Degradation of Carbamazepine from Aqueous Solutions via TiO2-Assisted Photo Catalyze
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Set-Up
2.2. Reagents
2.3. Analytical Methods
2.4. Toxicity and Genotoxicity Tests
3. Results
3.1. Influence of the Photocatalyst Type
3.2. Influence of Photocatalyst Dose and Irradiation Time
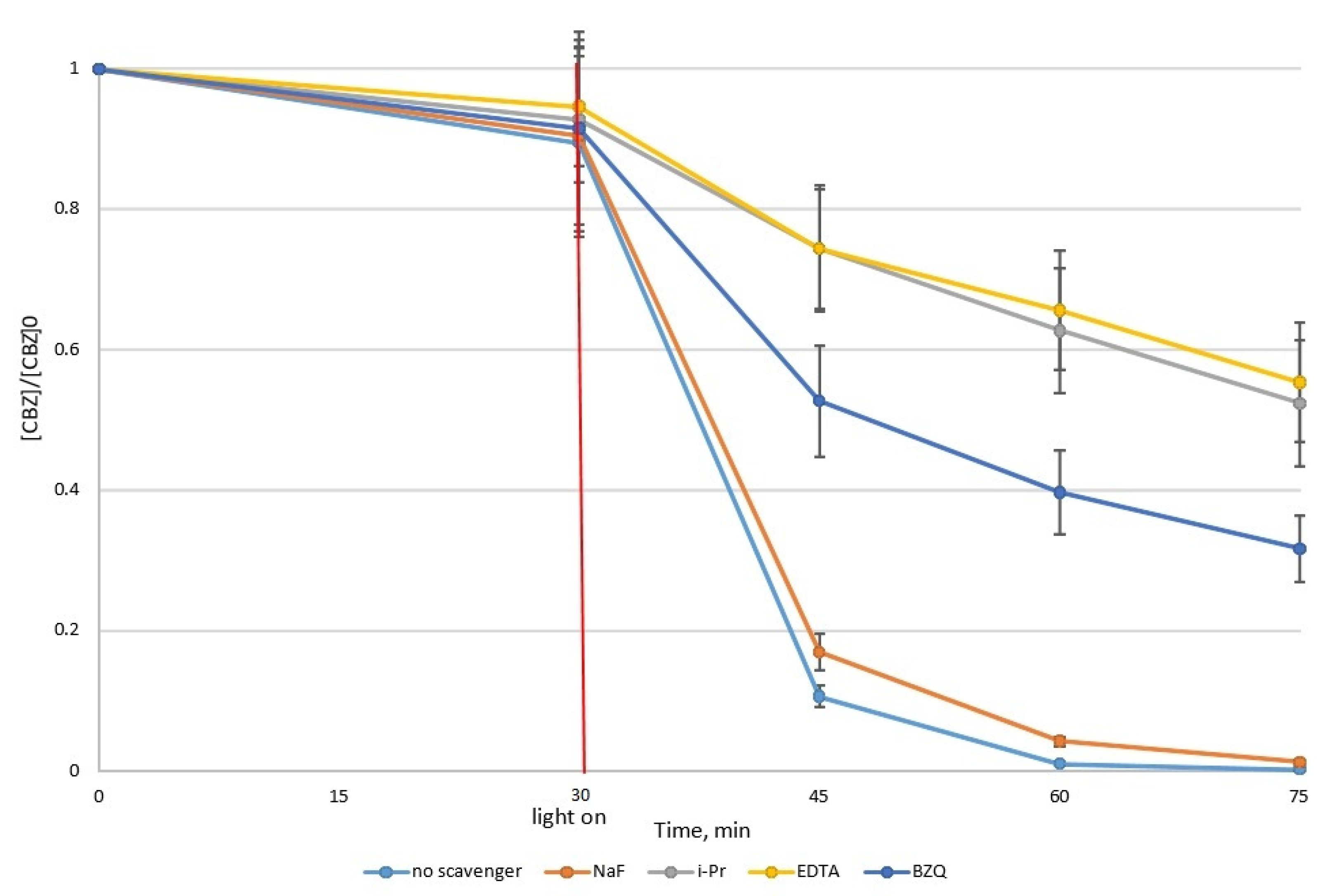
3.3. Effect of Scavengers’ Presence
- NaF (sodium fluoride) (50:1)—for blocking hydroxyl radicals from the photocatalyst particle’s surface;
- i-PrOH (isopropanol) (100:1)—for blocking free hydroxyl radicals HO;
- EDTA (disodium ethylenediaminetetracetate) (100:1)—for blocking holes h+;
- BQ (1,4-benzoquinone) (45:1)—for blocking superoxide radicals O2.
3.4. Identification of CBZ Degradation By-Products
3.5. Toxicity and Genotoxicity Tests
4. Discussion
4.1. Optimum Photocatalyst Type
4.2. Optimum Photocatalyst Dose
4.3. Kinetics
4.4. Main Reactive Species
4.5. Possible Degradation Pathway
4.6. Toxicity and Genotoxicity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Gu, X.; Zeng, Q.; Mao, Z. Acute and chronic toxicity of carbamazepine on the release of chitobiase, molting, and reproduction in Daphnia similis. Int. J. Environ. Res. Public Health 2019, 16, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.Y.; Zhao, J.-L.; Liu, Y.-S.; Liu, W.-R.; Zhang, Q.-Q.; Yao, L.; Hu, L.-X.; Zhang, J.-N.; Jiang, Y.-X.; Ying, G.-G. Pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) in surface and ground waters and their application as indication of wastewater contamination. Sci. Total Environ. 2018, 616–617, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Constantin, L.A.; Constantin, M.A.; Nitoi, I.; Chiriac, F.L.; Galaon, T.; Cristea, N.I. Possible pathway for ifosfamide degradation via Fe-TiO2 assisted photo catalysis. Rev. Chim.–Bucharest 2018, 69, 3234–3237. [Google Scholar] [CrossRef]
- Tran, R.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions–a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Azapagic, A. A methodology for estimating concentrations of pharmaceuticals and personal care products (PPCPs) in wastewater treatment plants and in freshwaters. Sci. Total Environ. 2018, 622–623, 1417–1430. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Geiben, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Batucan, N.S.P.; Tremblay, L.A.; Northcott, G.L.; Matthaei, C.D. Medicating the environment? A critical review on the risks of carbamazepine, diclofenac and ibuprofen to aquatic organisms. Environ. Adv. 2022, 7, 100164. [Google Scholar] [CrossRef]
- Compound Summary Carbamazepine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carbamazepine#section=Hazards-Identification (accessed on 17 March 2022).
- Carbamazepine. Available online: https://go.drugbank.com/drugs/DB00564 (accessed on 17 March 2022).
- Oetken, M.; Nentwig, G.; Löffler, D.; Ternes, T.; Oehlmann, J. Effects of pharmaceuticals on aquatic invertebrates. Part I. The antiepileptic drug carbamazepine. Arch. Environ. Contam Toxicol. 2005, 49, 353–361. [Google Scholar] [CrossRef]
- Jos, A.; Repetto, G.; Rios, J.C.; Hazen, M.J.; Molero, M.; del Peso, A.; Salguero, M.; Fernandez-Freire, P.; Perez-Martin, J.M.; Camean, A. Ecotoxicological evaluation of carbamazepine using six different model systems with eighteen endpoints. Toxicol. In Vitro 2003, 17, 525–532. [Google Scholar] [CrossRef]
- Gheorghe, S.; Petre, J.; Lucaciu, I.; Stoica, C.; Nita-Lazar, M. Risk screening of pharmaceutical compounds in Romanian aquatic environment. Environ. Monit. Assess. 2016, 188, 379. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Yan, Z.; Liu, J.; Wang, P.; Wang, Y. Bioaccumulation and trophic transfer of pharmaceuticals in food webs from a large freshwater lake. Environ. Pollut. 2017, 222, 356–366. [Google Scholar] [CrossRef]
- Ebrahimzadeh, S.; Castiglioni, S.; Riva, F.; Zuccato, E.; Azzellino, A. Carbamazepine Levels Related to the Demographic Indicators in Groundwater of Densely Populated Area. Water 2021, 13, 2539. [Google Scholar] [CrossRef]
- Petre, J.; Galaon, T.; Vasile, I.I.; Vasile, G.G.; Stanescu, E.; Pascu, L.F.; Simion, M.; Cruceru, L. Simultaneous analysis of selected dissolved pharmaceuticals in the water of the Danube river and its three major tributaries in Romania. Rev. Chim.-Bucharest 2016, 67, 1436–1440. [Google Scholar]
- Wang, S.; Wang, J. Degradation of carbamazepine by radiation-induced activation of peroxymonosulfate. Chem. Eng. J. 2018, 336, 595–601. [Google Scholar] [CrossRef]
- Nasir, N.M.; Talib, S.A.; Hashim, S.N.; Tay, C.C. Biodegradation of carbamazepine using fungi and bacteria. J. Fundam. Appl. Sci. 2017, 9, 124–146. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.F.; Qu, L.; Yang, H.; Chu, W. Degradation of carbamazepine by Fe (II) activated persulfate process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef]
- Calvert, J.G.; Pitts, J.N. Photochemistry; John Wiley & Sons Inc.: London, UK, 1966. [Google Scholar]
- Technical Committee ISO/TC 147/SC 5 Biological methods. ISO 6341:2012; Water Quality–Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)–Acute Toxicity Test. International Organization for Standardization: Geneva, Switzerland, 2012. Available online: www.iso.org/standard/54614.html (accessed on 10 February 2022).
- Technical Committee ISO/TC 147/SC 5 Biological methods. ISO 8692:2012; Water Quality–Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. International Organization for Standardization: Geneva, Switzerland, 2012. Available online: www.iso.org/standard/54150.html (accessed on 10 February 2022).
- EBPI. Specializing in Biomolecular Testing Kits for Toxicity, Mutagenicity, and Genotoxicity; Environmental Bio-Detection Products Inc. Ontario, Canada. Available online: https://www.biotoxicity.com/index.php/ebpi-toxicity-tests/sos-genotoxicity-tests/60-ebpi-toxicity-tests/201-sos-genotoxicity-tests (accessed on 17 March 2022).
- Kocak, E. Investigation of potential genotoxic activity using the SOS-Chromotest for real paracetamol wastewater and the wastewater treated by the Fenton process. J. Environ. Health Sci. Engineer 2015, 13, 66. [Google Scholar] [CrossRef] [Green Version]
- Carabin, A.; Drogui, P.; Robert, D. Photo-degradation of carbamazepine using TiO2 suspended photocataysts. J. Taiwan Inst. Chem. Eng. 2015, 54, 109–117. [Google Scholar] [CrossRef]
- Franz, S.; Falletta, E.; Arab, H.; Murgolo, S.; Bestetti, M.; Mascolo, G. Degradation of carbamazepine by photo(electro)catalysis on nanostructured TiO2 meshes: Transformation products and reaction pathways. Catalysts 2020, 10, 169. [Google Scholar] [CrossRef] [Green Version]
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of emerging organic pollutants in wastewater effluents by electrochemical photocatalysis on nanostructured TiO2 meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef]
- Xu, L.; Niu, J.; Xie, H.; Ma, X.; Zhu, Y.; Crittenden, J. Effective degradation of aqueous carbamazepine on a novel blue-colored TiO2 nanotube arrays membrane filter anode. J. Hazard. Mater. 2021, 402, 123530. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hao, X.; Yu, D.; Zhou, P.; Peng, Y.; Jia, Y.; Zhao, C.; He, J.; Zhan, C.; Lai, B. High visible-light catalytic activity of Bis-PDI-T@TiO2 for activating persulfate toward efficient degradation of carbamazepine. Sep. Purif. Technol. 2021, 263, 118384. [Google Scholar] [CrossRef]
- Yang, L.; Jia, Y.; Peng, Y.; Zhou, P.; Yu, D.; Zhao, C.; He, J.; Zhan, C.; Lai, B. Visible-light induced activation of persulfate by self-assembled EHPDI/TiO2 photocatalyst toward efficient degradation of carbamazepine. Sci. Total Environ. 2021, 783, 146996. [Google Scholar] [CrossRef] [PubMed]
- Dudziak, S.; Bielan, Z.; Kubica, P.; Zielinska-Jurek, A. Optimization of carbamazepine photodegradation on defective TiO2–based magnetic photocatalyst. J. Environ. Chem. Eng. 2021, 9, 105782. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; da Silva, L.P.; Zegaoui, O.; da Silva, J.C.G.E. Synthesis of Fe–and Co- doped TiO2 with improved photocatalytic activity under visible irradiation toward carbamazepine degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, B.; Paxeus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotox. Environ. Safe. 2003, 55, 359–370. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res. 2002, 36, 5012–5022. [Google Scholar] [CrossRef]
- Laville, N.; Ait-Aissa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Donner, E.; Kosjek, T.; Qualmann, S.; Kusk, K.O.; Michael Revitt, D.; Ledin, A.; Andersen, H.R. Ecotoxicity of carbamazepine and its UV photolysis transformation products. Sci. Total Environ. 2013, 443, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Ji, H.; Du, P.; Huang, T.; Wang, C.; Liu, W. Insights into catalytic activation of peroxymonosulfate for carbamazepine degradation by MnO2 nanoparticles in-situ anchored titanate nanotube: Mechanism, ecotoxicity and DFT study. J. Hazard. Mater. 2021, 402, 123779. [Google Scholar] [CrossRef]


| Process | [CBZ] | Removal Efficiency | Reference |
|---|---|---|---|
| Biodegradation with fungi | 4 μg/L–9 mg/L | 40–90% | [17] |
| Biodegradation with bacteria | 4 μg/L–9.5 mg/L | 15–60% | [17] |
| Chemical oxidation with Fe (II) activated persulfate | 0.025 mM | 78% | [18] |
| Photocatalyst Type | [CBZ], mg/L | Efficiency, % | Apparent First-Order Reaction Rate Constant (Mean Value) k, s−1 |
|---|---|---|---|
| P25 Degussa | 0.56 ± 0.09 | 93.60 ± 1.04 | 1.53 × 10−3 |
| Rovis Optics anatase | 1.05 ± 0.17 | 88.00 ± 2.81 | 1.18 × 10−3 |
| Merck | 1.31 ± 0.20 | 85.03 ± 2.49 | 1.06 × 10−3 |
| Kurt Lesker | 7.60 ± 0.40 | 13.14 ± 4.57 | 7.83 × 10−5 |
| Umicore | 8.01 ± 0.40 | 8.46 ± 4.68 | 4.91 × 10−5 |
| No | Structure Name | Retention Time, min | Molecular Ion, [M + H]+ | Molecular Formula | Chemical Structure |
|---|---|---|---|---|---|
| 1 | CBZ | 12.1 | 237 | C15H12N2O | 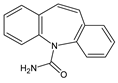 |
| 2 | TP268 | 3.15 | 269 | C15H12N2O3 | 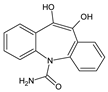 |
| 3 | TP266 | 3.37 | 267 | C15H10N2O3 |  |
| 4 | TP195 | 3.63 | 196 | C13H9NO | 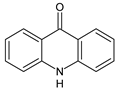 |
| 5 | TP222 | 3.66 | 223 | C14H10N2O |  |
| 6 | TP252-A | 4.42 | 253 | C15H12N2O2 |  |
| 7 | TP252-B | 4.79 | 253 | C15H12N2O2 | 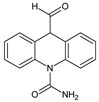 |
| 8 | TP250 | 5.25 | 251 | C15H10N2O2 | 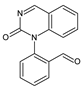 |
| 9 | TP223 | 5.32 | 224 | C14H9NO2 | 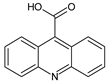 |
| Daphnia magna (Mortality %) | Pseudokirchneriella subcapitata (Algae Growth Inhibition Rate %) | Escherichia coli (Genotoxicity) | |||||
|---|---|---|---|---|---|---|---|
| Sample | 24 h | 48 h | 24 h | 48 h | 72 h | Survival Rate, % | Genotoxicity IF |
| Untreated [CBZ] = 8.75 mg/L | 95 ± 19 | 100 ± 20 | −48.18 ± (−14.4) | 1.77 ± 0.53 | 3.53 ± 1.05 | 107.34 ± 21.4 | 1.23 ± 0.23 (NO) |
| 15 min irradiation [CBZ] = 0.93 mg/L | 45 ± 9 | 95 ± 19 | −46.04 ± (−13.81) | 16.17 ± 4.85 | 8.40 ± 2.52 | 104.81 ± 20.96 | 1.20 ± 0.23 (NO) |
| 30 min irradiation [CBZ] = 0.09 mg/L | 25 ± 5 | 75 ± 15 | 77.91 ± 23.37 | −11.71 ± (−3.51) | −2.22 ± (−0.66) | N/A | N/A |
| 45 min irradiation [CBZ] = 0.02 mg/L | 10 ± 2 | 70 ± 14 | −211.69 ± (−63.3) | −9.39 ± (−2.81) | −2.62 ± (−0.78) | 98.27 ± 19.65 | 1.28 ± 0.20 (NO) |
| Controls No CBZ | 0 | 0 | 0 | 0 | 0 | NC 96.88 PC 100% | 1.05 ± 0.21 (NO) 3.68 ± 0.77 (YES) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, M.A.; Chiriac, F.L.; Gheorghe, S.; Constantin, L.A. Degradation of Carbamazepine from Aqueous Solutions via TiO2-Assisted Photo Catalyze. Toxics 2022, 10, 168. https://doi.org/10.3390/toxics10040168
Constantin MA, Chiriac FL, Gheorghe S, Constantin LA. Degradation of Carbamazepine from Aqueous Solutions via TiO2-Assisted Photo Catalyze. Toxics. 2022; 10(4):168. https://doi.org/10.3390/toxics10040168
Chicago/Turabian StyleConstantin, Mirela Alina, Florentina Laura Chiriac, Stefania Gheorghe, and Lucian Alexandru Constantin. 2022. "Degradation of Carbamazepine from Aqueous Solutions via TiO2-Assisted Photo Catalyze" Toxics 10, no. 4: 168. https://doi.org/10.3390/toxics10040168
APA StyleConstantin, M. A., Chiriac, F. L., Gheorghe, S., & Constantin, L. A. (2022). Degradation of Carbamazepine from Aqueous Solutions via TiO2-Assisted Photo Catalyze. Toxics, 10(4), 168. https://doi.org/10.3390/toxics10040168







