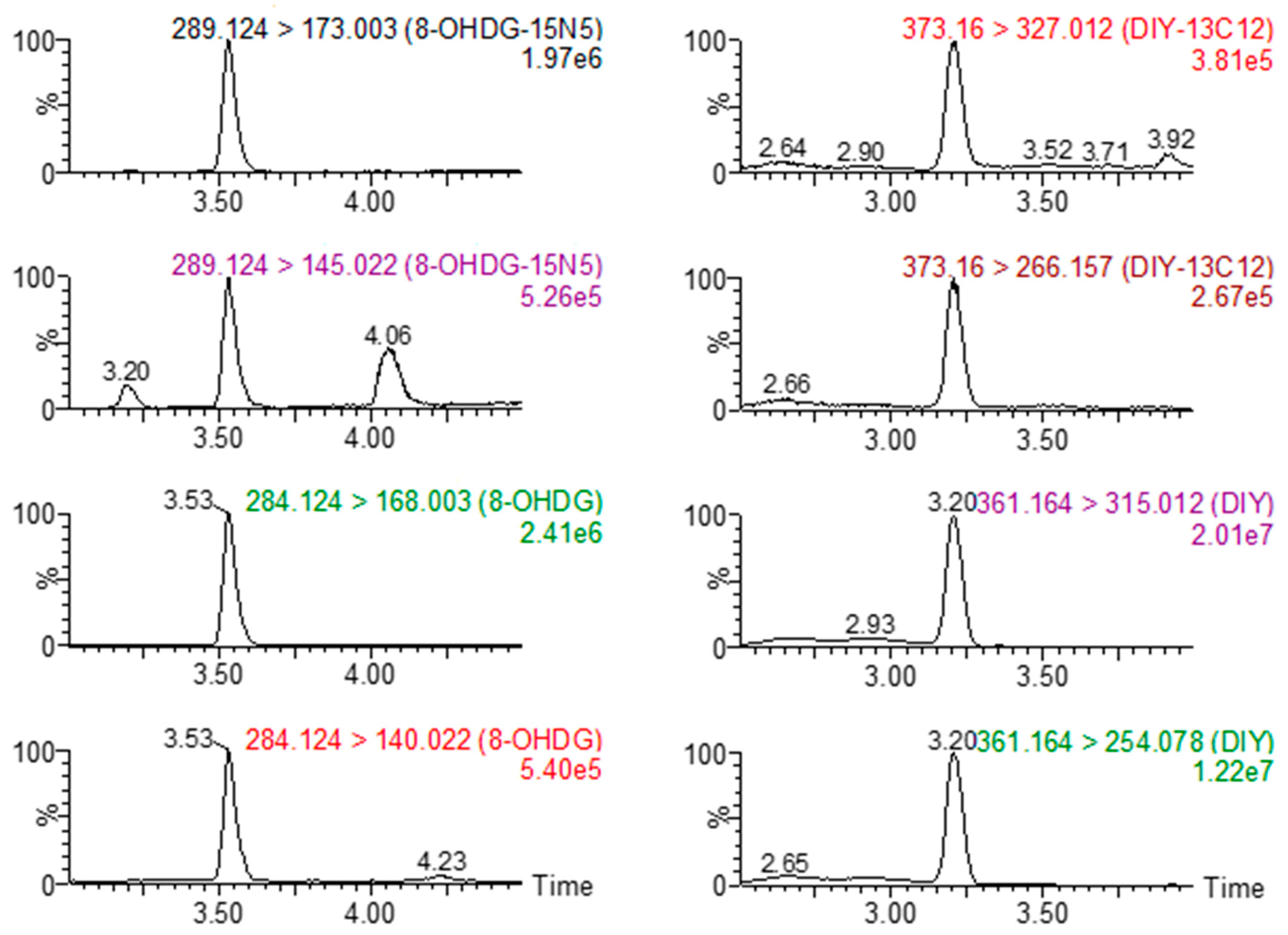
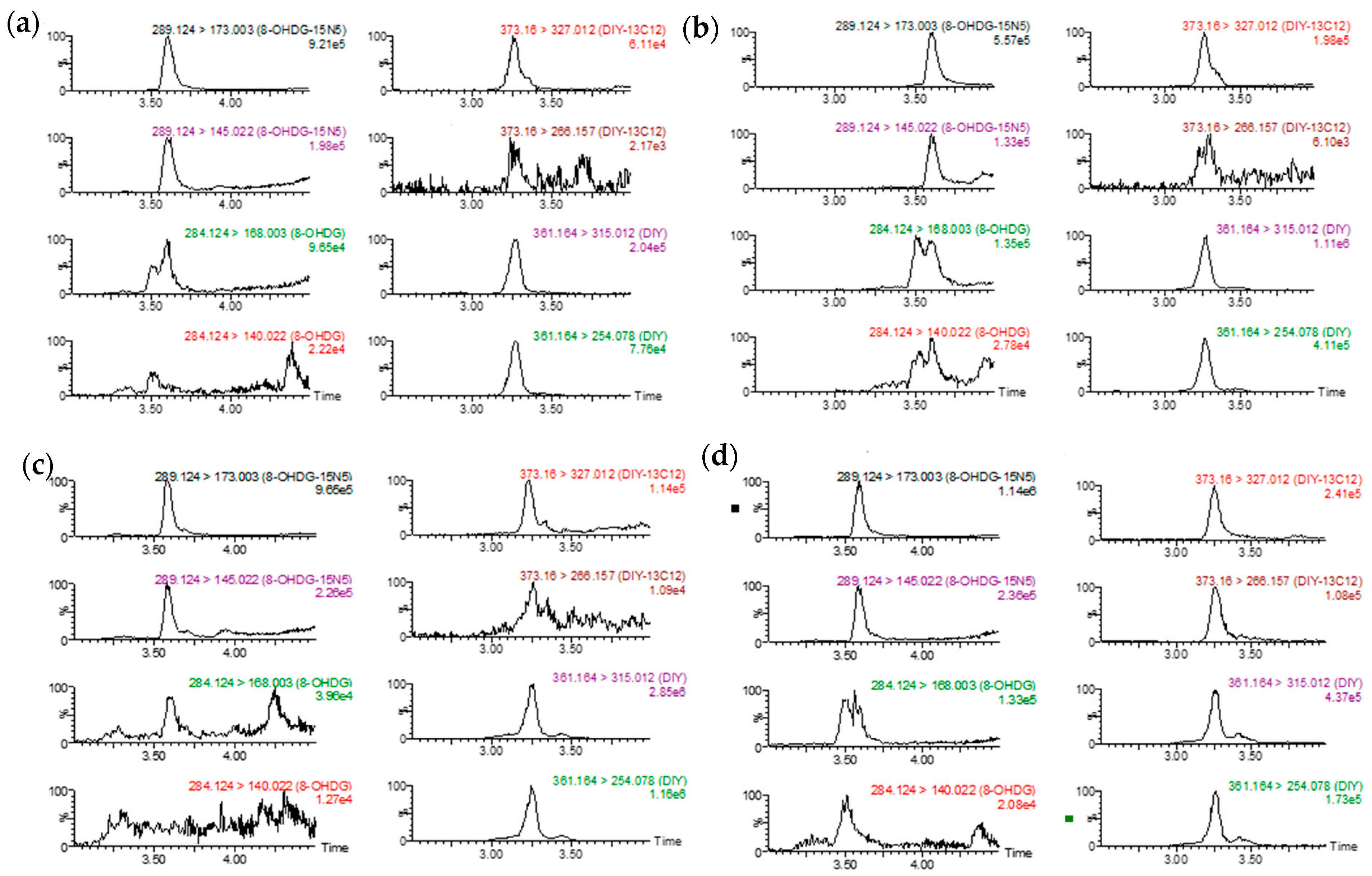
| Compound | Retention Time | Precursor Ion | Quantification/Confirmation | Cone Voltage (V) | Collision Energy (ev) |
|---|---|---|---|---|---|
| 8OHDG | 3.53 | 284.1 | 140.0/168.0 | 22 | 30/12 |
| DIY | 3.20 | 361.1 | 315.0/254.0 | 18 | 24/22 |
| 15N5-8OHDG | 3.53 | 289.1 | 145.0/173.0 | 22 | 30/12 |
| 13C12-DIY | 3.20 | 373.1 | 327.0/266.1 | 18 | 24/22 |
| Calibration Range | r2 | LOD (ng/g) | LOQ (ng/g) | Method Inter-day Precision a (%) | Instrumental Intra-Day Precision (%) a | |
|---|---|---|---|---|---|---|
| 8-OHDG | 0.02–20 | 0.99 | 0.11 | 0.37 | 5.62 | 3.21 |
| DIY | 0.1–200 | 0.99 | 1.37 | 4.57 | 5.18 | 2.49 |
| ng/g w.w. | Skin (n = 222) | Gills (n = 232) | Dorsal Fin (n = 225) | Liver (n = 228) | ||||
|---|---|---|---|---|---|---|---|---|
| 8OHDG | DIY | 8OHDG | DIY | 8OHDG | DIY | 8OHDG | DIY | |
| Max | 3.60 | 1518 | 914 | 1278 | 216 | 4050 | 10.0 | 13176 |
| Min | <0.11 | <1.37 | <0.11 | <1.37 | <0.11 | <1.37 | <0.11 | <1.37 |
| Mean ± st.dev. | 0.33 ± 0.37 | 197 ± 128 | 25 ± 75 | 468 ± 202 | 7.5 ± 23 | 402 ± 507 | 0.41 ± 0.97 | 3534 ± 2175 |
| 25th | <0.11 | 128 | 1.41 | 329 | 0.48 | 196 | <0.11 | 2020 |
| Median | 0.29 | 167 | 4.70 | 417 | 1.20 | 343 | <0.11 | 3012 |
| 75th | 0.42 | 229 | 18 | 568 | 3.2 | 498 | <0.11 | 4704 |
| DR% * | 71 | 99 | 95 | 99 | 90 | 78 | 24 | 97 |
| Compound | Fish | Sample | Analytical Technique | Concentrations | Ref. |
|---|---|---|---|---|---|
| 8OHDG | Alburnus tarichi Güldenstädt | Gills | Antibody | 25–37 ng/mL | [11] |
| 8OHDG | Cyprinus carpio | Liver | ELISA | 3.5–5.6 ng/mg prot | [12] |
| Gills | 5.5–7.5 ng/mg prot | ||||
| Muscle | 6.0–9.5 ng/mg prot | ||||
| Intestines | 2.8–5.2 ng/mg prot | ||||
| 8OHDG | Cyprinus carpio | Gills | ELISA | 4.8–6.6 ng/mg prot | [44] |
| 8OHDG | Oncorhynchus mykiss | Liver | ELISA | 0.45–1.05 ng/mg prot | [21] |
| 8OHDG | Salmo salar | Liver | UPLC–MS/MS | <0.11–10 ng/g | This study |
| Gills | <0.11–914 ng/g | ||||
| Skin | <0.11–3.6 ng.g | ||||
| Dorsal fin | <0.11–216 ng/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Sundfør, E.B.; Klokkerengen, R.; Gonzalez, S.V.; Mota, V.C.; Lazado, C.C.; Asimakopoulos, A.G. Determination of the Oxidative Stress Biomarkers of 8-Hydroxydeoxyguanosine and Dityrosine in the Gills, Skin, Dorsal Fin, and Liver Tissue of Atlantic Salmon (Salmo salar) Parr. Toxics 2022, 10, 509. https://doi.org/10.3390/toxics10090509
Zhang J, Sundfør EB, Klokkerengen R, Gonzalez SV, Mota VC, Lazado CC, Asimakopoulos AG. Determination of the Oxidative Stress Biomarkers of 8-Hydroxydeoxyguanosine and Dityrosine in the Gills, Skin, Dorsal Fin, and Liver Tissue of Atlantic Salmon (Salmo salar) Parr. Toxics. 2022; 10(9):509. https://doi.org/10.3390/toxics10090509
Chicago/Turabian StyleZhang, Junjie, Eivind B. Sundfør, Rolf Klokkerengen, Susana V. Gonzalez, Vasco C. Mota, Carlo C. Lazado, and Alexandros G. Asimakopoulos. 2022. "Determination of the Oxidative Stress Biomarkers of 8-Hydroxydeoxyguanosine and Dityrosine in the Gills, Skin, Dorsal Fin, and Liver Tissue of Atlantic Salmon (Salmo salar) Parr" Toxics 10, no. 9: 509. https://doi.org/10.3390/toxics10090509
APA StyleZhang, J., Sundfør, E. B., Klokkerengen, R., Gonzalez, S. V., Mota, V. C., Lazado, C. C., & Asimakopoulos, A. G. (2022). Determination of the Oxidative Stress Biomarkers of 8-Hydroxydeoxyguanosine and Dityrosine in the Gills, Skin, Dorsal Fin, and Liver Tissue of Atlantic Salmon (Salmo salar) Parr. Toxics, 10(9), 509. https://doi.org/10.3390/toxics10090509