Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Photosynthetic Parameters
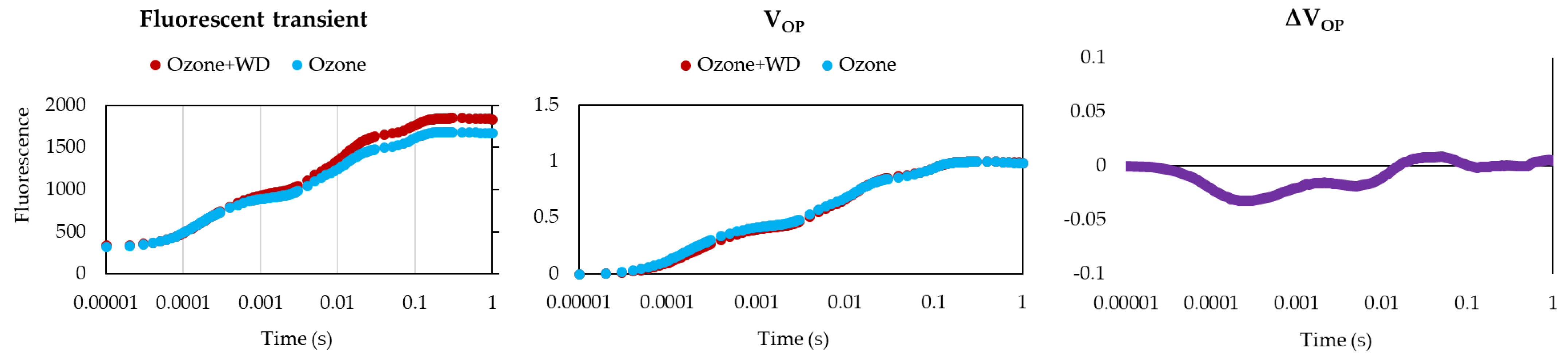
2.2.1. Chlorophyll Fluorescence Analyses
2.2.2. Analysis of the Chlorophyll Content
2.3. Expression of Antioxidants
2.3.1. Total Antioxidant Power
2.3.2. Content of Caffeic Acid and Quercetin
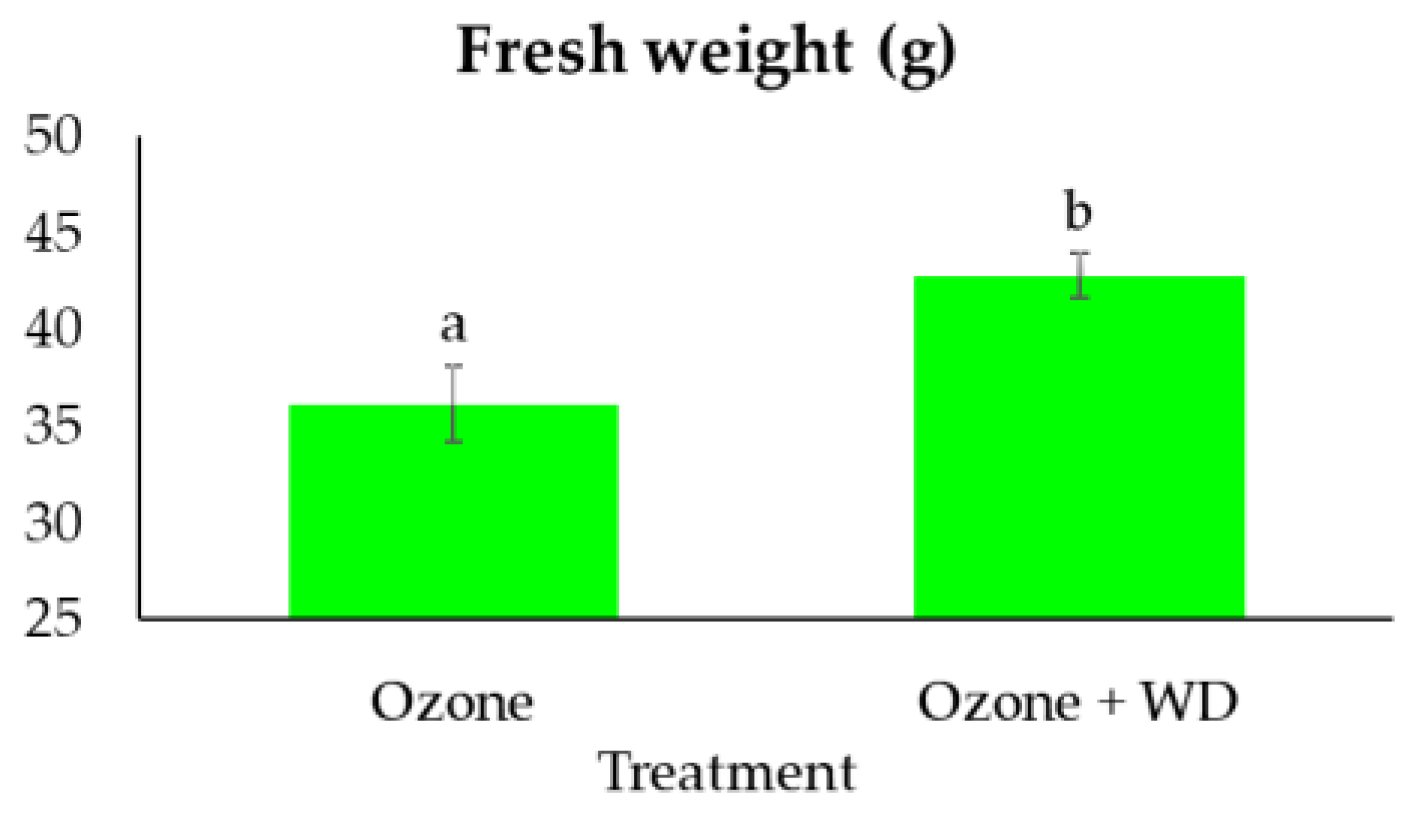
2.4. Edible Fresh Biomass
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, O.R.; Parrish, D.D.; Ziemke, J.R.; Balashov, N.V.; Cupeiro, M.; Galbally, I.; Gilge, S.; Horowitz, L.W.; Jensen, N.R.; Lamarque, J.-F.; et al. Global distribution and trends of tropospheric ozone: An observation-based review. Elementa Sci. Anthr. 2014, 2, 000029. [Google Scholar] [CrossRef]
- Emberson, L. Effects of ozone on agriculture, forests and grasslands. Philos. Trans. R. Soc. A 2020, 378, 20190327. [Google Scholar] [CrossRef]
- Grulke, N.E.; Heath, R.L. Ozone effects on plants in natural ecosystems. Plant Biol. 2019, 22, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C.; Fluhr, R. Ozone and Reactive Oxygen Species. eLS 2001. [Google Scholar] [CrossRef]
- Maliba, B.G.; Inbaraj, P.M.; Berner, J. The Use of OJIP Fluorescence Transients to Monitor the Effect of Elevated Ozone on Biomass of Canola Plants. Water Air Soil Pollut. 2019, 230, 75. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; De Marco, A.; Anav, A.; Gualtieri, M.; Sicard, P.; Tian, H.; Fornasier, F.; Tao, F.; Guo, A.; Paoletti, E. Economic losses due to ozone impacts on human health, forest productivity and crop yield across China. Environ. Int. 2019, 131, 104966. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Saitanis, C.J.; Agathokleous, E. Exogenous application of chemicals for protecting plants against ambient ozone pollution: What should come next? Curr. Opin. Environ. Sci. Health 2021, 19, 100215. [Google Scholar] [CrossRef] [PubMed]
- Didyk, N.P.; Blum, O.B. Natural antioxidants of plant origin against ozone damage of sensitive crops. Acta Physiol. Plant. 2010, 33, 25–34. [Google Scholar] [CrossRef]
- Bellini, E.; De Tullio, M.C. Ascorbic Acid and Ozone: Novel Perspectives to Explain an Elusive Relationship. Plants 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittipornkul, P.; Treesubsuntorn, C.; Thiravetyan, P. Effect of exogenous catechin and salicylic acid on rice productivity under ozone stress: The role of chlorophyll contents, lipid peroxidation, and antioxidant enzymes. Environ. Sci. Pollut. Res. 2020, 27, 25774–25784. [Google Scholar] [CrossRef] [PubMed]
- Macias-Benitez, S.; Navarro-Torre, S.; Caballero, P.; Martín, L.; Revilla, E.; Castaño, A.; Parrado, J. Biostimulant Capacity of an Enzymatic Extract From Rice Bran Against Ozone-Induced Damage in Capsicum annum. Front. Plant Sci. 2021, 12, 749422. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Zakaria, Z.A. Pyroligneous acid—the smoky acidic liquid from plant biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Mahmud, K.N.; Hashim, N.M.; Ani, F.N.; Zakaria, Z.A. Antioxidants, Toxicity, and Nitric Oxide Inhibition Properties of Pyroligneous Acid from Palm Kernel Shell Biomass. Waste Biomass Valorization 2020, 11, 6307–6319. [Google Scholar] [CrossRef]
- Vannini, A.; Moratelli, F.; Monaci, F.; Loppi, S. Effects of wood distillate and soy lecithin on the photosynthetic performance and growth of lettuce (Lactuca sativa L.). SN Appl. Sci. 2021, 3, 1–6. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117. [Google Scholar] [CrossRef] [PubMed]
- Biodea. Available online: https://biodea.bio/scheda-colturale/olive-trees/?lang=en (accessed on 15 January 2022).
- Chen, Y.-H.; Li, Y.-F.; Wei, H.; Li, X.-X.; Zheng, H.-T.; Dong, X.-Y.; Xu, T.-F.; Meng, J.-F. Inhibition efficiency of wood vinegar on grey mould of table grapes. Food Biosci. 2020, 38, 100755. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Uptake of Trace Elements in the Water Fern Azolla filiculoides after Short-Term Application of Chestnut Wood Distillate (Pyroligneous Acid). Plants 2020, 9, 1179. [Google Scholar] [CrossRef]
- Filippelli, A.; Ciccone, V.; Loppi, S.; Morbidelli, L. Characterization of the Safety Profile of Sweet Chestnut Wood Distillate Employed in Agriculture. Safety 2021, 7, 79. [Google Scholar] [CrossRef]
- Bussotti, F.; Strasser, R.J.; Schaub, M. Photosynthetic behavior of woody species under high ozone exposure probed with the JIP-test: A review. Environ. Pollut. 2007, 147, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis Mechanism, Regulation & Adaptation, 1st ed.; Mohammad, Y., Uday, P., Eds.; CRC Press: New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Gomes, M.T.G.; da Luz, A.C.; dos Santos, M.R.; Batitucci, M.d.C.P.; Silva, D.M.; Falqueto, A.R. Drought tolerance of passion fruit plants assessed by the OJIP chlorophyll a fluorescence transient. Sci. Hortic. 2012, 142, 49–56. [Google Scholar] [CrossRef]
- A Gitelson, A.; Buschmann, C.; Lichtenthaler, H.K. The Chlorophyll Fluorescence Ratio F735/F700 as an Accurate Measure of the Chlorophyll Content in Plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Vannini, A.; Canali, G.; Pica, M.; Nali, C.; Loppi, S. The Water Content Drives the Susceptibility of the Lichen Evernia prunastri and the Moss Brachythecium sp. to High Ozone Concentrations. Biology 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; Zaman, Q.U.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective Roles and Mechanisms of Caffeic Acid in Counter Plant Stress: A Mini Review. Pak. J. Agric. Res. 2018, 32, 32. [Google Scholar] [CrossRef]
- Tokuşoğlu, Ö.; Ünal, M.K.; Yıldırım, Z. HPLC-UV and GC-MS characterization of the fla-vonol aglycons quercetin, kaempferol, and myricetin in tomato pastes and other tomato-based products. Acta Chromatogr. 2003, 13, 196–207. [Google Scholar]
- Kumar, N.; Bhandari, P.; Singh, B.; Gupta, A.; Kaul, V.K. Reversed phase-HPLC for rapid determination of polyphenols in flowers of rose species. J. Sep. Sci. 2008, 31, 262–267. [Google Scholar] [CrossRef]
- Brauer, M.; Curtin, J.J. Linear mixed-effects models and the analysis of nonindependent data: A unified framework to analyze categorical and continuous independent variables that vary within-subjects and/or within-items. Psychol. Methods 2018, 23, 389–411. [Google Scholar] [CrossRef]
- Luke, S.G. Evaluating significance in linear mixed-effects models in R. Behav. Res. Methods 2016, 49, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 7 January 2022).
- Mills, G.; Buse, A.; Gimeno, B.; Bermejo, V.; Holland, M.; Emberson, L.; Pleijel, H. A synthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos. Environ. 2007, 41, 2630–2643. [Google Scholar] [CrossRef]
- Calatayud, A.; Ramirez, J.W.; Iglesias, D.J.; Barreno, E. Effects of ozone on photosynthetic CO2 exchange, chlorophyll a fluorescence and antioxidant systems in lettuce leaves. Physiol. Plant. 2002, 116, 308–316. [Google Scholar] [CrossRef]
- Calatayud, A.; Barreno, E. Response to ozone in two lettuce varieties on chlorophyll a fluorescence, photosynthetic pigments and lipid peroxidation. Plant Physiol. Biochem. 2004, 42, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.P.; Velez, J.P.A.; Pinzon, M.A.; Arango, J.A.M.; Muriel, A.P. Chemical Characterization and Antiradical Properties of Pyroligneous Acid from a Preserved Bamboo, Guadua angustifolia Kunth. Braz. Arch. Biol. Technol. 2021, 64, 21190730. [Google Scholar] [CrossRef]
- Benzon, H.R.L.; Lee, S.C. Pyroligneous Acids Enhance Phytoremediation of Heavy Metal-Contaminated Soils Using Mustard. Commun. Soil Sci. Plant Anal. 2017, 48, 2061–2073. [Google Scholar] [CrossRef]
- Berahim, Z.; Panhwar, Q.A.; Ismail, M.R.; Saud, H.M.; Mondal, A.; Naher, U.A.; Islam, R. Rice yield improvement by foliar application of phytohormone. J. Food Agric. Environ. 2018, 12, 399–404. [Google Scholar]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-Based Solutions for Agriculture: Foliar Application of Wood Distillate Alone and in Combination with Other Plant-Derived Corroborants Results in Different Effects on Lettuce (Lactuca Sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Theerakulpisut, P.; Kanawapee, N.; Panwong, B. Seed Priming Alleviated Salt Stress Effects on Rice Seedlings by Improving Na+/K+ and Maintaining Membrane Integrity. Int. J. Plant Biol. 2016, 7, 6402. [Google Scholar] [CrossRef] [Green Version]
- Tanase, C.; Bara, C.I.; Popa, V.I. Cytogenetical effect of some polyphenol compounds separated from industrial by-products on. Cell. Chem. Technol. 2015, 49, 799–805. [Google Scholar]
- Tanase, C.; Bujor, O.-C.; Popa, V.I. Phenolic Natural Compounds and Their Influence on Physiological Processes in Plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–58. [Google Scholar] [CrossRef]
- Wilkinson, S.; Mills, G.; Illidge, R.; Davies, W.J. How is ozone pollution reducing our food supply? J. Exp. Bot. 2012, 63, 527–536. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root Proteomics Reveals the Effects of Wood Vinegar on Wheat Growth and Subsequent Tolerance to Drought Stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef] [Green Version]
- Hassan, I.A.; Haiba, N.S.; Badr, R.H.; Basahi, J.M.; Almeelbi, T.; Ismail, I.M.; Taia, W.K. Effects of ambient ozone on reactive oxygen species and antioxidant metabolites in leaves of pea (Pisum sativum L.) plants. Pak. J. Bot. 2017, 49, 47–55. [Google Scholar]
- Diao, E.; Wang, J.; Li, X.; Wang, X.; Song, H.; Gao, D. Effects of ozone processing on patulin, phenolic compounds and organic acids in apple juice. J. Food Sci. Technol. 2019, 56, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Baek, S.-A.; Kim, N.S.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Elevated Ozone Levels Affect Metabolites and Related Biosynthetic Genes in Tartary Buckwheat. J. Agric. Food Chem. 2020, 68, 14758–14767. [Google Scholar] [CrossRef]
- Kreczmer, B.; Dyba, B.; Barbasz, A.; Rudolphi-Szydło, E. Advantageous/Unfavorable Effect of Quercetin on the Membranes of SK-N-SH Neuroblastoma Cells. Molecules 2021, 26, 4945. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Grzesiak, S. Possible contribution of cell-wall-bound ferulic acid in drought resistance and recovery in triticale seedlings. J. Plant Physiol. 2009, 166, 1720–1733. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Gandin, A.; Dizengremel, P.; Jolivet, Y. Integrative role of plant mitochondria facing oxidative stress: The case of ozone. Plant Physiol. Biochem. 2020, 159, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Kawasaki, M.; Taniguchi, M.; Miyake, H. Correlation between Chloroplast Ultrastructure and Chlorophyll Fluorescence Characteristics in the Leaves of Rice (Oryza sativa L.) Grown under Salinity. Plant Prod. Sci. 2008, 11, 139–145. [Google Scholar] [CrossRef]
- Lascano, H.; Melchiorre, M.N.; Luna, C.M.; Trippi, V.S. Effect of photooxidative stress induced by paraquat in two wheat cultivars with differential tolerance to water stress. Plant Sci. 2003, 164, 841–848. [Google Scholar] [CrossRef]
- Pellegrini, E.; Cotrozzi, L.; Neri, L.; Baraldi, R.; Carrari, E.; Nali, C.; Lorenzini, G.; Paoletti, E.; Hoshika, Y. Stress markers and physiochemical responses of the Mediterranean shrub Phillyrea angustifolia under current and future drought and ozone scenarios. Environ. Res. 2021, 201, 111615. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, E.; Francini, A.; Lorenzini, G.; Nali, C. Ecophysiological and antioxidant traits of Salvia officinalis under ozone stress. Environ. Sci. Pollut. Res. 2015, 22, 13083–13093. [Google Scholar] [CrossRef]
- Allen, J.; Williams, J. Photosynthetic reaction centers. FEBS Lett. 1998, 438, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Thwe, A.A.; Kasemsap, P. Quantification of OJIP Fluorescence Transient in Tomato Plants Under Acute Ozone Stress. Agric. Nat. Resour. 2014, 48, 665–675. [Google Scholar]
- Gottardini, E.; Cristofori, A.; Cristofolini, F.; Nali, C.; Pellegrini, E.; Bussotti, F.; Ferretti, M. Chlorophyll-related indicators are linked to visible ozone symptoms: Evidence from a field study on native Viburnum lantana L. plants in northern Italy. Ecol. Indic. 2014, 39, 65–74. [Google Scholar] [CrossRef]
- Kirschbaum, M.U. Does Enhanced Photosynthesis Enhance Growth? Lessons Learned from CO2 Enrichment Studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Description |
|---|---|
| ABS/CS0 | Absorbance for excited cross-section |
| TR0/CS0 | Trapping flux for excited cross-section |
| ET0/CS0 | Energy transmission for excited cross-section |
| RC/CS0 | Number of reaction centers for excited cross-section |
| DI0/CS0 | Heat dissipation for excited cross-section |
| FV/FM | Photosynthetic efficiency |
| PIABS | Performance index |
| Parameter | Ozone | Ozone + WD | p-Value |
|---|---|---|---|
| ABS/CS0 | 293 ± 2.5 a | 301 ± 2.6 b | p < 0.05 |
| DI0/CS0 | 51 ± 0.5 a | 49 ± 0.5 b | p < 0.01 |
| TR0/CS0 | 242 ± 2.0 a | 252 ± 2.1 b | p < 0.001 |
| ET0/CS0 | 131 ± 1.4 a | 141 ± 1.4 b | p < 0.001 |
| RC/CS0 | 105 ± 1.1 a | 117 ± 1.1 b | p < 0.001 |
| FV/FM | 0.826 ± 0.001 a | 0.837 ± 0.001 b | p < 0.001 |
| PIABS | 2.05 ± 0.03 a | 2.56 ± 0.04 b | p < 0.001 |
| Chlorophyll (mg/m2) | 200 ± 3.7 a | 225 ± 2.7 b | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannini, A.; Fedeli, R.; Guarnieri, M.; Loppi, S. Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca sativa L.). Toxics 2022, 10, 178. https://doi.org/10.3390/toxics10040178
Vannini A, Fedeli R, Guarnieri M, Loppi S. Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca sativa L.). Toxics. 2022; 10(4):178. https://doi.org/10.3390/toxics10040178
Chicago/Turabian StyleVannini, Andrea, Riccardo Fedeli, Massimo Guarnieri, and Stefano Loppi. 2022. "Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca sativa L.)" Toxics 10, no. 4: 178. https://doi.org/10.3390/toxics10040178
APA StyleVannini, A., Fedeli, R., Guarnieri, M., & Loppi, S. (2022). Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca sativa L.). Toxics, 10(4), 178. https://doi.org/10.3390/toxics10040178









