Antibiotics in Wastewater: Baseline of the Influent and Effluent Streams in Kuwait
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analytical Methods
2.3. Statistical Analyses
3. Results and Discussion
3.1. Removal Efficiencies from the Influents of the Two WWTPs
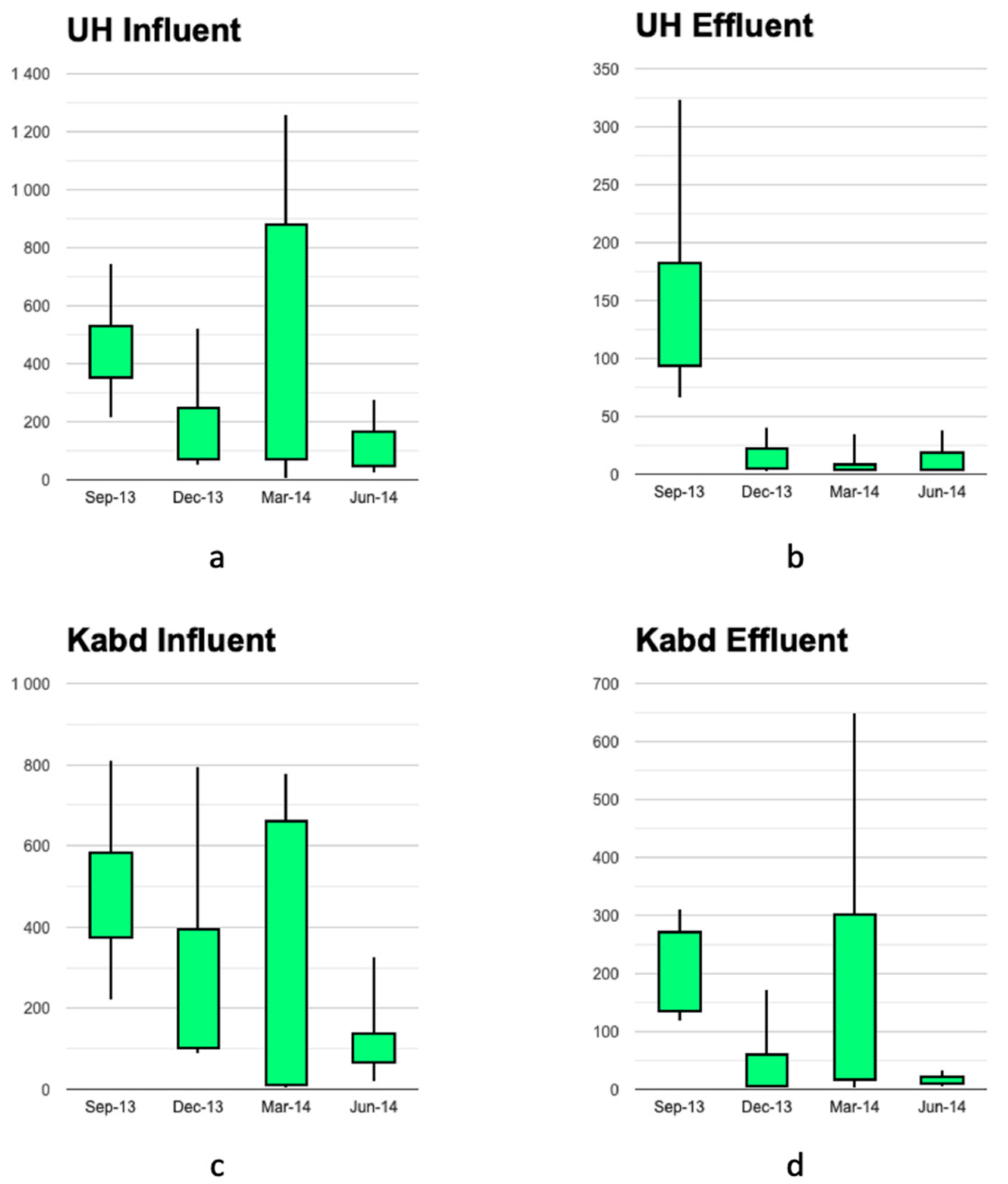
3.2. Temporal Variability in the Concentration of Antibiotics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, K.V.; Langford, K.H. Point Sources of Human Pharmaceuticals into the Aquatic Environment. In Green and Sustainable Pharmacy; Kümmerer, K., Hempel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 211–223. [Google Scholar]
- Daughton, C.G. Pharmaceuticals & Personal Care Products (PPCPs) in the Environment: Agents of Subtle Change? USEPA, NERL: Las Vegas, NV, USA, 2001. [Google Scholar]
- Daughton, C.G.; Jones-Lepp, T. Pharmaceuticals and Personal Care Products in the Environment: Scientific and Regulatory Issues; American Chemical Society: Washington, DC, USA, 2001; p. 396. [Google Scholar]
- Daughton, C.G.; Ruhoy, I.S. Reducing the Ecological Footprint of Pharmaceutical Usage: Linkages between Healthcare Practices and the Environment, in Green and Sustainable Pharmacy; Kümmerer, K., Hempel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 77–102. [Google Scholar]
- Brain, R.A.; Solomon, K.R.; Brooks, B.W. Targets, Effects and Risks in Aquatic Plants Exposed to Veterinary Antibiotics. In Veterinary Pharmaceuticals in the Environment; Coats, J., Henderson, K., Eds.; American Chemical Society: Washington, DC, USA, 2009. [Google Scholar]
- Brooks, B.W.; Ankley, G.L.; Hobson, J.F.; Lazorchak, J.M.; Meyerhoff, R.L.; Solomon, K.R. Aquatic hazard assessment of veterinary medicines. In Veterinary Pharmaceuticals in the Environment; Crane, M., Barrett, K., Boxall, A., Eds.; SETAC Press, Taylor and Francis: Pensicola, FL, USA, 2009. [Google Scholar]
- Brooks, B.W.; Huggett, D.; Boxall, A. Pharmaceuticals and Personal Care Products: Research Needs for the Next Decade. Environ. Toxicol. Chem. 2009, 28, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Brain, R.A.; Huggett, D.B.; Ankley, G.T. Risk assessment considerations for veterinary medicines in aquatic systems. In Veterinary Pharmaceuticals in the Environment; Coats, J., Henderson, K., Eds.; American Chemical Society: Washington, DC, USA, 2009; pp. 205–223. [Google Scholar]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della Giustina, S.V.; Llorca, M.; Barcelo, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, W.; Liang, H.; Gao, D. Occurrence and fate of typical antibiotics in wastewater treatment plants in Harbin, North-east China. Front. Environ. Sci. Eng. 2019, 13, 34. [Google Scholar] [CrossRef]
- Gevao, B.; Uddin, S.; Dupont, S. Baseline concentrations of pharmaceuticals in Kuwait’s coastal marine environment. Mar. Pollut. Bull. 2021, 173, 113040. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.F. Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Kümmerer, K.; Henninger, A. Promoting resistance by the emission of antibiotics from hospitals and households into effluent. Clin. Microbiol. Infect. 2003, 9, 1203–1214. [Google Scholar] [CrossRef]
- Blair, B.D.; Crago, J.P.; Hedmna, C.J.; Klaper, R.D. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere 2013, 93, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Binh, V.N.; Dang, N.; Kieu Anh, N.T.; Ky, L.X.; Thai, P.K. Antibiotics in the aquatic environment of Vietnam: Sources, concentrations, risk and control strategy. Chemosphere 2018, 197, 438–450. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, I.-S.; Oh, J.-E. Human and veterinary pharmaceuticals in the marine environment including fish farms in Korea. Sci. Total Environ. 2017, 579, 940–949. [Google Scholar] [CrossRef]
- Du, J.; Zhao, H.; Liu, S.; Xie, H.; Wang, Y.; Chen, J. Antibiotics in the coastal water of the South. Yellow Sea in China: Occurrence, distribution and ecological risks. Sci. Total Environ. 2017, 595, 521–527. [Google Scholar]
- Zhang, R.; Yu, K.; Wang, Y.; Huang, X.; Pei, J.; Wei, C.; Pan, Z.; Qin, Z.; Zhang, G. Occurrence, sources and transport of antibiotics in the surface water of coral reef regions in the South. China Sea: Potential risk to coral growth. Environ. Pollut. 2018, 232, 450–457. [Google Scholar] [CrossRef]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2012, 1248, 104–121. [Google Scholar] [PubMed]
- Daughton, C.G.; Ruhoy, I.S. Environmental footprint of pharmaceuticals: The significance of factors beyond direct excretion to sewers. Environ. Toxicol. Chem. 2009, 28, 2495–2521. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Pharmaceuticals in the Environment: Knowing and Managing the Risks, in a Healthy Future—Pharmaceuticals in a Sustainable Society; MistraPharma, and Stockholm County Council: Stockholm, Sweden, 2009; pp. 124–147. [Google Scholar]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef]
- Palli, L.; Spina, F.; Varese, C.; Romagnolo, A.; Bonari, A.; Bossi, I.; Pompilio, I.; Dugheri, S.; Tilli, S.; Scozzafava, A.; et al. Pharmaceuticals in Wastewater Treatment Plants of Tuscany: Occurrence and Toxicity; Springer: Cham, Switzerland, 2017; pp. 308–312. [Google Scholar]
- Halling-Sorensen, B.; Nielsen, S.N.; Lanzky, P.F.; Ingerslev, F.; Lutzhoft, H.C.; Jorgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Di Guardo, A.; Calamari, D.; Benfenati, E.; Halling-Sorensen, B.; Zuccato, E.; Fanelli, R. Pharmaceuticals as Environmental Contaminants: Modelling Distribution and Fate, in Pharmaceuticals in the Environment—Sources, Fate, Effects and Risks; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 183–194. [Google Scholar]
- Aubertheau, E.; Stalder, T.; Mondamert, L.; Ploy, M.-C.; Dagot, C.; Labanowski, J. Impact of wastewater treatment plant discharge on the contamination of river biofilms by pharmaceuticals and antibiotic resistance. Sci. Total Environ. 2017, 579, 1387–1398. [Google Scholar] [CrossRef]
- Uddin, S.; Aba, A.; Behbahani, M. Baseline concentration of 210Po and 210Pb in Sargassum from the northern Gulf. Mar. Pollut. Bull. 2015, 90, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Castiglioni, S.; Fanelli, R.; Reitano, G.; Bagnati, R.; Chiabrando, C.; Pomati, F.; Rossetti, C.; Calamari, D. Pharmaceuticals in the environment in Italy: Causes, occurrence, effects and control. Environ. Sci. Pollut. Res. 2006, 13, 15–21. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of Pharmaceuticals in Sewage Treatment Plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Silva, L.J.G.; Laranjeiro, C.S.M.; Meisel, L.M.; Lino, C.M.; Pena, A. Human pharmaceuticals in Portuguese rivers: The impact of water scarcity in the environmental risk. Sci. Total Environ. 2017, 609, 1182–1191. [Google Scholar] [CrossRef]
- Younes, H.A.; Mahmoud, H.M.; Abdelrahman, M.M.; Nassar, H.F. Seasonal occurrence, removal efficiency and associated ecological risk assessment of three antibiotics in a municipal wastewater treatment plant in Egypt. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100239. [Google Scholar] [CrossRef]
- Abbassi, B.; Abusaleem, M.; Zytner, R.; Gharabaghi, B.; Rudra, R. Antibiotics in wastewater: Their degradation and effect on wastewater treatment efficiency. J. Food Agric. Environ. 2016, 14, 95–99. [Google Scholar]
- Denver, J.; Dick, M.E.; Gervin, L.; Hughes, S.; McCumby, H.K.; Didriksen North, K.; Valiela, L.; Ventura, A. Discussion Paper on Pharmaceutical Disposal to Sewer Systems; Emerging Contaminants Workgroup of the Santa Clara Basin Watershed Management Initiative: San Jose, CA, USA, 2005; p. 18. [Google Scholar]
- Di Marcantonio, C.; Chiavola, A.; Dossi, S.; Cecchini, G.; Leoni, S.; Frugis, A.; Spizzirri, M.; Boni, M.R. Occurrence, seasonal variations and removal of Organic Micropollutants in 76 Wastewater Treatment Plants. Process. Saf. Environ. Prot. 2020, 141, 61–72. [Google Scholar] [CrossRef]
- Kreuzinger, N.; Clara, M.; Strenn, B.; Vogel, B. Investigation on the behaviour of selected pharmaceuticals in the groundwater after infiltration of treated wastewater. Water Sci. Technol. 2004, 50, 221–228. [Google Scholar] [CrossRef][Green Version]
- Clara, M.; Kreuzinger, N.; Strenn, B.; Gans, O.; Kroiss, H. The solids retention time—A suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Miège, C.; Choubert, J.M.; Ribeiro, L.; Eusèbe, M.; Coquery, M. Fate of pharmaceuticals and personal care products in wastewater treatment plants—Conception of a database and first results. Environ. Pollut. 2009, 157, 1721–1726. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Behbehani, M. An assessment of microplastic inputs into the aquatic environment from wastewater streams. Mar. Pollut. Bull. 2020, 160, 111538. [Google Scholar] [CrossRef]
- Naji, A.; Azadkhah, S.; Farahani, H.; Uddin, S.; Khan, F.R. Microplastics in wastewater outlets of Bandar Abbas city (Iran.): A potential point source of microplastics into the Persian Gulf. Chemosphere 2021, 262, 128039. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Behbehani, M. Micro-Nano Plastic in the Aquatic Environment: Methodological Problems and Challenges. Animals 2022, 12, 297. [Google Scholar] [CrossRef]
- Kotwani, A.; Joshi, J.; Kaloni, D. Pharmaceutical effluent: A critical link in the interconnected ecosystem promoting antimicrobial resistance. Environ. Sci. Pollut. Res. Int. 2021, 28, 32111–32124. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, W.; Korzeniewska, E.; Harnisz, M.; Drzymała, J.; Felis, E.; Bajkacz, S. Wastewater treatment plants as a reservoir of integrase and antibiotic resistance genes—An epidemiological threat to workers and environment. Environ. Int. 2021, 156, 106641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, P.; Yang, Q. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci. Total Environ. 2018, 621, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Occurrence of antibiotics and antibiotic resistance genes in soils from wastewater irrigation areas in the Pearl River Delta region, southern China. Sci. Total Environ. 2018, 624, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Wang, J.; Cao, R.; Yang, M.; Zhang, Y.; Qiang, Z. Distribution of antibiotic resistance in the effluents of ten municipal wastewater treatment plants in China and the effect of treatment processes. Chemosphere 2017, 172, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Daqing, M.; Rysz, M.; Qixing, Z.; Hongjie, Z.; Lin, X.; Alvarez, P.J.J. Trends in Antibiotic Resistance Genes Occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 44, 7220–7225. [Google Scholar]
- Sabri, N.A.; Schmitt, H.; Van der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in The Netherlands. J. Environ. Chem. Eng. 2020, 8, 102245. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; Ciavola, M.; Rizzo, L.; McDowell, D.A.; Byrne, J.A. Effect of photocatalysis on the transfer of antibiotic resistance genes in urban wastewater. Catal. Today 2015, 240, 55–60. [Google Scholar] [CrossRef]
- Anthony, A.; Adekunle, A.F.; Thor, S. Residual antibiotics, antibiotic resistant superbugs and antibiotic resistance genes in surface water catchments: Public health impact. Phys. Chem. Earth 2018, 105, 177–183. [Google Scholar] [CrossRef]
- Jendrzejewska, N.; Karwowska, E. The influence of antibiotics on wastewater treatment processes and the development of antibiotic-resistant bacteria. Water Sci. Technol. 2018, 77, 2320–2326. [Google Scholar] [CrossRef]
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ. Sci. Technol. 2005, 39, 8220–8226. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography-tandem mass spectrometry detection. J. Chromatogr. A 2006, 1134, 101–111. [Google Scholar] [CrossRef]
- van Nuijs, A.L.N.; Pecceu, B.; Theunis, L.; Covaci, A.; Bervoets, L.; Dubois, N.; Jorens, P.G.; Blust, R.; Charlier, C.; Neels, H. Cocaine and metabolites in waste and surface water across Belgium. Environ. Pollut. 2009, 157, 123–129. [Google Scholar] [CrossRef]
- van Nuijs, A.L.N.; Castiglioni, S.; Tarcomnicu, I.; Postigo, C.; de Alda, M.L.; Neels, H.; Zuccato, E.; Barcelo, D.; Covaci, A. Illicit drug consumption estimations derived from wastewater analysis: A critical review. Sci. Total Environ. 2011, 409, 3564–3577. [Google Scholar] [CrossRef]
- Kim, J.-W.; Ishibashi, H.; Yamauchi, R.; Ichikawa, N.; Takao, Y.; Hirano, M.; Koga, M.; Arizono, K. Acute toxicity of pharmaceutical and personal care products on freshwater crustacean (Thamnocephalus platyurus) and fish (Oryzias latipes). J. Toxicol. Sci. 2009, 34, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Al-Murad, M.; Uddin, S.; Rashid, T.; Al-Qallaf, H.; Bushehri, A. Waterlogging in Arid Agriculture Areas Due to Improper Groundwater Management—An Example from Kuwait. Sustainability 2017, 9, 2131. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Afonso-Olivares, C.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. An Assessment of the Concentrations of Pharmaceutical Compounds in Wastewater Treatment Plants on the Island of Gran Canaria (Spain). SpringerPlus 2013, 2, 24. [Google Scholar] [CrossRef][Green Version]
- Praveena, S.M.; Shaifuddin, S.N.M.; Sukiman, S.; Nasir, F.A.M.; Hanafi, Z.; Kamarudin, N.; Ismail, T.H.T.; Aris, A.Z. Pharmaceuticals residues in selected tropical surface water bodies from Selangor (Malaysia): Occurrence and potential risk assessments. Sci. Total Environ. 2018, 642, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Balakrishna, K.; Sinha, R.K.; Yamashita, N.; Balasubramanian, V.G.; Kannan, K. Mass loading and removal of pharmaceuticals and personal care products, including psychoactive and illicit drugs and artificial sweeteners, in five sewage treatment plants in India. J. Environ. Chem. Eng. 2015, 3, 2882–2891. [Google Scholar] [CrossRef]
- Ali, A.; Rønning, H.; Alarif, W.; Kallenborn, R.; Al-Lihaibi, S. Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 2017, 175, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Occurrence of antibiotics and risk of antibiotic resistance evolution in selected Kenyan wastewaters, surface waters and sediments. Sci. Total Environ. 2020, 720, 137580. [Google Scholar] [CrossRef] [PubMed]
- Semerjian, L.; Shanableh, A.; Semreen, M.H.; Samarai, M. Human health risk assessment of pharmaceuticals in treated wastewater reused for non-potable applications in Sharjah, United Arab Emirates. Environ. Int. 2018, 121, 325–331. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Deng, S.; Chen, W.; Yu, G. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes. Environ. Sci. Technol. 2011, 45, 3341–3348. [Google Scholar] [CrossRef]
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment—A review. J. Water Process. Eng. 2022, 45, 102474. [Google Scholar]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Voulvoulis, N.; Lesten, J.N. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res. 2002, 36, 5013–5022. [Google Scholar] [CrossRef]
- Jones, S. Assessing the effectiveness of one-stop dispensing. Hosp. Pharm. 2002, 9, 237–239. [Google Scholar]
- Sun, Q.; Lv, M.; Hu, A.; Yang, X.; Yu, C.-P. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J. Hazard. Mater. 2014, 277, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, L.; Fernandez-Fontaina, E.; Singh, R.R.; Lema, J.M.; Carballa, M.; Aga, D.S. Feeding composition and sludge retention time both affect (co-)metabolic biotransformation of pharmaceutical compounds in activated sludge systems. J. Environ. Chem. Eng. 2021, 9, 105123. [Google Scholar] [CrossRef]
- Li, W.J.; Lan, M.; Peng, X. Removal of antibiotics from swine wastewater by UV/H2O2 combined oxidation. Environ. Pollut. Control. 2011, 33, 25–28. [Google Scholar]
- Yu, Y.; Wu, L.; Chang, A.C. Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants. Sci. Total Environ. 2013, 442, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yuan, T.; Hu, J.; Wang, W. Seasonal occurrence and behavior of synthetic musks (SMs) during wastewater treatment process in Shanghai, China. Sci. Total Environ. 2010, 408, 4170–4176. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, A.; Svanfelt, J.; Kronberg, L.; Prévost, M.; Weyhenmeyer, G.A. Seasonal variations in the occurrence and fate of basic and neutral pharmaceuticals in a Swedish river-lake system. Chemosphere 2010, 80, 301–309. [Google Scholar] [CrossRef]
- Daneshvar, A.; Svanfelt, J.; Kronberg, L.; Weyhenmeyer, G. Winter accumulation of acidic pharmaceuticals in a Swedish river. Environ. Sci. Pollut. Res. 2010, 17, 908–916. [Google Scholar] [CrossRef]
- van Nuijs, A.L.N.; Pecceu, B.; Theunis, L.; Dubois, N.; Charlier, C.; Jorens, P.G.; Bervoets, L.; Blust, R.; Neels, H.; Covaci, A. Spatial and temporal variations in the occurrence of cocaine and benzoylecgonine in waste- and surface water from Belgium and removal during wastewater treatment. Water Res. 2009, 43, 1341–1349. [Google Scholar] [CrossRef]
- Elkadiri, R.; Manche, C.; Sultan, M.; Al-Dousari, A.; Uddin, S.; Chouinard, K.; Abotalib, A.Z. Development of a Coupled Spatiotemporal Algal Bloom Model. for Coastal Areas: A Remote Sensing and Data Mining-Based Approach. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 5159–5171. [Google Scholar] [CrossRef]
| Umm Al Hayman WWTP | Kabd WWTP | |
|---|---|---|
| Flow rate (m3/day) | 27,000 | 180,000 |
| Primary treatment | Screening and grit removal | Screening, grit removal, oil and grease removal |
| Secondary treatment | Aeration tanks (oxidation ditches) | Vertical activated sludge process |
| Principle | Extended aeration | Hybrid aerated anoxic-aerobic oxidation |
| MLSS (mg/L) | 3000 | 6000 |
| SRT (d) | 25 | 8 |
| HRT (h) | 11 | 10 |
| Secondary calcifiers | ||
| No tanks | 4 | 6 |
| MLSS (mg/d) | 2800 | 4000 |
| HRT (h) | 1.96 | 3.5 |
| Tertiary treatment | Sand filtration | Disc filtration |
| No. of units | 12 | 8 |
| Surface area (m2) | 20 | 100 |
| Advance Treatment | Chlorination and UV radiation | Chlorination and UV radiation |
| Residual chlorine (mg/L) | 0.5 | 0.5–1 |
| Population of catchment | 173,000 | 1,200,000 |
| Compounds | Recoveries % (n = 3) | MDL (ng/L) | MQL (ng/L) | |||
|---|---|---|---|---|---|---|
| Effluent | Influent | WWE | WWI | WWE | WWI | |
| Eryhromycin | 137 ± 18.0 | 110 ± 18.0 | 1.1 | 2.1 | 3.5 | 6.9 |
| Azithromycin | 111 ± 11.6 | 74 ± 8.2 | 0.4 | 2 | 1.2 | 6.6 |
| Clarithromycin | 106 ± 12.5 | 75 ± 7.2 | 1.3 | 3.1 | 4.3 | 10.4 |
| Tetracycline | 127 ± 6.3 | 101 ± 2.2 | 7 | 26 | 23 | 60 |
| Ofloxacin | 116 ± 15.9 | 74 ± 14.3 | 0.6 | 3.7 | 1.8 | 12.1 |
| Ciprofloxacin | 140 ± 21.2 | 122 ± 21.9 | 7 | 10 | 23 | 35 |
| Cefalexin | 70 ± 8.6 | 108 ± 6.7 | 5 | 8 | 16.6 | 26.8 |
| Sulfamethoxazole | 81 ± 11.3 | 84 ± 5.5 | 5.5 | 7.1 | 18 | 23.7 |
| Trimethoprim | 67 ± 7.1 | 65 ± 6.9 | 2.4 | 7.1 | 8.1 | 20 |
| Dimetridazole | 109 ± 11.1 | 79 ± 2.0 | 15 | 20 | 50 | 68 |
| Metronidazole | 109 ± 4.7 | 127 ± 4.5 | 26 | 50 | 44 | 70 |
| Metronidazole-OH | 43 ± 8.2 | 61 ± 12.8 | 14 | 25 | 48 | 70 |
| Ronidazole | 108 ± 10.1 | 51 ± 2.8 | 15 | 17 | 51 | 53 |
| Influent Concentration (ng/L) | ||||||||
| Umm Al Hayman WWTP | Kabd WWTP | |||||||
| Average | Minimum | Maximum | Median | Average | Minimum | Maximum | Median | |
| Azithromycin | 174 | 82 | 355 | 129 | 157 | <MDL | 466 | 78 |
| Cefalexin | 491 | 412 | 536 | 525 | 598 | 481 | 794 | 519 |
| Ciprofloxacin | 672 | 256 | 1335 | 548 | 865 | 237 | 1492 | 865 |
| Clarithromycin | 592 | 25 | 1258 | 493 | 949 | 37 | 1999 | 810 |
| Dimetridazole | 209 | <MDL | 415 | 210 | 169 | <MDL | 466 | 103 |
| Erythromycin | 111 | 6 | 216 | 111 | 112 | <MDL | 219 | 112 |
| Metronidazole | 238 | 145 | 331 | 238 | 246 | 144 | 356 | 236 |
| Metronidazole-OH | 128 | 30 | 365 | 59 | 184 | 96 | 384 | 128 |
| Ofloxacin | 446 | 64 | 889 | 415 | 446 | 87 | 779 | 460 |
| Ronidazole | 169 | 6 | 332 | 169 | 178 | 6 | 350 | 178 |
| Sulfamethoxazole | 852 | 264 | 1231 | 956 | 520 | 328 | 743 | 505 |
| Tetracycline | 293 | 48 | 537 | 293 | 249 | 21 | 562 | 164 |
| Trimethoprim | 262 | 117 | 419 | 257 | 234 | 112 | 479 | 172 |
| Effluent Concentration (ng/L) | ||||||||
| Umm Al Hayman WWTP | Kabd WWTP | |||||||
| Average | Minimum | Maximum | Median | Average | Minimum | Maximum | Median | |
| Azithromycin | 26 | <MDL | 74 | 14 | 48 | 6 | 119 | 32 |
| Cefalexin | 69 | <MDL | 203 | 2 | 213 | <MDL | 444 | 192 |
| Ciprofloxacin | 153 | <MDL | 533 | 40 | 192 | <MDL | 535 | 115 |
| Clarithromycin | 39 | <MDL | 80 | 35 | 189 | 14 | 420 | 134 |
| Dimetridazole | 96 | <MDL | 237 | 48 | 274 | 76 | 527 | 246 |
| Erythromycin | 34 | <MDL | 66 | 34 | 57 | 25 | 118 | 28 |
| Metronidazole | 93 | 29 | 157 | 93 | 93 | 34 | 182 | 64 |
| Metronidazole-OH | 62 | <MDL | 165 | 18 | 61 | 20 | 179 | 23 |
| Ofloxacin | 120 | <MDL | 324 | 76 | 127 | <MDL | 311 | 98 |
| Ronidazole | 80 | <MDL | 156 | 80 | 78 | <MDL | 153 | 78 |
| Sulfamethoxazole | 212 | 105 | 272 | 236 | 338 | 174 | 649 | 264 |
| Tetracycline | 75 | 24 | 126 | 75 | 112 | <MDL | 307 | 27 |
| Trimethoprim | 41 | <MDL | 141 | 10 | 48 | 6 | 131 | 28 |
| Source | F | p |
|---|---|---|
| Model | F12,169 = 10.05 | <0.0001 |
| Location | F1 = 1.98 | 0.16 |
| Influent/effluent | F1 = 75.18 | <0.0001 |
| Time | F1 = 118.32 | <0.0001 |
| Location × influent/effluent | F1 = 1.86 | 0.17 |
| Location × time | F3 = 0.16 | 0.93 |
| Influent/effluent × time | F3 = 2.38 | 0.07 |
| Location x influent/effluent × time | F3 = 0.76 | 0.52 |
| Umm Hayman | Kabd | |||||||
|---|---|---|---|---|---|---|---|---|
| September 2013 | December 2013 | March 2014 | June 2014 | Sepember 2013 | December 2013 | March 2014 | June 2014 | |
| Azithromycin | 79.08 | 85.40 | 89.93 | 97.79 | 74.41 | 66.60 | 87.38 | 86.45 |
| Cefalexin | 62.15 | 99.62 | 99.51 | 60.00 | 99.75 | 14.42 | ||
| Ciprofloxacin | 60.10 | 83.97 | 99.51 | 94.47 | 64.13 | 13.14 | 99.70 | 97.70 |
| Clarithromycin | 83.87 | 97.23 | 91.86 | 83.50 | 78.98 | 62.97 | ||
| Dimetridazole | 42.93 | 91.67 | 99.05 | 44.75 | 85.67 | 98.29 | 98.02 | |
| Erythromycin | 69.61 | 67.74 | 46.26 | 85.71 | ||||
| Metronidazole | 52.42 | 80.07 | 49.03 | 73.01 | 76.63 | |||
| Metronidazole-OH | 54.82 | 64.85 | 93.42 | 53.43 | 75.39 | 82.87 | 84.21 | |
| Ofloxacin | 53.34 | 96.88 | 84.67 | 88.33 | 60.05 | 97.69 | 75.69 | 95.97 |
| Ronidazole | 53.00 | 50.79 | 56.39 | 45.16 | ||||
| Sulfamethoxazolle | 85.98 | 10.87 | 77.95 | 79.64 | 52.52 | 56.36 | 12.59 | 27.44 |
| Tetracycline | 76.55 | 50.06 | 45.40 | 98.78 | 21.79 | |||
| Trimethoprim | 66.28 | 84.89 | 99.15 | 99.28 | 72.75 | 81.41 | 85.50 | 94.49 |
| Umm Al Hayman WWTP | Kabd WWTP | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | |||||||||||||
| September 2013 | December 2013 | March 2014 | June 2014 | September 2013 | December 2013 | March 2014 | June 2014 | September 2013 | December 2013 | March 2014 | June 2014 | September 2013 | December 2013 | March 2014 | June 2014 | |
| Azithromycin | 355.2 | 81.5 | 166.8 | 90.66 | 74.3 | 11.9 | 16.8 | 2 | 465.8 | 94 | 31.7 | 62.2 | 119.2 | 31.4 | 4 | 8.43 |
| Cefalexin | 535.5 | 524.6 | 412.3 | Nd | 202.7 | 2 | 2 | nd | 480.5 | 794.2 | 518.9 | nd | 192.2 | 2 | 444.1 | nd |
| Ciprofloxacin | 1335.4 | 255.8 | 406.7 | 688.34 | 532.8 | 41 | 2 | 38.08 | 1491.8 | 236.7 | 665.6 | 1064.9 | 535.1 | 205.6 | 2 | 24.53 |
| Clarithromycin | 492.9 | Nd | 1258.1 | 24.57 | 79.5 | nd | 34.8 | 2 | 810.1 | Nd | 1998.9 | 37.29 | 133.7 | nd | 420.1 | 13.81 |
| Dimetridazole | 414.6 | 48 | Blq | 209.62 | 236.6 | 4 | blq | 2 | 466.4 | 527 | 233.97 | 202.2 | 257.7 | 75.5 | 4 | 4 |
| Erythromicin | 215.5 | Nd | 6.2 | Nd | 65.5 | nd | 2 | nd | 219.4 | Nd | 28 | nd | 117.9 | nd | 4 | 25.4 |
| Metronidazole | 330.6 | 145 | Blq | Nd | 157.3 | 28.9 | blq | nd | 356.3 | 236 | blq | 144.28 | 181.6 | 63.7 | blq | 33.72 |
| Metronidazole-OH | 365 | 51.5 | 65.5 | 30.41 | 164.9 | 18.1 | nd | 2 | 384.4 | 96.3 | 130.2 | 126.69 | 179 | 23.7 | 22.3 | 20.01 |
| Ofloxacin | 695.2 | 64.1 | 888.5 | 135.34 | 324.4 | 2 | 136.2 | 15.79 | 777.8 | 86.7 | 779.1 | 141.38 | 310.7 | 2 | 189.4 | 5.7 |
| Ronidazole | 331.9 | Nd | 6.3 | Nd | 156 | nd | 3.1 | nd | 350.4 | Nd | 6.2 | nd | 152.8 | nd | 3.4 | Nd |
| Sulfamethoxazolle | 747.6 | 264.1 | 1231.1 | 1163.94 | 104.8 | 235.4 | 271.5 | 236.92 | 612 | 398.3 | 742.9 | 328.13 | 290.6 | 173.8 | 649.4 | 238.1 |
| Tetracycline | 536.9 | Nd | Blq | 48.16 | 125.9 | nd | blq | 24.05 | 561.5 | 164.4 | blq | 26.8 | 306.6 | 2 | blq | 20.96 |
| Trimethoprim | 419.3 | 116.5 | 235.9 | 278.14 | 141.4 | 17.6 | 2 | 2 | 479.2 | 171.6 | 172.4 | 111.88 | 130.6 | 31.9 | 25 | 6.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevao, B.; Uddin, S.; Krishnan, D.; Rajagopalan, S.; Habibi, N. Antibiotics in Wastewater: Baseline of the Influent and Effluent Streams in Kuwait. Toxics 2022, 10, 174. https://doi.org/10.3390/toxics10040174
Gevao B, Uddin S, Krishnan D, Rajagopalan S, Habibi N. Antibiotics in Wastewater: Baseline of the Influent and Effluent Streams in Kuwait. Toxics. 2022; 10(4):174. https://doi.org/10.3390/toxics10040174
Chicago/Turabian StyleGevao, Bondi, Saif Uddin, Divya Krishnan, Smitha Rajagopalan, and Nazima Habibi. 2022. "Antibiotics in Wastewater: Baseline of the Influent and Effluent Streams in Kuwait" Toxics 10, no. 4: 174. https://doi.org/10.3390/toxics10040174
APA StyleGevao, B., Uddin, S., Krishnan, D., Rajagopalan, S., & Habibi, N. (2022). Antibiotics in Wastewater: Baseline of the Influent and Effluent Streams in Kuwait. Toxics, 10(4), 174. https://doi.org/10.3390/toxics10040174








