Cytotoxicity of 9,10-Phenanthrenequinone Impairs Mitotic Progression and Spindle Assembly Independent of ROS Production in HeLa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Immunofluorescence
2.3. Immunoblotting
2.4. Cell Viability Assay
2.5. Statistical Analyses
3. Results
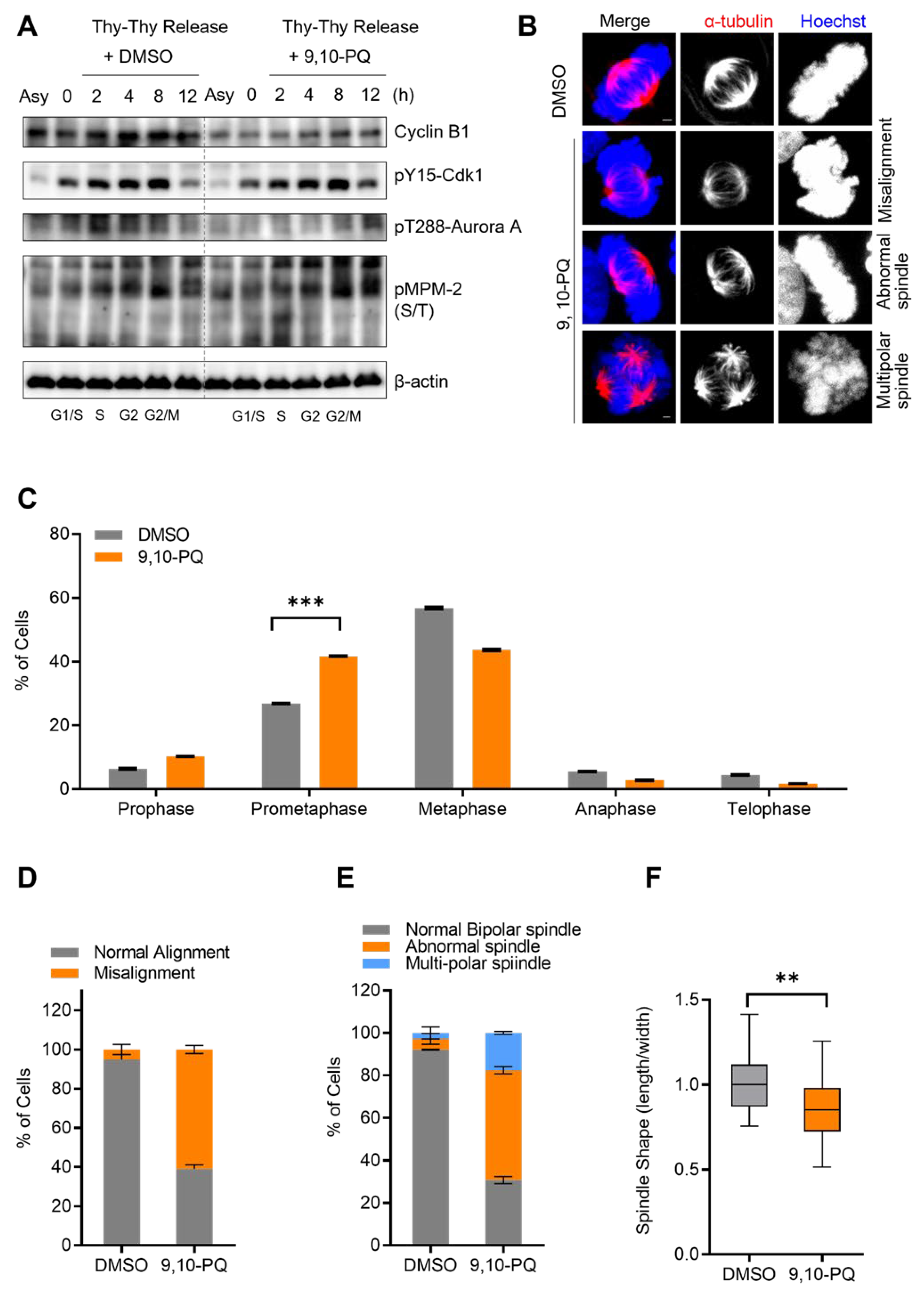
3.1. Exposure to 9,10-PQ Disturbs Mitotic Progression
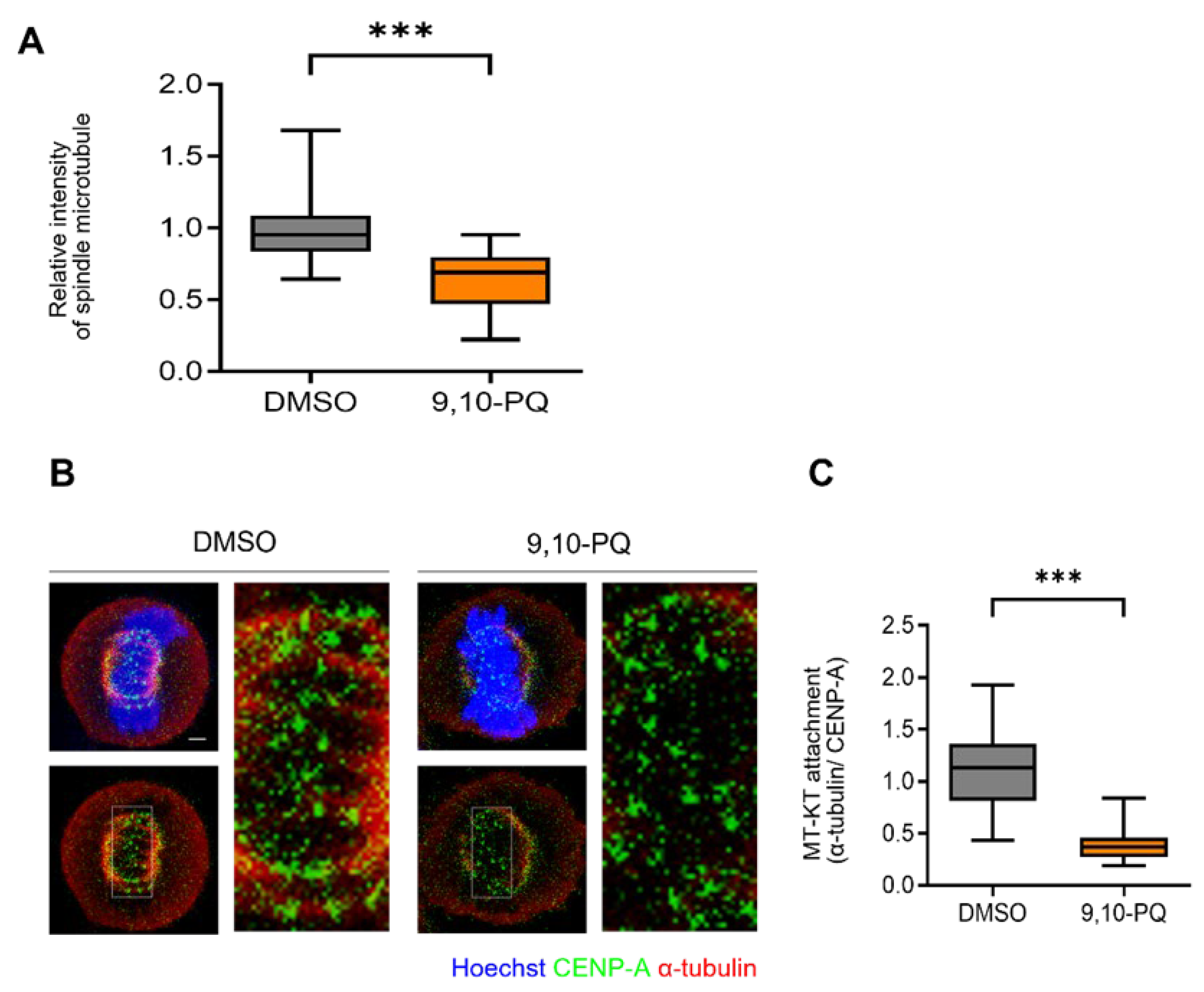
3.2. 9,10-PQ Exposure Impairs kMT Attachment during Mitosis
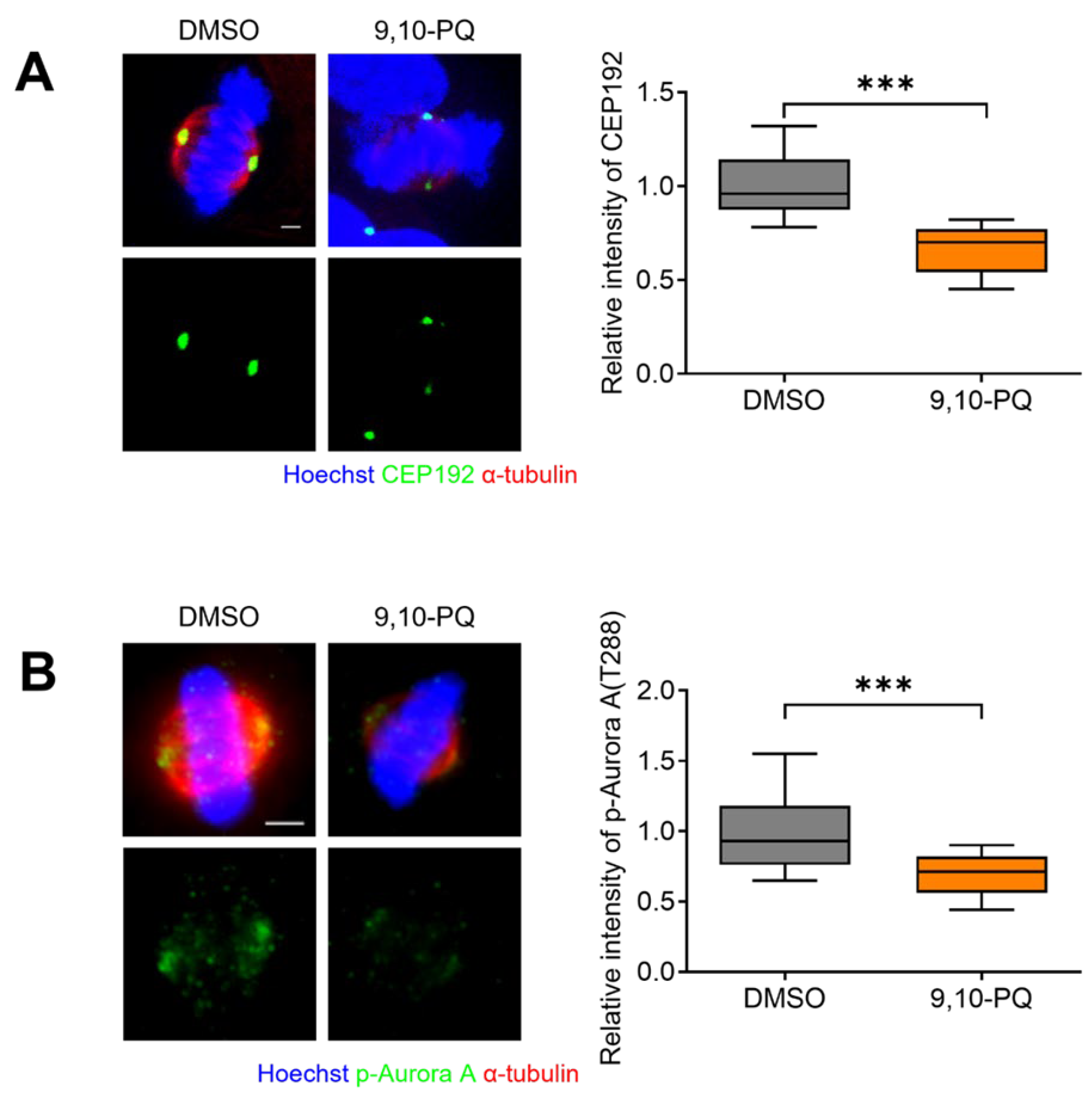
3.3. 9,10-PQ Exposure Decreases Centrosome Integrity and Spindle Assembly during Mitosis
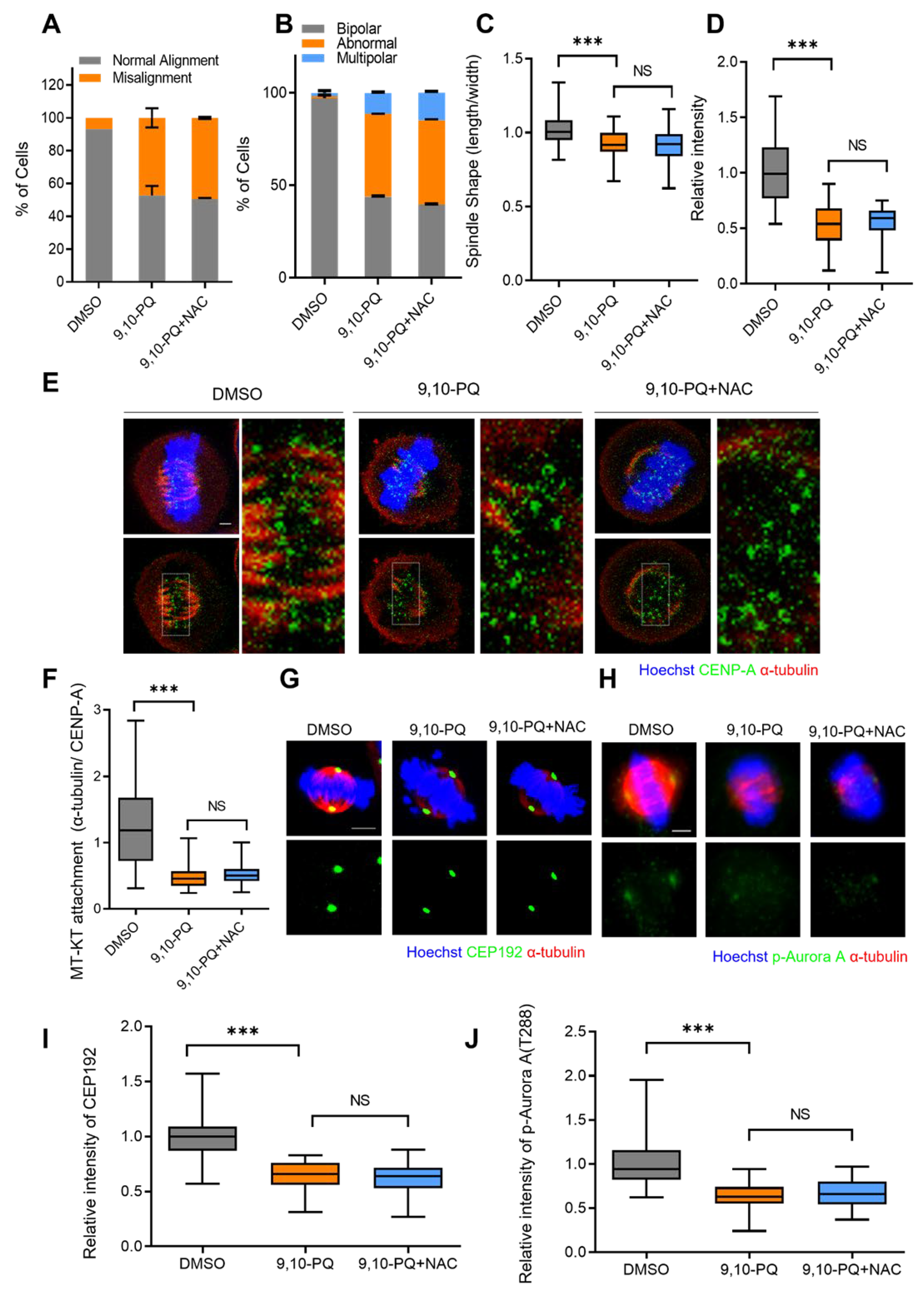
3.4. Mitotic Defects Induced by 9,10-PQ Exposure Are Not Associated with ROS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvi, S.S.; Frew, A.; Holgate, S. Is diesel exhaust a cause for increasing allergies? Clinical and experimental allergy. J. Br. Soc. Allergy Clin. Immunol. 1999, 29, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J. Epidemiologic evidence for asthma and exposure to air toxics: Linkages between occupational, indoor, and community air pollution research. Environ. Health Perspect. 2002, 110 (Suppl. S4), 573–589. [Google Scholar] [CrossRef] [PubMed]
- Berube, K.A.; Pjones, T.; Williamson, B. Electron microscopy of urban airborne particulate matter. Microsc. Anal. 1997, 61, 11–14. [Google Scholar]
- Marano, F.; Boland, S.; Bonvallot, V.; Baulig, A.; Baeza-Squiban, A. Human airway epithelial cells in culture for studying the molecular mechanisms of the inflammatory response triggered by diesel exhaust particles. Cell Biol. Toxicol. 2002, 18, 315–320. [Google Scholar] [CrossRef]
- Kerminen, V.-M.; Mäkelä, T.E.; Ojanen, C.H.; Hillamo, R.E.; Vilhunen, J.K.; Rantanen, L.; Havers, N.; von Bohlen, A.; Klockow, D. Characterization of the Particulate Phase in the Exhaust from a Diesel Car. Environ. Sci. Technol. 1997, 31, 1883–1889. [Google Scholar] [CrossRef]
- Clifford, R.L.; Jones, M.J.; MacIsaac, J.L.; McEwen, L.M.; Goodman, S.J.; Mostafavi, S.; Kobor, M.S.; Carlsten, C. Inhalation of diesel exhaust and allergen alters human bronchial epithelium DNA methylation. J. Allergy Clin. Immunol. 2017, 139, 112–121. [Google Scholar] [CrossRef]
- Steiner, S.; Bisig, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Diesel exhaust: Current knowledge of adverse effects and underlying cellular mechanisms. Arch. Toxicol. 2016, 90, 1541–1553. [Google Scholar] [CrossRef]
- Cho, A.K.; Di Stefano, E.; You, Y.; Rodriguez, C.E.; Schmitz, D.A.; Kumagai, Y.; Miguel, A.H.; Eiguren-Fernandez, A.; Kobayashi, T.; Avol, E.; et al. Determination of Four Quinones in Diesel Exhaust Particles, SRM 1649a, and Atmospheric PM2.5 Special Issue of Aerosol Science and Technology on Findings from the Fine Particulate Matter Supersites Program. Aerosol Sci. Technol. 2004, 38 (Suppl. 1), 68–81. [Google Scholar] [CrossRef]
- Chung, M.Y.; Lazaro, R.A.; Lim, D.; Jackson, J.; Lyon, J.; Rendulic, D.; Hasson, A.S. Aerosol-Borne Quinones and Reactive Oxygen Species Generation by Particulate Matter Extracts. Environ. Sci. Technol. 2006, 40, 4880–4886. [Google Scholar] [CrossRef]
- Taguchi, K.; Shimada, M.; Fujii, S.; Sumi, D.; Pan, X.; Yamano, S.; Nishiyama, T.; Hiratsuka, A.; Yamamoto, M.; Cho, A.K.; et al. Redox cycling of 9, 10-phenanthraquinone to cause oxidative stress is terminated through its monoglucuronide conjugation in human pulmonary epithelial A549 cells. Free Radic. Biol. Med. 2008, 44, 1645–1655. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Yang, M.; Ahmed, H.; Wu, W.; Jiang, B.; Jia, Z. Cytotoxicity of Air Pollutant 9, 10-Phenanthrenequinone: Role of Reactive Oxygen Species and Redox Signaling. BioMed Res. Int. 2018, 2018, 9523968. [Google Scholar]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Toyooka, T.; Shinmen, T.; Aarts, J.M.; Ibuki, Y. Dual effects of N-acetyl-L-cysteine dependent on NQO1 activity: Suppressive or promotive of 9, 10-phenanthrenequinone-induced toxicity. Toxicol. Appl. Pharmacol. 2012, 264, 404–412. [Google Scholar] [CrossRef]
- Matsunaga, T.; Morikawa, Y.; Haga, M.; Endo, S.; Soda, M.; Yamamura, K.; El-Kabbani, O.; Tajima, K.; Ikari, A.; Hara, A. Exposure to 9, 10-phenanthrenequinone accelerates malignant progression of lung cancer cells through up-regulation of aldo-keto reductase 1B10. Toxicol. Appl. Pharmacol. 2014, 278, 180–189. [Google Scholar] [CrossRef]
- Hatae, N.; Nakamura, J.; Okujima, T.; Ishikura, M.; Abe, T.; Hibino, S.; Choshi, T.; Okada, C.; Yamada, H.; Uno, H.; et al. Effect of the orthoquinone moiety in 9, 10-phenanthrenequinone on its ability to induce apoptosis in HCT-116 and HL-60 cells. Bioorg. Med. Chem. Lett. 2013, 23, 4637–4640. [Google Scholar] [CrossRef]
- Kamase, K.; Taguchi, M.; Ikari, A.; Endo, S.; Matsunaga, T. 9,10-Phenanthrenequinone provokes dysfunction of brain endothelial barrier through down-regulating expression of claudin-5. Toxicology 2021, 461, 152896. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.C.; Dao, K.; Roque, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology 2017, 59, 133–139. [Google Scholar] [CrossRef]
- Sullivan, M.; Morgan, D.O. Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 2007, 8, 894–903. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Sieber, O.; Heinimann, K.; Tomlinson, I. Genomic stability and tumorigenesis. Semin. Cancer Biol. 2005, 15, 61–66. [Google Scholar] [CrossRef]
- Dabas, N.; Byrnes, D.M.; Rosa, A.M.; Eller, M.S.; Grichnik, J.M. Diagnostic role of chromosomal instability in melanoma. J. Skin Cancer 2012, 2012, 914267. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, A.; Salmon, E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007, 8, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.E.; Carroll, S.B.; Zhang, X.F.; Mackey, M.A. Spontaneous premature chromosome condensation, micronucleus formation, and non-apoptotic cell death in heated HeLa S3 cells. Ultrastructural observations. Am. J. Pathol. 1995, 146, 963–971. [Google Scholar] [PubMed]
- Kutuk, O.; Letai, A. Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res. 2008, 68, 7985–7994. [Google Scholar] [CrossRef]
- King, R.W.; Jackson, P.K.; Kirschner, M.W. Mitosis in transition. Cell 1994, 79, 563–571. [Google Scholar] [CrossRef]
- Zheng, Z.L. Cyclin-Dependent Kinases and CTD Phosphatases in Cell Cycle Transcriptional Control: Conservation across Eukaryotic Kingdoms and Uniqueness to Plants. Cells 2022, 11, 279. [Google Scholar] [CrossRef]
- Sazonova, E.V.; Petrichuk, S.V.; Kopeina, G.S.; Zhivotovsky, B. A link between mitotic defects and mitotic catastrophe: Detection and cell fate. Biol. Direct 2021, 16, 25. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Medema, R.H. Mechanisms of centrosome separation and bipolar spindle assembly. Dev. Cell. 2010, 19, 797–806. [Google Scholar] [CrossRef]
- Joukov, V.; Walter, J.C.; De Nicolo, A. The Cep192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol. Cell 2014, 55, 578–591. [Google Scholar] [CrossRef]
- Gomez-Ferreria, M.A.; Rath, U.; Buster, D.W.; Chanda, S.K.; Caldwell, J.S.; Rines, D.R.; Sharp, D.J. Human Cep192 is required for mitotic centrosome and spindle assembly. Curr. Biol. 2007, 17, 1960–1966. [Google Scholar] [CrossRef]
- Joukov, V.; De Nicolo, A. Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci. Signal 2018, 11, eaar4195. [Google Scholar] [CrossRef]
- Skubnik, J.; Jurasek, M.; Ruml, T.; Rimpelova, S. Mitotic Poisons in Research and Medicine. Molecules 2020, 25, 4632. [Google Scholar] [CrossRef]
- Nakayama, Y.; Inoue, T. Antiproliferative Fate of the Tetraploid Formed after Mitotic Slippage and Its Promotion, A Novel Target for Cancer Therapy Based on Microtubule Poisons. Molecules 2016, 21, 663. [Google Scholar] [CrossRef]
- Krenning, L.; van den Berg, J.; Medema, R.H. Life or Death after a Break: What Determines the Choice? Mol. Cell 2019, 76, 346–358. [Google Scholar] [CrossRef]
- Chen, G.; Deng, X. Cell Synchronization by Double Thymidine Block. Bio-Protocol 2018, 8, e2994. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 2015, 15, 682. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, H.J.; Lee, Y.J.; Lim, Y.B.; Sim, W.J.; Jang, J.H.; Heo, H.R.; Lim, H.J.; Jung, J.W.; Kim, J.S. Determination of Genotoxicity Attributed to Diesel Exhaust Particles in Normal Human Embryonic Lung Cell (WI-38) Line. Biomolecules 2021, 11, 291. [Google Scholar] [CrossRef]
- Douki, T.; Corbiere, C.; Preterre, D.; Martin, P.J.; Lecureur, V.; Andre, V.; Landkocz, Y.; Pottier, I.; Keravec, V.; Fardel, O.; et al. Comparative study of diesel and biodiesel exhausts on lung oxidative stress and genotoxicity in rats. Environ. Pollut. 2018, 235, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Bennabi, I.; Queguiner, I.; Kolano, A.; Boudier, T.; Mailly, P.; Verlhac, M.H.; Terret, M.E. Shifting meiotic to mitotic spindle assembly in oocytes disrupts chromosome alignment. EMBO Rep. 2018, 19, 368–381. [Google Scholar] [CrossRef]
- Kline-Smith, S.L.; Walczak, C.E. Mitotic spindle assembly and chromosome segregation: Refocusing on microtubule dynamics. Mol. Cell 2004, 15, 317–327. [Google Scholar] [CrossRef]
- Silva, P.; Barbosa, J.; Nascimento, A.V.; Faria, J.; Reis, R.; Bousbaa, H. Monitoring the fidelity of mitotic chromosome segregation by the spindle assembly checkpoint. Cell Prolif. 2011, 44, 391–400. [Google Scholar] [CrossRef]
- Prosser, S.L.; Pelletier, L. Mitotic spindle assembly in animal cells: A fine balancing act. Nat. Rev. Mol. Cell Biol. 2017, 18, 187–201. [Google Scholar] [CrossRef]
- Kralova, V.; Hanusova, V.; Rudolf, E.; Canova, K.; Skalova, L. Flubendazole induces mitotic catastrophe and senescence in colon cancer cells in vitro. J. Pharm. Pharmacol. 2016, 68, 208–218. [Google Scholar] [CrossRef]
- Khing, T.M.; Choi, W.S.; Kim, D.M.; Po, W.W.; Thein, W.; Shin, C.Y.; Sohn, U.D. The effect of paclitaxel on apoptosis, autophagy and mitotic catastrophe in AGS cells. Sci. Rep. 2021, 11, 23490. [Google Scholar] [CrossRef]
- Florio, R.; Veschi, S.; di Giacomo, V.; Pagotto, S.; Carradori, S.; Verginelli, F.; Cirilli, R.; Casulli, A.; Grassadonia, A.; Tinari, N.; et al. The Benzimidazole-Based Anthelmintic Parbendazole: A Repurposed Drug Candidate That Synergizes with Gemcitabine in Pancreatic Cancer. Cancers 2019, 11, 2042. [Google Scholar] [CrossRef]
- Patterson, J.C.; Joughin, B.A.; van de Kooij, B.; Lim, D.C.; Lauffenburger, D.A.; Yaffe, M.B. ROS and Oxidative Stress Are Elevated in Mitosis during Asynchronous Cell Cycle Progression and Are Exacerbated by Mitotic Arrest. Cell Syst. 2019, 8, 163–167. [Google Scholar] [CrossRef]
- Savitsky, P.A.; Finkel, T. Redox regulation of Cdc25C. J. Biol. Chem. 2002, 277, 20535–20540. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, M.; Candas, D.; Zhang, T.Q.; Qin, L.; Eldridge, A.; Wachsmann-Hogiu, S.; Ahmed, K.M.; Chromy, B.A.; Nantajit, D.; et al. Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev. Cell 2014, 29, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Havens, C.G.; Ho, A.; Yoshioka, N.; Dowdy, S.F. Regulation of late G1/S phase transition and APC Cdh1 by reactive oxygen species. Mol. Cell. Biol. 2006, 26, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Lee, K.S.; Woo, H.A.; Kang, D.; Rhee, S.G. Control of the pericentrosomal H2O2 level by peroxiredoxin I is critical for mitotic progression. J. Cell Biol. 2015, 210, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Conour, J.E.; Graham, W.V.; Gaskins, H.R. A combined in vitro/bioinformatic investigation of redox regulatory mechanisms governing cell cycle progression. Physiol. Genom. 2004, 18, 196–205. [Google Scholar] [CrossRef][Green Version]
- Helmke, K.J.; Heald, R.; Wilbur, J.D. Interplay between Spindle Architecture and Function; Elsevier: Amsterdam, The Netherlands, 2013; pp. 83–125. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Leem, J.; Oh, J.S.; Kim, J.-S. Cytotoxicity of 9,10-Phenanthrenequinone Impairs Mitotic Progression and Spindle Assembly Independent of ROS Production in HeLa Cells. Toxics 2022, 10, 327. https://doi.org/10.3390/toxics10060327
Kim S, Leem J, Oh JS, Kim J-S. Cytotoxicity of 9,10-Phenanthrenequinone Impairs Mitotic Progression and Spindle Assembly Independent of ROS Production in HeLa Cells. Toxics. 2022; 10(6):327. https://doi.org/10.3390/toxics10060327
Chicago/Turabian StyleKim, Seul, Jiyeon Leem, Jeong Su Oh, and Jae-Sung Kim. 2022. "Cytotoxicity of 9,10-Phenanthrenequinone Impairs Mitotic Progression and Spindle Assembly Independent of ROS Production in HeLa Cells" Toxics 10, no. 6: 327. https://doi.org/10.3390/toxics10060327
APA StyleKim, S., Leem, J., Oh, J. S., & Kim, J.-S. (2022). Cytotoxicity of 9,10-Phenanthrenequinone Impairs Mitotic Progression and Spindle Assembly Independent of ROS Production in HeLa Cells. Toxics, 10(6), 327. https://doi.org/10.3390/toxics10060327





