Abstract
Technical xylene is a compound of massive production that is used in applications such as petrochemical and healthcare laboratories. Exposure to xylene can cause acute and chronic effects in humans and animals. Currently available studies regarding xylene’s adverse effects with credible designs were dated almost twenty years ago. This systematic review summarizes the findings regarding the detrimental effects of technical xylene from human, animal, and in vitro studies. It recapitulated available studies with respect to the effects of xylene on the female reproductive system to stress the need for updating the current data and guidelines. Based on pre-specified criteria, 22 studies from journal databases exploring the toxic effects of xylene on menstruation, endocrine endpoints, fetal development, and reproductive functions were included for the review. It was found that related studies with a specific focus on the effects of technical xylene on the female reproductive system were insufficient. Therefore, further studies are necessary to update the existing data, thus improving the quality and reliability of risk assessment of exposure to xylene in pregnant women
1. Introduction
Technical xylene, or simply referred to as xylene or xylol [1,2], is an organic chemical compound from the benzene family. With a chemical formula of C8H10 and a molecular weight of 106.2 g/mol, technical xylene is a mixture of three individual isomers (Figure 1), namely ortho-xylene (o-xylene), meta-xylene (m-xylene), and para-xylene (p-xylene) [1]. Being a cyclic aromatic hydrocarbon, xylene is a flammable, colorless liquid with a pleasant odor [1,3].
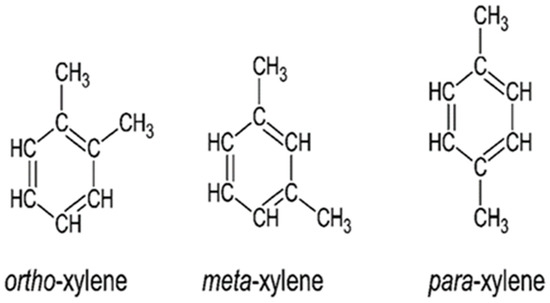
Figure 1.
Xylene isomers.
Xylene is produced through the extraction of crude oil and raw natural gas, where the compound is extracted from the reformulation of naphta during the petroleum refining process [1,4]. It is widely used in industries, household products, and healthcare laboratories. Approximately 30 million tons of xylene are used across the globe each year, and it is one of the most highly manufactured chemicals in the United States. It is broadly used as an organic solvent and chemical feedstock in manufacturing industries [1,2]. In healthcare settings, xylene is one of the major chemicals commonly used in histological laboratories. The analytical grade of xylene, with a purity of 99.7% of xylene constituents, is used by healthcare histology technicians and medical laboratory technologists for tissue processing, staining, and cover-slipping the stained slides in order to permanently preserve the tissue samples for further examinations by the pathologists [5]. Xylene acts as a clearing agent, with the purpose of making the histological tissue sections clear or transparent so that the detailed morphological structure of the tissues can be examined [6].
There are many published articles showing the evidence of the health effects of xylene as a constituent of organic solvents to both humans and animals. Previous studies have demonstrated that the individual isomers of xylene have almost similar toxicokinetic characteristics and toxicological effects [7]. The harmful effects of xylene have been investigated by several authors [2,3,4,5,6]. Exposure to xylene affects the central nervous system, respiratory system, and hepatic system. The adverse effects include increased dopamine in the brain, irritation of the nose and throat, and increased liver weight [3]. In relation to the reproductive effects of xylene, there were some deficiencies in the previously published studies regarding the duration of animal exposure to conclude the no-observed-adverse-effect level (NOAEL) [3,8,9]. In addition, reviews on the reproductive effects of technical xylene are still lacking with their focus on the female reproductive outcomes, morphologically and histologically. This systematic review will highlight the currently available studies assessing the detrimental effects of technical xylene exposure to the female reproductive system, despite the presumptions that data are growingly up to date.
2. Materials and Methods
This section describes the strategy applied to retrieve articles within the scope of the field of interest. The step-by-step review process was achieved with the aid of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The process also comprise the search of published articles within the key area of interest, which were sourced from several online databases. Later, the searched articles were narrowed down based on the inclusion and exclusion criteria. Through a detailed systematic review, the final retrieval of study articles was accomplished for data mining and analyses.
2.1. PRISMA
PRISMA is frequently adopted within medical research and health care [10] because of its ability to identify the inclusion and exclusion criteria [11]. On top of that, PRISMA allows systematic searches by identifying clear research questions. From a large database of scientific literature, it can examine the records within a specified time [12]. Due to these advantages, PRISMA was a useful tool to accurately gather the articles and to identify the current studies investigating the toxicity of technical xylene, particularly on the female reproductive system.
2.2. Search Strategy
Research articles for the review were resourced from six journal databases: Web of Science (WoS), Scopus, Science Direct, PubMed, Dimension, and Google Scholar. The use of Dimension and Google Scholar allowed both indexed and non-indexed studies to be retrieved, thus providing a comprehensive, non-biased result [13]. The systematic review process began with the identification of research articles with a high potential of being parallel to the objectives of the review. To achieve this, a search strategy was applied by identifying the relevant keywords and phrases depicting the subject of interest. Guided by previous studies to find all relevant articles, the search was further strengthened using the keywords’ similar term with the aid of a thesaurus, dictionaries, and the Medical Subject Headings (MeSH) function to target keywords such as “technical xylene exposure”, “xylene administration”, “xylol inhalation”, “mixed xylene” combined with “reproductive toxicity”, “developmental toxicity”, “detrimental effects”, “endocrine fate” and “female reproduction”. Table 1 shows the search string used for article searching from two major databases. A similar query or syntax was used for other databases.

Table 1.
The search string. An asterisk (*) is used for multiple character searching.
The next stage was the screening of all available identified articles. Accordingly, a total of 411 articles were obtained from a primary search of the previously stated databases. Aided by PRISMA, Figure 2 illustrates the step-by-step process of identifying and screening the articles eligible for the systematic review.
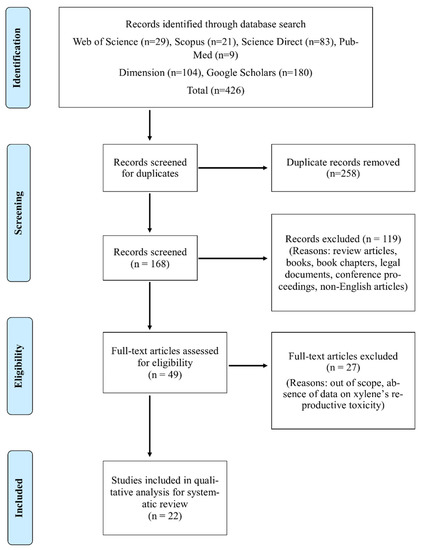
Figure 2.
Article search and identification using PRISMA [14].
2.3. Inclusion and Exclusion Criteria
The criteria of selection and exclusion were determined for the review process according to population, exposure, comparator, and outcomes, or the PECO model [15]. Only research articles containing empirical data on the reproductive effects of technical xylene in women of reproductive age, pregnant animals, and ovarian cell lines were selected (Table 2).

Table 2.
PECO (population, exposure, comparator, and outcome) model.
Review articles, books or book chapters, legal documents, patents, and conference proceedings were excluded. Secondly, to avoid mistranslation and confusion with the non-English papers, e.g., Chinese and Arabic, only research articles published in English were included. Additionally, this review only included literature published within a 30-year timeline, which was from 1990 to 2022, to evaluate the trend of studies and publications in the key subject area. In terms of geographical eligibility, the review included research articles from across the globe. A summary of the inclusion and exclusion criteria is shown in Table 3.

Table 3.
Inclusion and exclusion criteria.
2.4. Data Retrieval and Analysis
All remaining 22 research articles were thoroughly assessed based on their objectives, methodologies, and findings. Research papers regarding the toxicity of xylene compounds to the female reproductive system were categorized into in vitro studies, in vivo studies, and human studies.
3. Results
Through a rigorous search sourced from several databases, a total of 22 research articles, highlighting the detrimental effects of xylene on female reproduction, were obtained. These articles were divided into three major categories based on their methodologies, namely in vitro studies, in vivo studies, and human studies, as summarized by Table 4, Table 5 and Table 6.

Table 4.
In vitro studies on the toxicity of technical xylene to the female reproductive system from 1990 to 2022.

Table 5.
In vivo studies on the toxicity of technical xylene to the female reproductive system from 1990 to 2022.

Table 6.
Human studies on the toxicity of technical xylene to the female reproductive system from 1990 to 2022.
3.1. In Vitro and In Vivo Studies
Studies were carried out on ovarian cell lines to investigate the effect of xylene on different reproductive parameters, such as cellular replications and sexual hormones. In addition, in vivo studies pertaining to the toxicity secondary to xylene exposure on the female reproductive system were conducted using different animal models. The xylene compounds used for these studies were either xylene alone or as a mixture with other organic solvents.
3.1.1. Ovarian Cell Toxicity
Disturbances in maternal ovarian functions have been studied as part of the embryotoxic mechanisms of xylene during pregnancy [37], as the mammalian ovarian gland is the ultimate point for the sympathetic and sensory neurons responsible for the establishment and maintenance of ovarian functions [38]. Xylene had demonstrated to be hindering the basic cellular functions of ovaries, namely cellular replication, hormone production, and apoptotic activities. Such findings were discovered by Tarko et al. [18] through in vitro studies using Holstein cows’ ovarian granulosa cell culture. Via immunoassay and immunocytochemical analysis, they found that in vitro exposure of ovarian cells to xylene could interfere with the release of progesterone and testosterone. Simultaneously, xylene increased cellular multiplication and insulin growth factor 1 (IGF-1) production. The latter suggested that xylene could exert cancerous change characterized by amplified cellular replication [17,18].
Using the cultured porcine ovarian granulosa cells, Sirotkin et al. [19] found that xylene stimulated the build-up of proliferating cell nuclear antigen (PCNA), an indication of increased cellular replication. The result was found to be contrary in their later study, where xylene reduced the PCNA expression [21]. A xylene compound also surged the apoptotic activity, as shown by an elevated apoptotic marker, Bax, thus reducing the cellular viability. The levels of progesterone and oxytocin were also elevated following exposure of the cell cultures to 0.1% of xylene [19]. These findings also proved that the cultured ovarian cells are suitable to be used to assess the ovarian responses to external stressors. It was evident by the ability of cells to eliminate the Trypan blue and the presence of proliferation- and apoptosis-related compounds [19]. In another study, the team established that xylene, as part of BTEX, could induce apoptosis in porcine and bovine ovarian cell cultures, as indicated by the elevation of apoptotic markers, namely Bax and p53 [16]. They further investigated the adverse effects of xylene by comparing the expression of apoptotic and proliferation markers produced by ovarian cells harvested from cows of different body condition scores. They found that ovarian cells from cows having a higher body condition score were more resistant to the harmful effects of xylene compared to emaciated cows [20]. These in vitro studies provided useful data regarding the notorious effects of xylene on female reproductive functions by directly exposing the target cells to the compound.
3.1.2. Maternal Toxicity
Xylene and its isomers had been found to cause a significant decrease in the maternal body weight and food consumption at a concentration of 1000 parts per million [9]. The study, however, did not reflect an occupational setting due to the continuous daily exposure of technical xylene to the tested female rats [9]. Inhalation daily exposure to technical xylene at 200 ppm during the organogenesis period did not cause maternal toxicity [22].
3.1.3. Developmental Toxicity
Due to its lipid solubility characteristic, xylene is able to reach the embryos from maternal exposure through placental transfer [39,40]. Xylene and its isomers had been found to cause a significant decrease in fetal body weight at a minimum concentration of 500 parts per million [9]. Adverse fetal effects were manifested as malformations such as skeletal variations, visceral deformities, and abnormal ossification [9,22]. Saillenfait’s research was designed in accordance with OECD guidelines for the female reproductive system toxicity study (OECD, 2001) [41]. In different studies, the compound, as a mixture with other toxic solvents, e.g., methyl ketone and toluene, caused developmental malformations such as diaphragmatic hernia, absence of tail, and skeletal variations. These studies, however, were weakly associated with a xylene compound [23,24].
Prenatal inhalation exposure to xylene, as part of the thinner mixture during the whole gestational period of pregnant mice, was also found to cause developmental toxicity and behavioral changes in mice offspring, according to Malloul’s study [26]. Significant adverse effects were observed following prenatal exposure to paint thinner and other xylene-containing solvent mixtures at 100 ppm to 2000 ppm. Several notable effects include shortened offspring’s body length, retarded sensorimotor development, anxiety, and depression [26,42]. Her study emphasized the inhalation exposure that mimicked inhaled solvent abuse (thinner sniffing) among pregnant women, rather than exposure from an occupational setting.
Singh et al. [25] tested the potential cellular toxicity of xylene, benzene, and toluene on Drosophila melanogaster (D. melanogaster) larvae and found that these compounds induced heat shock proteins and the reactive oxygen species (ROS), which subsequently delayed the development of adult flies for 48 h in the xylene-treated groups. In addition, xylene caused a significant decrease in the number of eggs laid by female D. melanogaster [25].
3.2. Human Studies
The associations between xylene exposure and female reproductive functions in humans were investigated through cross-sectional population studies. None of this research was conducted with the specificity of investigating xylene alone as the compound of interest, but rather as a mixture of organic solvents or volatile organic compounds (VOCs). Nevertheless, there is a high possibility of interaction between xylene and other organic solvents due to its ability to act as inducer or inhibitor of the microsomal cytochrome P450 enzymes in the liver. Interactions of xylene with other compounds are challenging due to differences in metabolism among the exposed species [7]. The effect of xylene and alcohol mixture was, however, extensively studied, and it was found that alcohol consumption may inhibit xylene metabolism due to competition for mixed oxidases in the liver [7]. Other factors that contribute to xylene metabolisms and its effects include exercises, environmental factors, and pathological predispositions [43].
3.2.1. Menstrual Disturbances
The female menstrual cycle has been accounted for as a vital parameter in assessing reproductive health [27,44]. Alteration of menstrual patterns is an indicator that can be used as a prediction of the internal state of the endocrine function, which may be linked to other chronic diseases such as breast cancer [44]. Merklinger-Gruchala et al. [45] used the menstrual cycle characteristics as a prognostic factor of women’s reproductive health. They stated that any disturbances in the menstrual cycle may hinder the oocyte quality, ovulation process, fertilization, implantation, or the fate of embryos [45].
The most recent study was carried out by Moradi et al. [33] using a cross-sectional population study. They investigated the occupational exposure of xylene, together with other compounds of benzene, toluene, ethylbenzene, and xylene (BTEX), among 36 beauty salon workers in the main city of Tehran [33]. They measured both salon indoor and outdoor BTEX levels using the National Institute of Occupational Safety and Health (NIOSH) 1501 method [46]. Urine BTEX metabolites collected from the beauty salon workers were also quantified using the headspace solid-phase micro extraction (HS-SPME) coupled with gas chromatography-mass spectrometry. The analysis results were used to correlate the BTEX exposure with menstrual disturbance indices, namely menstrual cycle length and menstrual flow. The urinary concentrations of m-xylene and o-xylene metabolites were between 99.3 ng and 276.3 ng/L and 57.6 ng/L and 149.2 ng/L, respectively. It was concluded that m-xylene and o-xylene caused menstrual disturbances, with a prevalence of 47% among beauty salons workers, which might be caused by day-to-day salon activities such as hair coloring, nail treatment, and hair removal [33,47]. Nevertheless, the threshold limit value (TLV) or the permissible exposure limit (PEL) for individual compounds in this study was not suitable when applied to non-industrial workplaces due to shorter exposure duration and inadequate protective equipment [33].
Using the same study approach, another research team studied the relationship between occupational exposure to xylene, as a mixture of four organic solvents (xylene, benzene, styrene, and toluene), and menstrual cycle length among 1408 female petrochemical workers in China [27]. Applying the logistic regression, they scrutinized the correlation between the low-level exposure of xylene, with other organic solvents and prolonged menstrual cycle length, or medically termed as oligomenorrhea. Oligomenorrhea was defined, for the study, as an average cycle length longer than 35 days. This also included a menstrual cycle lasting over 90 days [44,48,49]. The assessment was made through an interviewer-delivered questionnaire to the enrolled subjects. The study concluded that the exposure of xylene alone, at a low level, was associated with a prolonged menstrual cycle in 284 enrolled females, accounting for 14.1% of the total subjects. Although this study claimed that the exposure was at a low level, there were no quantitative analyses conducted to present the data with accurate values. The inference was made based on a report provided by an industrial hygienist in the research team [27].
3.2.2. Endocrine Disruption
Endocrine disruptors are defined as exogenous agents that hinder the normal regulations of natural hormones that are responsible for the preservation of homeostasis and the developmental process [50,51]. Endocrine characteristics, particularly hormonal variables, are associated with female fecundability and, thus, fertility [52]. Studies have proven that reproductive hormonal quality may predict the possibility of pregnancy in women within their ovulatory menstrual cycle [28,52]. In 2002, a cross-sectional population study was conducted by Reutman and their team to explore the potential endocrine reproductive effects of mixed xylene exposures amongst female United States Air Force (USAF) employees. This study, however, did not investigate xylene, per se, but as a combination of solvents and fuel [28]. Four urinary endocrine endpoints were used, namely follicular progesterone, mid-luteal progesterone, luteinizing hormone, and estradiol, based on an established algorithm to assess the reproductive endocrine characteristics [28,52,53]. It was evident, from this study, that the occupational exposure of m-xylene, p-xylene, and o-xylene within the aromatic hydrocarbons significantly reduced the pre-ovulatory luteinizing hormone (15.8 vs. 22.0 mIU LH/mg creatinine) and almost significantly lowered the mid-luteal progesterone. The data were gathered and analyzed from 100 eligible participants. Such disturbances in the regulation of reproductive endocrine may carry predictive values in women’s reproductive performance [28].
3.2.3. Adverse Birth Outcomes and Other Potential Reproductive Health Risks
Adverse birth outcomes, such as oral clefts and neural tube defects (NTDs), have been associated with maternal exposure to hazardous air pollutants (HAP) [54,55,56]. Lupo et al. [30] conducted a case-control population study to determine whether maternal exposure to ambient levels of BTEX is linked to NTDs among offspring in Texas, United States. They collected 1108 birth defect data from the Texas Birth Defects Registry from January 1999 to December 2004. The study, however, concluded that there was no significant relationship between ambient levels of xylene and offspring suffering from NTDs [30]. Although the potential confounders and etiologic heterogeneity were identified to minimize the errors, a few limitations were identified. In relation to exposure assessment, the 1999 United States Environmental Protection Agency (USEPA) Assessment System for Population Exposure Nationwide (ASPEN) model might cause misclassification to the data collected after 1999 due to environmental changes, such as roadways and industrial emissions [30].
Studies by Ghosh et al. [31] and Serrano-Lomelin et al. [34] showed strong correlations between low birth weight and xylene-containing traffic pollutants, as well as industrial air pollutants. In Serrano-Lomelin’s study, a significant spatial association (OR, 1.16) was discovered between low birth weight and xylene-containing industrial air contaminants among 333,247 singleton births from different postal code regions in Canada from 2006 until 2012 [34]. Their study also found associations between these pollutants and other adverse birth outcomes, namely preterm birth and small for gestational age [34]. These findings were supported by Aguilera et al. [29] in their cohort study, using the data from 562 women exposed to BTEX and nitrogen dioxide (NO2). Their study found a negative association between BTEX and the biparietal diameter of the subjects’ fetuses when exposed from week 20 to week 22 [29]. Furthermore, Ghosh et al. [31] discovered higher chances of delivering low-birth-weight infants among mothers who were exposed to BTEX-containing traffic pollutants during the third trimester. They collected 8181 low-birth-weight data and 370,922 normal-birth-weight data from 1995 to 2006 [31]. In addition, evidence of an association between BTEX exposure and preterm newborns was discovered by Cassidy-Bushrow et al. [35]. They found that preterm births, along with other maternal factors such as maternal age and ethnicity, were strongly linked (OR > 1.00) with ambient BTEX exposure [35].
In 2017, Santiago et al. [32] presented two case reports of acquired chromosomal aberrations in female gas station workers with chronic exposure to BTEX. Both females had a history of miscarriage during the first half of their pregnancy. Fluorescence in situ Hybridization (FISH) revealed complex chromosomal rearrangements (CCR) involving the derivatization and insertion of chromosomal breakpoints that were positively associated with reproductive risk and early pregnancy loss [32]. They suggested that chromosomal aberrations may be an indication of chemosensitivity induced by environmental contaminants such as BTEX [32].
The long-standing concerns regarding the reproductive outcomes from exposure to organic solvents in general and xylene in specific has been influenced by the ubiquitous nature of these compounds. Humans were at risk of being exposed through environmental, occupational [27,33,57,58], and other sources, including commercialized consumer products. In the most recent study, Ding et al. [36] conducted a cross-sectional population study to establish an association between exposure to m-xylene and p-xylene, as part of the volatile organic compounds and the application of feminine hygiene products among American women in their reproductive age [36]. Trace levels of m-xylene and p-xylene were found among women who used vaginal douches for a period of six months. Ding et al. [36] admitted that the study had several drawbacks in terms of the reliability of the results. The cross-sectional data gathered for their study did not address other possible sources of volatile organic compounds. In addition, the population data used were retrospective in nature, which were dated from 2001 until 2004. Thus, the norms of feminine hygiene practices 15 years later might not be accurately reflected in misclassification and recall bias [36].
4. Discussion
This review paper was aimed to systematically assess the existing studies on the toxicity of xylene exposure to the female reproductive system. Nevertheless, technical xylene is also known to adversely affect the male reproductive system. Previously published studies showed that xylene caused histological alterations in the testes, such as increased intratubular spaces, reduced number of Leydig cells, and the degeneration of the spermatozoids in male rats [59]. In addition, a study on male rats reported that xylene may disturb the pubertal Leydig cell development by elevating the production of reactive oxygen species (ROS) [60]. Figure 3 highlights the proposed technical xylene’s mechanism of toxicity on the female reproductive system.
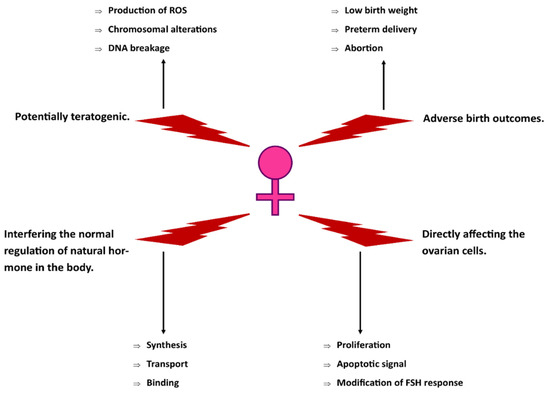
Figure 3.
Suggested toxicity mechanisms of technical xylene on the female reproductive system.
Most of the studies were conducted using animal and in vitro models to understand the effects of xylene on female reproductive functions. Two major areas were being focused on in the studies: developmental toxicities and ovarian cell toxicities. Developmental toxicities of xylene were scrutinized from the aspect of the maternal inhalation route. On the other hand, in vitro studies were the experimental approach to investigating ovarian toxicity resulting from xylene exposures. Literature that specifically highlighted the toxicity of technical xylene as an individual compound, not as a mixture with other organic solvents, were only available from animal studies [17,18,26].
Only one scholarly research article was identified regarding the female reproductive toxicity of technical xylene and its isomeric compounds of m-xylene, p-xylene, and o-xylene. Saillenfait et al. [9] studied the maternal inhalation exposure of technical xylene and its toxicity on rat development [9]. Maternal toxicities were reported as reduced body weight and declined food consumption at high levels of exposure (1000 ppm) from m-, p-, and o-xylene. In terms of scope and design, the study was considered comprehensive in exploring the maternal and developmental effects of technical xylene, since most of the previous studies were not tailored according to the current recommendations for chemical testing [41]. The study also managed to suggest the No Observed Adverse Effect Level (NOAEL) of technical xylene and its isomers for the rats and their offspring [9]. According to this study, the NOAELs of technical xylene and its isomers for maternal toxicity were between 100 ppm and 500 ppm [9]. Although continually cited in many research and review papers, as well as legal documents for many years as references related to xylene toxicity, there were gaps in this study suggestive of future research to address the unexplored variables. For instance, the maternal toxicities of xylene were only observed as decreases in weight and food consumption.
Due to their lipophilic properties, xylene isomers are quickly absorbed and distributed within the body, followed by the Cytochrome P450 (CYP450)-mediated oxidation to methylbenzyl alcohol, with urinary metabolites of 2-methylhippuric acid (2MHA), 3-methylhippuric acid (3MHA), and 4-methylhippuric acid (4MHA) from o-, m-, and p-xylene, respectively, of which they serve as an indicator for xylene exposure [7,61]. Much less is known about the exposure to mixed xylenes or the individual isomers and the populations at risk [62]. Based on earlier studies, oral administration of p-xylene at high concentration (2000 mg/kg/day) caused death in tested rats [63]. It was suggested that the biotransformation of p-xylene into a toxic tolualdehyde caused a 65% loss of microsomal hydroxylase activity [64]. The mechanism of how the technical xylene hampered the fetal development, resulting from maternal toxicity of xylene, was not thoroughly investigated. It was proposed by Miller and Edwards in 1999 that there were potential interactions between xylene isomers and the mechanism of their toxicities, which are yet to be revealed [65]. In terms of xylene exposure to the animal subjects, it did not mimic the occupational setting due to the constant daily exposure of xylene subjected to the rats, which was unlikely to happen in an occupational setting [9]. Furthermore, integrating animal data to establish the occupational exposure limits in humans involves several key uncertainties, such as interspecies variabilities and the establishment of safe levels from shorter duration studies to a lifetime exposure [66]. Inadequacies in the overall health effects database, where the most sensitive adverse effects are not investigated, also contribute to the insufficiencies [66]. To date, new studies to bridge these gaps remain insufficient.
Prenatal exposure to xylene, in the form of a mixture with other compounds such as thinner, has resulted in depression-like behaviors, impaired learning, and memory disturbance in mice’s offspring. Xylene, however, comprised only 15.5% of the product. Such a study was conducted to extrapolate the effects of prenatal and postnatal exposure to inhalant abuse among pregnant women to their babies [26]. However, the toxicity of xylene alone was not evaluated in these studies.
In vitro studies explored further the protective effects of certain vegetables and medicinal plants against xylene toxicity using bovine and porcine ovarian granulosa cells [17,18,21]. Nevertheless, the mechanism of xylene toxicity when absorbed into human and animal bodies is not well understood. It had been suggested that the biotransformation of xylene takes place in the liver through several enzymatic processes that produce a toxic aldehyde called p-tolualdehyde, which is believed to be the compound that exerts the toxic effects [65]. Preferably, the studies regarding the potential protective effects against xylene must be later verified by conducting suitable in vivo studies [18].
From in vitro studies using bovine ovarian cells, technical xylene was found to trigger the release of insulin-like growth factor hormone, IGF-1, and have the potential of inducing malignancy [17]. The study also found that xylene reduced the release of progesterone. Further investigation revealed that plant-derived flavonoids, called quercetin, and a medicinal plant, chia seeds, have protective effects against the deleterious action of xylene in vitro [17,18]. In contrast, other studies demonstrated stimulatory effects of xylene on the release of progesterone, while simultaneously hampering the cell survival indicated by an increase in apoptotic signal. These findings were evident through studies by Sirotkin and his team. Using a closely resembled experimental design, they assessed the in situ effects of xylene, as a mixture of environmental contaminants, on several mammalian ovarian cell cultures [16,19].
It is important that in vitro studies are validated through sufficient and reliable in vivo studies to reinforce the vital weaknesses of the non-physiological environment of in vitro cultures. It must be re-emphasized that the in vitro environment does not reflect the living animals’ body temperature, electrolyte concentrations, and metabolisms [18,67]. These in vitro studies also did not justify the selected concentrations of xylene used for the treatment as analogous to that of actual exposure to living animals. Furthermore, the scholars did not take into account that xylene is believed to affect female reproduction negatively through its metabolized aldehyde form, which occurs in the liver by microsomal enzymes [64,65]. In vitro tests were even, in some literature, referred to as impracticable for regulatory purposes [67]. Nevertheless, these scholarly articles have been useful in updating the currently available data related to the subject matter. The results could be potentially congruent to the readily documented data or future studies to explore in-depth the undesirable outcome of technical xylene to female reproductive functions.
From human studies, it was concluded that xylene affects menstruation by prolonging its cycle. This finding was evidenced through a couple of cross-sectional population studies aiming to explore the negative effects of occupational exposure of organic solvents and BTEX to female workers [27,33]. Oligomenorrhoea, following exposure to organic solvents at low levels, was also prevalent among the female employees of petroleum and chemical processing plants in China [27]. The occurrence of menstrual cycle disturbances among females following exposure to xylene was also probed within a smaller occupational setting. Additionally, the use of BTEX compounds in confined spaces such as beauty salons increased the ambient levels of xylene in the working space. Exposure to xylene in these smaller occupational settings was also found to cause menstrual disturbances with other health problems amongst female workers, e.g., skin irritations, headaches, and increased heart rate [33].
Apart from menstrual cycle disturbance, endocrine disruption was also found to occur following xylene exposure. It was evidenced from a study that occupational exposure to xylene and its isomers did affect the outcome of reproductive endocrine regulations [28]. In Reutman’s population study, several endocrine findings proved that xylene has an endocrine disruptive characteristic [28]. The endocrine functions of female employees working in the Air Force Base of the United States were assessed through questionnaires, interviews, and quantitation of urinary endocrine hormones, namely progesterone, luteinizing hormone, and estradiol. These endocrine landmarks were selected because of their predictive values of conception among women at their reproductive ages [28,52,53]. Although there have been reports on endocrine-related adverse outcomes (e.g., breast cancer and prostate cancer) of environmental pollutants, including xylene, scientific ambiguities remain on the cause of these reported effects [50]. Nevertheless, it has been suggested that endocrine is adversely affected by organic solvents and other environmental toxicants through hormone disruption where the chemical with a similar structure to endogenous molecules enters the reproductive organs and hampers the normal cellular processes, e.g., mitosis, meiosis, apoptosis, migration, and cellular signaling [68]. In agreement, environmental toxicants may induce cellular chemosensitivity, which leads to aberrant chromosomal alterations with an adverse outcome to female reproduction [32].
Due to the ubiquity of the source of exposure to the volatile organic compounds, there was an assessment conducted to evaluate the content of these chemicals in the feminine hygiene products being used by reproductive-aged women. Concerns regarding reproductive health were raised when feminine products such as vaginal douches, tampons, sanitary napkins, and feminine sprays were found to be the probable source of m-xylene and p-xylene exposures with other volatile organic compounds [36]. This recently published study, however, utilized the data regarding feminine hygiene products collected over a period of four years, more than 15 years ago. This study did not eliminate other contributing factors that might be responsible for compound exposure due to data absence. Furthermore, the nature of the cross-sectional data used for this study did not reflect the current pattern of the subjects being studied. In addition, the questionnaire, based on which the data were collected, was vulnerable to bias and inaccurate categorization [36].
The effects of xylene exposure on the female reproductive functions from human studies investigate only some of the xylene-containing organic solvents to which women are exposed in occupational settings. In addition, all studies were conducted as cross-sectional population studies, in which the study populations were selected based on pre-designed inclusion or exclusion criteria [27,28,33]. Due to the nature of occupational settings, from which the recruited subjects were exposed to the compounds of interest, none of these studies investigated technical xylene mixture in specific. Hence, it is highly recommended, as a future direction, that the reproductive effects of xylene should be studied as an individual compound, within an occupational setting such as the healthcare setting. In healthcare facilities, particularly histology processing laboratories, technical xylene is extensively used, not just as a pure compound but in massive volumes by laboratory personnel on a routine basis. This is in line with several published scholarly opinions that future studies must contribute in addition to the existing work and form the ground for another goal rather than reiterating the subject being studied [69,70].
Quoted from George E.P. Box, a renowned British statistician; “All models are wrong, some models are useful” [66], it should be taken into the fundamental understanding that scientific data from various study designs used in regulatory toxicology are subjected to limitations and drawbacks. Their core reliance on mechanistic models to reach any decisions remains unchanged significantly over decades; therefore, making animal studies the methods of choice in gathering toxicological data [67]. A huge number of toxicant-caused health problems are modeled by animals where their physiological states and tissues being tested, but a duplication of observed interactions are not attainable in non-animal models [67]. Human and animal (in vivo) studies and in vitro assays have been identified as the experimental approaches to fit the purpose of assessing the adverse effects of xylene on female reproductive outcomes. Many aspects of females’ reproduction were explored, i.e., menstrual regulation, ovarian functions, endocrine characteristics, as well as prenatal and postnatal developmental aspects [9,17,18,19,27,28,33,36,58].
In toxicology studies using animal models to determine the exposure limits, the toxicity responses are often assessed as a dichotomous response (e.g., presence or absence of malignancy), continuous measures (e.g., organ weight), or ordinal units (e.g., pain score) [71]. Although the dose-related effects are clearly defined, such studies are subject to remarkable uncertainties such as species differences, routes, and duration of exposure. Besides, the potency of the selected exposures in humans is highly unpredictable [71]. Generally, the occupational exposure limits can be established confidently by setting up a high-quality epidemiology study or an inhalation study using human volunteers under a fully regulated setting [72]. In chemical toxicity studies, both human and animal models pose challenges but are necessary to improve scientific certainty by fulfilling the requirements to integrate the chemical and species-specific data into the risk evaluation [72].
From the existing research articles taken for this systematic review, it can be summarized that the acquirement of data regarding the reproductive toxicity of xylene was vastly achieved through epidemiology and toxicology. Of these two disciplines, the latter provides more comprehensive knowledge pertaining to human exposures and adverse effects. None of these studies investigated xylene toxicities at the histological aspect of the female reproduction that could aid further understanding of xylene’s mechanism of toxicity. It was also stated that toxicological properties of xylene have not been thoroughly investigated [73]. Thus, these data must be kept updated with improved study approaches that are methodologically substantial to support and renew the existing information from previous literature.
Concerning occupational exposure to xylene, this compound was only studied as a joining member from a larger class of compounds, i.e., organic solvents, volatile organic compounds, or environmental pollutants [27,28,33]. There was no study found over the past 30 years that thoroughly investigated the effects of technical xylene as an individually used compound within occupational settings, except for the animal developmental toxicity study conducted by Saillenfait et al. [9]. In addition, the purpose of this review was to evaluate how much of the data regarding female reproductive and developmental toxicity of technical xylene has been updated. There were limited published studies that specifically focused on the toxicities of technical xylene to the female reproductive system. The process of incorporating toxicological science into human risk assessment is a complex effort. This review perhaps shall be able to provide justifications for future studies to fill the gaps of uncertainties regarding the adverse outcomes from xylene exposure to the female reproductive system. Furthermore, Langman [74] highlighted that the reported effects of xylene on pregnancy outcome and fertility in terms of spontaneous abortions and congenital defects are ambivalent [74].
5. Conclusions
This systematic review highlighted the current literature regarding the toxicity of technical xylene or mixed xylene on the female reproductive system. Based on human, animal, and in vitro studies, technical xylene affects the female reproductive system mainly from inhalational exposure. Technical xylene caused disturbances in menstrual regulation, endocrine performance, and ovarian cell functions. All human studies investigated the detrimental effects of xylene as organic solvent mixtures, which were appropriately in place according to the occupational settings of the studies. In the form of individual compounds, reproductive toxicities of xylene were only found from animal studies. Technical xylene exerts its toxicity to the female reproductive system possibly by interrupting hormone regulation or causing direct injuries to the ovarian cells (Figure 3). We recommend that future studies are necessary to clarify the uncertainties with regards to the toxicological effects of technical xylene exposure on the female reproductive system, both morphologically and functionally. Therefore, more relevant studies with improved study designs, methodologies, and scopes of research should be encouraged. In addition, further studies are needed to explore the mechanisms of the reproductive toxicity of xylene using appropriate animal models.
Author Contributions
Conceptualization, N.A.S.; methodology, M.A.A.; software, N.A.S.; validation, S.R.M.Z. and M.A.A.; formal analysis, N.A.S.; investigation, N.A.S.; resources, S.R.M.Z.; data curation, M.A.A.; writing—original draft preparation, N.A.S.; writing—review and editing, S.R.M.Z., M.A.A., M.H.M. and S.-Z.H.; visualization, N.A.S.; supervision, S.R.M.Z. and M.A.A.; project administration, N.A.S.; funding acquisition, S.R.M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Grant Scheme, FRGS-FP113-2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Myers, R.L. The 100 Most Important Chemical Compounds: A Reference Guide; ABC-CLIO: Santa Barbara, CA, USA, 2007. [Google Scholar]
- Rajan, S.T.; Malathi, N. Health hazards of xylene: A literature review. J. Clin. Diagn. Res. JCDR 2014, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Niaz, K.; Bahadar, H.; Maqbool, F.; Abdollahi, M. A review of environmental and occupational exposure to xylene and its health concerns. EXCLI J. 2015, 14, 1167. [Google Scholar] [PubMed]
- Bolden, A.L.; Kwiatkowski, C.F.; Colborn, T. New look at BTEX: Are ambient levels a problem? Environ. Sci. Technol. 2015, 49, 5261–5276. [Google Scholar] [CrossRef] [PubMed]
- Kandyala, R.; Sumanth Phani, S.; Raghavendra, C.S.; Rajasekharan, T. Xylene: An Overview of Its Health Hazards and Preventive Measures. J. Oral Maxilofac. Pathol. 2010, 14, 1–5. [Google Scholar] [CrossRef]
- Buesa, R.J.; Peshkov, M.V. Histology without xylene. Ann. Diagn. Pathol. 2009, 13, 246–256. [Google Scholar] [CrossRef]
- Fay, M.; Risher, J.; Wilson, J.D. Toxicological Profile for Xylene; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007. [Google Scholar]
- Balogh, T.; Tatrai, E.; Barczai, G. Study of the embryotoxic effect of xylene mixtures. Egeszsegtudomany 1982, 26, 42–48. [Google Scholar]
- Saillenfait, A.; Gallissot, F.; Morel, G.; Bonnet, P. Developmental toxicities of ethylbenzene, ortho-, meta-, para-xylene and technical xylene in rats following inhalation exposure. Food Chem. Toxicol. 2003, 41, 415–429. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Shaffril, H.A.M.; Samah, A.A.; Samsuddin, S.F.; Ali, Z. Mirror-mirror on the wall, what climate change adaptation strategies are practiced by the Asian’s fishermen of all? J. Clean. Prod. 2019, 232, 104–117. [Google Scholar] [CrossRef]
- Shaffril, H.A.M.; Krauss, S.E.; Samsuddin, S.F. A systematic review on Asian’s farmers’ adaptation practices towards climate change. Sci. Total Environ. 2018, 644, 683–695. [Google Scholar] [CrossRef]
- Germenis, A.; Kokkinides, P.; Stavropoulos-Giokas, C. Non-indexed medical journals in the Web: New perspectives in the medical literature. Int. J. Med. Inform. 1997, 47, 65–68. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Kádasi, A.; Baláži, A.; Baková, Z.; Harrath, A.H.; Makarevich, A.V.; Kolesárová, A.; Chrenek, P.; Kotwica, J.; Tóth, T. Influence of petrochemical industry environmental contaminants on animal ovarian cells. J. Microbiol. Biotechnol. Food Sci. 2012, 9, 517–525. [Google Scholar]
- Tarko, A.; Stochmalova, A.; Hrabovszka, S.; Vachanova, A.; Harrath, A.; Grossman, R.; Sirotkin, A. Can xylene and chia (Salvia hispanica L.) seed extract directly affect basic bovine ovarian cell functions? J. Anim. Feed Sci. 2017, 26, 109–115. [Google Scholar] [CrossRef]
- Tarko, A.; Štochmalova, A.; Hrabovszka, S.; Vachanova, A.; Harrath, A.H.; Alwasel, S.; Grossman, R.; Sirotkin, A.V. Can xylene and quercetin directly affect basic ovarian cell functions? Res. Vet. Sci. 2018, 119, 308–312. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kadasi, A.; Baláži, A.; Kotwica, J.; Alrezaki, A.; Harrath, A.H. Mechanisms of the direct effects of oil-related contaminants on ovarian cells. Environ. Sci. Pollut. Res. 2019, 27, 5314–5322. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Makarevich, A.V.; Kubovicova, E.; Medvedova, M.; Kolesarova, A.; Harrath, A.H. Relationship between body conditions and environmental contaminants in bovine ovarian cells. Theriogenology 2020, 147, 77–84. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Macejková, M.; Tarko, A.; Fabova, Z.; Alwasel, S.; Harrath, A.H. Buckwheat, rooibos, and vitex extracts can mitigate adverse effects of xylene on ovarian cells in vitro. Environ. Sci. Pollut. Res. 2021, 28, 7431–7439. [Google Scholar] [CrossRef]
- Hass, U.; Jakobsen, B.M. Prenatal toxicity of xylene inhalation in the rat: A teratogenicity and postnatal study. Pharmacol. Toxicol. 1993, 73, 20–23. [Google Scholar] [CrossRef]
- Saillenfait, A.; Gallissot, F.; Sabaté, J.-P.; Bourges-Abella, N.; Cadot, R.; Morel, G.; Lambert, A. Developmental toxicity of combined ethylbenzene and methylethylketone administered by inhalation to rats. Food Chem. Toxicol. 2006, 44, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.; Gallissot, F.; Sabaté, J.P.; Bourges-Abella, N.; Muller, S. Developmental toxic effects of ethylbenzene or toluene alone and in combination with butyl acetate in rats after inhalation exposure. J. Appl. Toxicol. Int. J. 2007, 27, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Reddy, M.K.; Mathur, N.; Saxena, D.; Chowdhuri, D.K. Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: Role of ROS generation. Toxicol. Appl. Pharmacol. 2009, 235, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Malloul, H.; Mahdani, F.M.; Bennis, M.; Ba-M’hamed, S. Prenatal exposure to paint thinner alters postnatal development and behavior in mice. Front. Behav. Neurosci. 2017, 11, 171. [Google Scholar] [CrossRef]
- Cho, S.-I.; Damokosh, A.I.; Ryan, L.M.; Chen, D.; Hu, Y.A.; Smith, T.J.; Christiani, D.C.; Xu, X. Effects of exposure to organic solvents on menstrual cycle length. J. Occup. Environ. Med. 2001, 43, 567–575. [Google Scholar] [CrossRef]
- Reutman, S.R.; LeMasters, G.K.; Knecht, E.A.; Shukla, R.; Lockey, J.E.; Burroughs, G.E.; Kesner, J.S. Evidence of reproductive endocrine effects in women with occupational fuel and solvent exposures. Environ. Health Perspect. 2002, 110, 805–811. [Google Scholar] [CrossRef]
- Aguilera, I.; Garcia-Esteban, R.; Iñiguez, C.; Nieuwenhuijsen, M.J.; Rodríguez, À.; Paez, M.; Ballester, F.; Sunyer, J. Prenatal exposure to traffic-related air pollution and ultrasound measures of fetal growth in the INMA Sabadell cohort. Environ. Health Perspect. 2010, 118, 705–711. [Google Scholar] [CrossRef]
- Lupo, P.J.; Symanski, E.; Waller, D.K.; Chan, W.; Langlois, P.H.; Canfield, M.A.; Mitchell, L.E. Maternal exposure to ambient levels of benzene and neural tube defects among offspring: Texas, 1999–2004. Environ. Health Perspect. 2011, 119, 397–402. [Google Scholar] [CrossRef]
- Ghosh, J.K.C.; Wilhelm, M.; Su, J.; Goldberg, D.; Cockburn, M.; Jerrett, M.; Ritz, B. Assessing the influence of traffic-related air pollution on risk of term low birth weight on the basis of land-use-based regression models and measures of air toxics. Am. J. Epidemiol. 2012, 175, 1262–1274. [Google Scholar] [CrossRef]
- Santiago, F.; Lima, S.; Pinheiro, T.; Silvestre, R.T.; Otero, U.B.; Tabalipa, M.M.; Kosyakova, N.; Ornellas, M.H.; Liehr, T.; Alves, G. Benzene poisoning, clinical and blood abnormalities in two Brazilian female gas station attendants: Two case reports. BMC Res. Notes 2017, 10, 52. [Google Scholar] [CrossRef]
- Moradi, M.; Hopke, P.; Hadei, M.; Eslami, A.; Rastkari, N.; Naghdali, Z.; Kermani, M.; Emam, B.; Farhadi, M.; Shahsavani, A. Exposure to BTEX in beauty salons: Biomonitoring, urinary excretion, clinical symptoms, and health risk assessments. Environ. Monit. Assess. 2019, 191, 286. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Lomelin, J.; Nielsen, C.C.; Jabbar, M.S.M.; Wine, O.; Bellinger, C.; Villeneuve, P.J.; Stieb, D.; Aelicks, N.; Aziz, K.; Buka, I. Interdisciplinary-driven hypotheses on spatial associations of mixtures of industrial air pollutants with adverse birth outcomes. Environ. Int. 2019, 131, 104972. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Bushrow, A.E.; Burmeister, C.; Lamerato, L.; Lemke, L.D.; Mathieu, M.; O’Leary, B.F.; Sperone, F.G.; Straughen, J.K.; Reiners, J.J., Jr. Prenatal airshed pollutants and preterm birth in an observational birth cohort study in Detroit, Michigan, USA. Environ. Res. 2020, 189, 109845. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Batterman, S.; Park, S.K. Exposure to Volatile Organic Compounds and Use of Feminine Hygiene Products among Reproductive-Aged Women in the United States. J. Women’s Health 2020, 29, 65–73. [Google Scholar] [CrossRef]
- Ungváry, G.; Varga, B.; Horváth, E.; Tátrai, E.; Folly, G. Study on the role of maternal sex steroid production and metabolism in the embryotoxicity of para-xylene. Toxicology 1981, 19, 263–268. [Google Scholar] [CrossRef]
- Dissen, G.A.; Paredes, A.; Romero, C.; Dees, L.W.; Ojeda, S.R. The Ovary, 2nd ed.; Elsevier Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Dowty, B.J.; Laseter, J.L.; Storer, J. The transplacental migration and accumulation in blood of volatile organic constituents. Pediatric. Res. 1976, 10, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Ungváry, G.; Tátrai, E.; Hudák, A.; Barcza, G.; Lõrincz, M. Studies on the embryotoxic effects of ortho-, meta-and para-xylene. Toxicology 1980, 18, 61–74. [Google Scholar] [CrossRef]
- OECD. Test Guideline 414. OECD Guideline for Testing of Chemicals, Prenatal Developmental Toxicity Study. 2001. [CrossRef]
- Faber, W.D.; Roberts, L.S.; Stump, D.G.; Beck, M.; Kirkpatrick, D.; Regan, K.S.; Tort, M.; Moran, E.; Banton, M. Inhalation developmental neurotoxicity study of ethylbenzene in Crl-CD rats. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2007, 80, 34–48. [Google Scholar] [CrossRef]
- Langman, J.M. Xylene: Its toxicity, measurement of exposure levels, absorption, metabolism and clearance. Pathology 1994, 26, 301–309. [Google Scholar] [CrossRef]
- Harlow, S.D.; Ephross, S.A. Epidemiology of menstruation and its relevance to women’s health. Epidemiol. Rev. 1995, 17, 265. [Google Scholar] [CrossRef]
- Merklinger-Gruchala, A.; Jasienska, G.; Kapiszewska, M. Effect of air pollution on menstrual cycle length—A prognostic factor of women’s reproductive health. Int. J. Environ. Res. Public Health 2017, 14, 816. [Google Scholar] [CrossRef] [PubMed]
- NIOSH. National Institute for Occupational Safety and Health. Hydrocarbons, Aromatic: Method 1501; NIOSH: Cincinnati, OH, USA, 1994. [Google Scholar]
- Song, H.N.; Kim, C.H.; Lee, W.Y.; Cho, S.H. Simultaneous determination of volatile organic compounds with a wide range of polarities in urine by headspace solid-phase microextraction coupled to gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; Mishell, D.; Stenchever, M.; Droegemueller, W. Comprehensive Gynecology; Mosby: St. Louis, MO, USA, 1992; pp. 79–140. [Google Scholar]
- Speroff, L.; Fritz, M.A. Clinical Gynecologic Endocrinology and Infertility; Lippincott Williams & wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Kavlock, R.J.; Daston, G.P.; DeRosa, C.; Fenner-Crisp, P.; Gray, L.E.; Kaattari, S.; Lucier, G.; Luster, M.; Mac, M.J.; Maczka, C. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the US EPA-sponsored workshop. Environ. Health Perspect. 1996, 104, 715–740. [Google Scholar]
- Verma, Y.; Rana, S.V.S. Endocrinal toxicity of industrial solvents—A mini review. Indian J. Exp. Biol. 2009, 47, 537–549. [Google Scholar] [PubMed]
- Baird, D.D. Characteristics of fertile menstrual cycles. Scand. J. Work. Environ. Health 1999, 25, 20–22. [Google Scholar]
- Baird, D.D.; Weinberg, C.R.; Zhou, H.; Kamel, F.; McConnaughey, D.R.; Kesner, J.S.; Wilcox, A.J. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil. Steril. 1999, 71, 40–49. [Google Scholar] [CrossRef]
- Brender, J.; Suarez, L.; Hendricks, K.; Baetz, R.A.; Larsen, R. Parental occupation and neural tube defect–affected pregnancies among Mexican Americans. J. Occup. Environ. Med. 2002, 44, 650–656. [Google Scholar] [CrossRef]
- McMartin, K.I.; Chu, M.; Kopecky, E.; Einarson, T.R.; Koren, G. Pregnancy outcome following maternal organic solvent exposure: A meta-analysis of epidemiologic studies. Am. J. Ind. Med. 1998, 34, 288–292. [Google Scholar] [CrossRef]
- Wennborg, H.; Magnusson, L.L.; Bonde, J.P.; Olsen, J. Congenital malformations related to maternal exposure to specific agents in biomedical research laboratories. J. Occup. Environ. Med. 2005, 47, 11–19. [Google Scholar] [CrossRef]
- Johnson, B.L. A review of the effects of hazardous waste on reproductive health. Am. J. Obstet. Gynecol. 1999, 181, S12–S16. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Influence of oil-related environmental pollutants on female reproduction. Reprod. Toxicol. 2017, 71, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.F.; Othman, G.O.; Othman, N.H.; Hassan, B.A. Study the Effects of Formaldehyde and Xylene Vapor on Lung and Testicular Tissue with Sperm Morphology of Adult Albino Rats. Polytech. J. 2021, 11, 46–51. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, S.; Wen, Z.; Li, H.; Huang, B.; Chen, Y.; Li, X.; Lin, H.; Wang, Y.; Ge, R.-S. Xylene delays the development of Leydig cells in pubertal rats by inducing reactive oxidative species. Toxicology 2021, 454, 152740. [Google Scholar] [CrossRef]
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Fishbein, L. An overview of environmental and toxicological aspects of aromatic hydrocarbons III. Xylene. Sci. Total Environ. 1985, 43, 165–183. [Google Scholar] [CrossRef]
- Condle, L.; Hill, J.; Borzelleca, J. Oral toxicology studies with xylene isomers and mixed xylenes. Drug Chem. Toxicol. 1988, 11, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Harper, C.; Drew, R. The biotransformation of p-xylene to a toxic aldehyde. Drug Metab. Dispos. 1978, 6, 368–374. [Google Scholar] [PubMed]
- Miller, M.; Edwards, J. Possible preferential metabolism of xylene isomers following occupational exposure to mixed xylenes. Int. Arch. Occup. Environ. Health 1999, 72, 89–97. [Google Scholar] [CrossRef]
- Box, G.E. Science and statistics. J. Am. Stat. Assoc. 1976, 71, 791–799. [Google Scholar] [CrossRef]
- Hartung, T.; Daston, G. Are in vitro tests suitable for regulatory use? Toxicol. Sci. 2009, 111, 233–237. [Google Scholar] [CrossRef]
- Sharara, F.I.; Seifer, D.B.; Flaws, J.A. Environmental toxicants and female reproduction. Fertil. Steril. 1998, 70, 613–622. [Google Scholar] [CrossRef]
- Cronin, P.; Ryan, F.; Coughlan, M. Undertaking a literature review: A step-by-step approach. Br. J. Nurs. 2008, 17, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ramdhani, A.; Ramdhani, M.A.; Amin, A.S. Writing a Literature Review Research Paper: A step-by-step approach. Int. J. Basic Appl. Sci. 2014, 3, 47–56. [Google Scholar]
- Wheeler, M.; Park, R.; Bailer, A.; Whittaker, C. Historical context and recent advances in exposure-response estimation for deriving occupational exposure limits. J. Occup. Environ. Hyg. 2015, 12, S7–S17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dankovic, D.; Naumann, B.; Maier, A.; Dourson, M.; Levy, L. The scientific basis of uncertainty factors used in setting occupational exposure limits. J. Occup. Environ. Hyg. 2015, 12, S55–S68. [Google Scholar] [CrossRef]
- Sigma Aldrich. Safety Data Sheet. Xylenes; MiliporeSigma, Ed.; Sigma Aldrich: Petaling Jaya, Malaysia, 2020. [Google Scholar]
- Langman, J. d-Limonene: Is it a safe, effective alternative to xylene? J. Histotechnol. 1995, 18, 131–137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).