The Relationship of Dioxin Levels in Serum of 9-Year-Old Vietnamese Children and Their Mothers’ Breast Milk
Abstract
1. Introduction
2. Materials and Methods
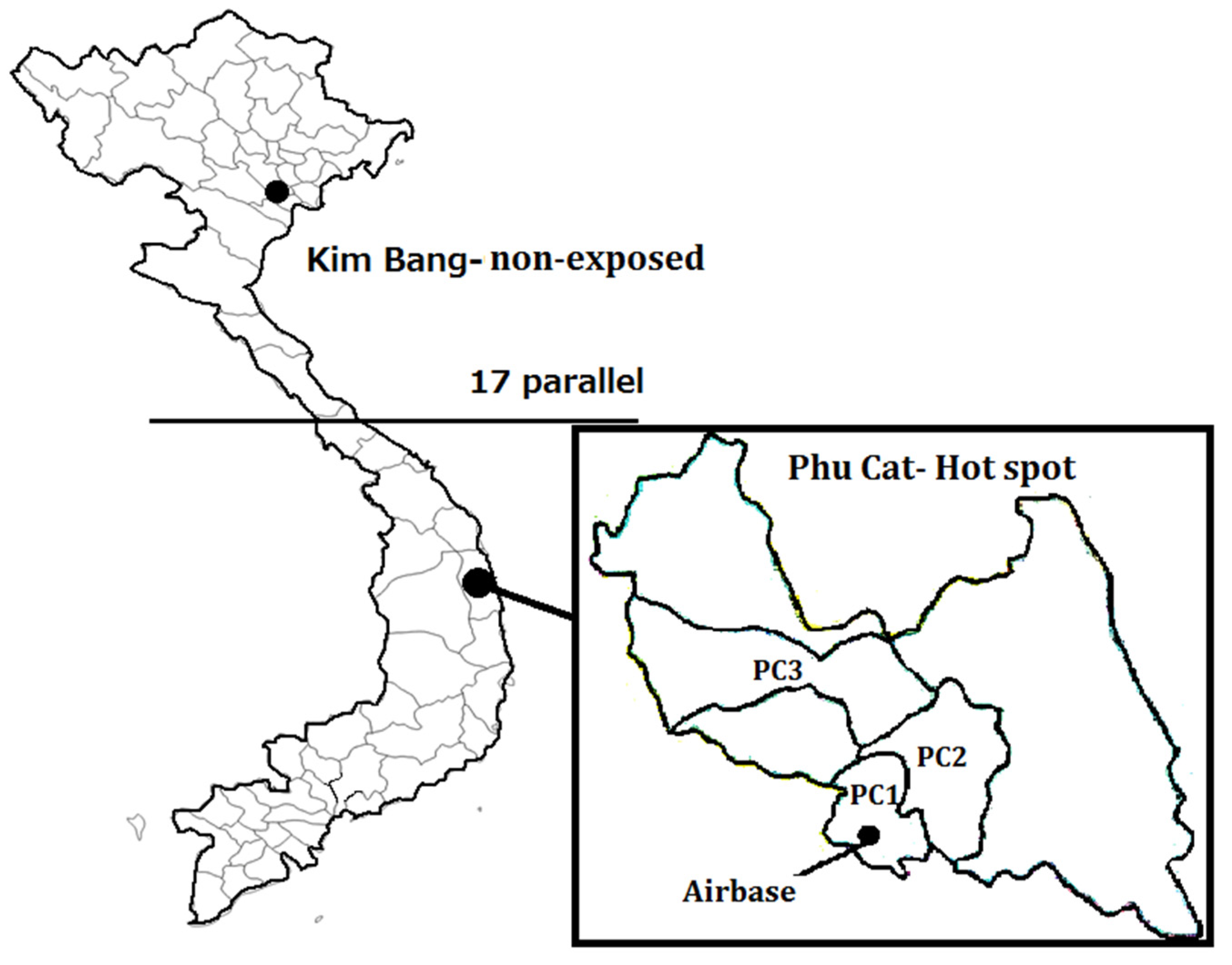
2.1. Study Areas
2.2. Study Participants
2.3. Dioxin Analysis
2.4. Data Analysis
3. Results and Discussion
3.1. Serum Concentration of PCDD/Fs in Children
3.2. Correlation of Dioxin in Children’s Blood and Mothers’ Breast Milk
3.3. Comparison of Dioxin Congener Patterns in Children, Mothers, and Men in the Hotspot Area
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stellman, J.M.; Stellman, S.D.; Christian, R.; Weber, T.; Tomasallo, C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature 2003, 422, 681–687. [Google Scholar] [CrossRef]
- Tawara, K.; Nishijo, M.; Maruzeni, S.; Nakagawa, H.; Kido, T.; Naganuma, R.; Suzuki, H.; Nhu, D.D.; Hung, N.N.; Thom, L.T.H. Residual congener pattern of dioxins in human breast milk in southern Vietnam. Chemosphere 2011, 84, 979–986. [Google Scholar] [CrossRef]
- Dwernychuk, L.W. Dioxin hot spots in Vietnam. Chemosphere 2005, 60, 998–999. [Google Scholar] [CrossRef]
- Dwernychuk, L.W.; Cau, H.D.; Hatfield, C.T.; Boivin, T.G.; Hung, T.M.; Dung, P.T.; Thai, N.D. Dioxin reservoirs in southern Viet Nam--a legacy of Agent Orange. Chemosphere 2002, 47, 117–137. [Google Scholar] [CrossRef]
- Dwernychuk, L.; Hung, T.; Boivin, T. The Agent Orange dioxin issue in Vietnam: A manageable problem. Organohalogen 2006, 68, 312–315. [Google Scholar]
- Le, L.T.H.; Dat, N.D.; Minh, N.H.; Nguyen, K.A. Characteristics of PCDD/Fs in soil and sediment samples collected from A-So former airbase in Central Vietnam. Sci. Total Environ. 2019, 661, 27–34. [Google Scholar] [CrossRef]
- Hue, N.T.M.; Nam, V.D.; Thuong, N.V.; Huyen, N.T.; Phuong, N.T.H.; Hung, N.X.; Tuan, N.H.; Son, L.K.; Minh, N.H. Determination of PCDD/Fs in breast milk of women living in the vicinities of Da Nang Agent Orange hot spot (Vietnam) and estimation of the infant’s daily intake. Sci. Total Environ. 2014, 491-492, 212–218. [Google Scholar] [CrossRef]
- Hue, N.T.M.; Van Thuong, N.; Mai, P.T.N.; Minh, N.H. Site-specific bioaccumulation of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/PCDFs) in mothers and their infants living in vicinity of Bien Hoa airbase, Southern Vietnam. Environ. Geochem. Health 2018, 40, 2539–2549. [Google Scholar] [CrossRef]
- Manh, H.D.; Kido, T.; Naganuma, R.; Xian Liang, S.; Supratman, S.; Anh, L.T.; Maruzeni, S.; Nishijo, M.; Nakagawa, H.; Honma, S.; et al. Serum Dioxin Levels in Vietnamese Men more than 40 Years after Herbicide Spraying. Environ. Sci. Technol. 2014, 48, 3496–3503. [Google Scholar] [CrossRef]
- Manh, H.D.; Kido, T.; Tai, P.T.; Okamoto, R.; Honma, S.; Liang, S.X.; Anh, L.T.; Maruzeni, S.; Nghi, T.N.; Nishijo, M.; et al. Levels of polychlorinated dibenzodioxins and polychlorinated dibenzofurans in breast milk samples from three dioxin-contaminated hotspots of Vietnam. Sci. Total Environ. 2015, 511, 416–422. [Google Scholar] [CrossRef]
- Nghi, T.N.; Nishijo, M.; Manh, H.D.; Tai, P.T.; Van Luong, H.; Anh, T.H.; Thao, P.N.; Trung, N.V.; Waseda, T.; Nakagawa, H.; et al. Dioxins and nonortho PCBs in breast milk of vietnamese mothers living in the largest hot spot of dioxin contamination. Environ. Sci. Technol. 2015, 49, 5732–5742. [Google Scholar] [CrossRef]
- Oyama, Y.; Phuc, H.D.; Honma, S.; Oanh, N.T.P.; Hung, N.X.; Anh, L.T.; Manh, H.D.; Van Tung, D.; Nhu, D.D.; Tan, N.M.; et al. Decreased serum testosterone levels associated with 17β-hydroxysteroid dehydrogenase activity in 7-year-old children from a dioxin-exposed area of Vietnam. Sci. Total Environ. 2021, 783, 146701. [Google Scholar] [CrossRef]
- Pham, D.T.; Nguyen, H.M.; Boivin, T.G.; Zajacova, A.; Huzurbazar, S.V.; Bergman, H.L. Predictors for dioxin accumulation in residents living in Da Nang and Bien Hoa, Vietnam, many years after Agent Orange use. Chemosphere 2015, 118, 277–283. [Google Scholar] [CrossRef]
- Tai, P.T.; Nishijo, M.; Kido, T.; Nakagawa, H.; Maruzeni, S.; Naganuma, R.; Anh, N.T.N.; Morikawa, Y.; Van Luong, H.; Anh, T.H.; et al. Dioxin concentrations in breast milk of Vietnamese nursing mothers: A survey four decades after the herbicide spraying. Environ. Sci. Technol. 2011, 45, 6625–6632. [Google Scholar] [CrossRef]
- Van Manh, P.; Tai, P.T.; Phuong, N.M.; Nishijo, M.; Trung, D.M.; Thao, P.N.; Son, H.A.; Van Tuan, T.; Van Chuyen, N.; Van Long, N.; et al. Serum dioxin concentrations in military workers at three dioxin-contaminated airbases in Vietnam. Chemosphere 2021, 266, 129024. [Google Scholar] [CrossRef]
- Anh, L.T.; Kido, T.; Honma, S.; Manh, H.D.; Koike, I.; Oyama, Y.; Phuc, H.D.; Okamoto, R.; Nakagawa, H.; Nakayama, S.F.; et al. A relationship in adrenal androgen levels between mothers and their children from a dioxin-exposed region in Vietnam. Sci. Total Environ. 2017, 607–608, 32–41. [Google Scholar] [CrossRef]
- Kido, T.; Honma, S.; Nhu, D.D.; Manh, H.D.; Van Tung, D.; Liang, S.X.; Anh, L.T.; Okamoto, R.; Maruzeni, S.; Nakagawa, H.; et al. Inverse association of highly chlorinated dioxin congeners in maternal breast milk with dehydroepiandrosterone levels in three-year-old Vietnamese children. Sci. Total Environ. 2016, 550, 248–255. [Google Scholar] [CrossRef]
- Nishijo, M.; Pham, T.T.; Nguyen, A.T.N.; Tran, N.N.; Nakagawa, H.; Hoang, L.V.; Tran, A.H.; Morikawa, Y.; Ho, M.D.; Kido, T.; et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin in breast milk increases autistic traits of 3-year-old children in Vietnam. Mol. Psychiatry 2014, 19, 1220–1226. [Google Scholar] [CrossRef]
- Oanh, N.T.P.; Kido, T.; Honma, S.; Oyama, Y.; Anh, L.T.; Phuc, H.D.; Viet, N.H.; Manh, H.D.; Okamoto, R.; Nakagawa, H.; et al. Androgen disruption by dioxin exposure in 5-year-old Vietnamese children: Decrease in serum testosterone level. Sci. Total Environ. 2018, 640–641, 466–474. [Google Scholar] [CrossRef]
- Tai, P.T.; Nishijo, M.; Nghi, T.N.; Nakagawa, H.; Van Luong, H.; Anh, T.H.; Nishijo, H. Effects of Perinatal Dioxin Exposure on Development of Children during the First 3 Years of Life. J. Pediatr. 2016, 175, 159–166.e2. [Google Scholar] [CrossRef]
- Van Tung, D.; Kido, T.; Honma, S.; Manh, H.D.; Nhu, D.D.; Okamoto, R.; Maruzeni, S.; Nishijo, M.; Nakagawa, H.; Ngoc, P.T.; et al. Low birth weight of Vietnamese infants is related to their mother’s dioxin and glucocorticoid levels. Environ. Sci. Pollut. Res. 2016, 23, 10922–10929. [Google Scholar] [CrossRef] [PubMed]
- Sergeyev, O.; Burns, J.S.; Williams, P.L.; Korrick, S.A.; Lee, M.M.; Revich, B.; Hauser, R. The association of peripubertal serum concentrations of organochlorine chemicals and blood lead with growth and pubertal development in a longitudinal cohort of boys: A review of published results from the Russian Children’s Study. Rev. Environ. Health 2017, 32, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mocarelli, P.; Gerthoux, P.M.; Patterson, D.G.; Milani, S.; Limonta, G.; Bertona, M.; Signorini, S.; Tramacere, P.; Colombo, L.; Crespi, C.; et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ. Health Perspect. 2008, 116, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Vietnam-Russia Tropical Center and Hatfield Consultants. Evaluation of Contamination At the Agent Orange Dioxin Hot Spots in Bien Hoa, Phu Cat and Vicinity, Viet Nam. 2009. Available online: https://www.hatfieldgroup.com/wp-content/uploads/2020/05/undp1391-final-vrt-report-and-appendices.pdf (accessed on 20 February 2022).
- Takasuga, T.; Senthilkumar, K.; Takemori, H.; Ohi, E.; Tsuji, H.; Nagayama, J. Impact of FEBRA (fermented brown rice with Aspergillus oryzae) intake and concentrations of PCDDs, PCDFs and PCBs in blood of humans from Japan. Chemosphere 2004, 57, 1409–1426. [Google Scholar] [CrossRef]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef]
- Burns, J.S.; Williams, P.L.; Sergeyev, O.; Korrick, S.; Lee, M.M.; Revich, B.; Altshul, L.; Patterson, D.G.; Turner, W.E.; Needham, L.L.; et al. Predictors of serum dioxins and PCBs among peripubertal Russian boys. Environ. Health Perspect. 2009, 117, 1593–1599. [Google Scholar] [CrossRef]
- Tohyama, C.; Uchiyama, I.; Hoshi, S.; Hijia, M.; Miyata, H.; Nagai, M.; Nakai, S.; Yauchi, M.; Ohkubo, S. Polychlorinated dioxins, furans, and biphenyls in blood of children and adults living in a dioxin-contaminated area in Tokyo. Environ. Health Prev. Med. 2011, 16, 6–15. [Google Scholar] [CrossRef][Green Version]
- Link, B.; Gabrio, T.; Zoellner, I.; Piechotowski, I.; Paepke, O.; Herrmann, T.; Felder-Kennel, A.; Maisner, V.; Schick, K.H.; Schrimpf, M.; et al. Biomonitoring of persistent organochlorine pesticides, PCDD/PCDFs and dioxin-like PCBs in blood of children from South West Germany (Baden-Wuerttemberg) from 1993 to 2003. Chemosphere 2005, 58, 1185–1201. [Google Scholar] [CrossRef]
- Minh, N.H.; Tran, T.M.; Hue, N.T.M.; Minh, T.B.; Tuyet-Hanh, T.T. Bioaccumulation of PCDD/Fs in foodstuffs near Bien Hoa and Da Nang airbases: Assessment on sources and distribution. Environ. Sci. Pollut. Res. 2019, 26, 28852–28859. [Google Scholar] [CrossRef]
- Tuyet-Hanh, T.T.; Minh, N.H.; Vu-Anh, L.; Dunne, M.; Toms, L.M.; Tenkate, T.; Thi, M.H.N.; Harden, F. Environmental health risk assessment of dioxin in foods at the two most severe dioxin hot spots in Vietnam. Int. J. Hyg. Environ. Health 2015, 218, 471–478. [Google Scholar] [CrossRef]
- Boivin, T.; Hang, T.M. Evaluation of Dioxin Project Impact To Environment and People Evaluation of Dioxin Project Impact on the Environment and People. 2015. Available online: https://www.vn.undp.org/content/vietnam/en/home/library/environment_climate/evaluation_of_dioxin_project_impact_to_environment_and_people.html (accessed on 22 February 2022).

| pg/g Lipid | Hotspot (n = 45, Individual Samples) | Nonexposed (n = 12, Pooled Samples) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % over LOD | GM | GSD | Median | % over LOD | GM | GSD | Median | p-Value | |
| PCDD congeners | |||||||||
| 1,2,3,7,8-PeCDD | 44 | 2.3 | 1.7 | 2.0 | 33 | 1.0 | 1.4 | 1.0 | <0.0001 a |
| 1,2,3,6,7,8-HxCDD | 60 | 6.0 | 1.8 | 7.0 | 8 | 1.6 | 1.1 | 1.5 | <0.0001 a |
| 1,2,3,4,6,7,8-HpCDD | 91 | 10.7 | 1.8 | 10.0 | 58 | 2.5 | 1.6 | 3.0 | <0.0001 a |
| OCDD | 100 | 161.6 | 1.6 | 160.0 | 100 | 64.3 | 1.3 | 63.0 | <0.0001 a |
| PCDF congeners | |||||||||
| 2,3,4,7,8-PeCDF | 98 | 5.6 | 1.5 | 6.0 | 92 | 2.5 | 1.6 | 3.0 | <0.0001 a |
| 1,2,3,4,7,8-HxCDF | 96 | 12.8 | 1.7 | 12.0 | 8 | 1.6 | 1.1 | 1.5 | <0.0001 a |
| 1,2,3,6,7,8-HxCDF | 87 | 8.9 | 1.8 | 9.0 | 8 | 1.6 | 1.1 | 1.5 | <0.0001 a |
| 1,2,3,4,6,7,8-HpCDF | 100 | 19.7 | 1.8 | 18.0 | 0 | 1.5 | 1.2 | 1.5 | <0.0001 a |
| TEQ pg/g lipid | |||||||||
| TEQ PCDDs | 5.5 | 1.4 | 5.3 | 1.8 | 1.3 | 1.5 | <0.0001 a | ||
| TEQ PCDFs | 5.1 | 1.4 | 4.7 | 1.6 | 1.3 | 1.6 | <0.0001 a | ||
| TEQ PCDD/Fs | 10.7 | 1.3 | 10.0 | 3.3 | 1.2 | 3.1 | <0.0001 a | ||
| pg/g Lipid | Gender | p Value a | Subareas | p Value b | |||
|---|---|---|---|---|---|---|---|
| Male (n = 26) | Female (n = 18) | PC1 (n = 18) | PC2 (n = 15) | PC3 (n = 11) | |||
| PCDD congeners | |||||||
| 1,2,3,7,8-PeCDD | 2.2 | 2.6 | 0.2 | 2.3 | 2.2 | 2.6 | 0.9 |
| 1,2,3,6,7,8-HxCDD | 5.9 | 6.1 | 0.7 | 5.7 | 5.3 | 7.4 | 0.2 |
| 1,2,3,4,6,7,8-HpCDD | 11.0 | 10.0 | 0.4 | 11.2 | 9.8 | 10.8 | 0.8 |
| OCDD | 163.5 | 153.8 | 0.7 | 158.6 | 165.0 | 153.5 | 1.0 |
| PCDF congeners | |||||||
| 2,3,4,7,8-PeCDF | 5.9 | 5.1 | 0.4 | 5.4 | 5.3 | 6.5 | 0.4 |
| 1,2,3,4,7,8-HxCDF | 13.4 | 11.7 | 0.3 | 11.8 | 11.5 | 16.3 | 0.1 |
| 1,2,3,6,7,8-HxCDF | 10.0 | 7.5 | 0.1 | 8.6 | 7.6 | 11.9 | 0.1 |
| 1,2,3,4,6,7,8-HpCDF | 19.7 | 19.6 | 0.7 | 18.0 | 20.1 | 22.0 | 0.3 |
| TEQ pg/g lipid | |||||||
| TEQ PCDDs | 5.3 | 5.7 | 0.5 | 5.2 | 5.3 | 6.1 | 0.5 |
| TEQ PCDFs | 5.3 | 4.7 | 0.1 | 4.7 | 4.8 | 6.1 | 0.1 |
| TEQ PCDD/Fs | 10.7 | 10.6 | 1.0 | 10.0 | 10.3 | 12.3 | 0.1 |
| Hotspot (n = 44) | Nonexposed (Pooled Samples, n = 10) | |||
|---|---|---|---|---|
| r | p | r | p | |
| PCDD congeners | ||||
| 1,2,3,7,8-PeCDD | −0.073 | 0.637 | 0.461 | 0.180 |
| 1,2,3,6,7,8-HxCDD | 0.159 | 0.303 | −0.115 | 0.753 |
| 1,2,3,4,6,7,8-HpCDD | 0.402 | 0.007 | 0.370 | 0.293 |
| OCDD | 0.140 | 0.365 | 0.675 | 0.032 |
| PCDF congeners | ||||
| 2,3,4,7,8-PeCDF | 0.075 | 0.628 | 0.467 | 0.173 |
| 1,2,3,4,7,8-HxCDF | 0.410 | 0.006 | 0.306 | 0.390 |
| 1,2,3,6,7,8-HxCDF | 0.333 | 0.027 | 0.309 | 0.386 |
| 1,2,3,4,6,7,8-HpCDF | 0.440 | 0.003 | 0.090 | 0.804 |
| PCDDs TEQ | −0.047 | 0.763 | 0.663 | 0.037 |
| PCDFs TEQ | 0.237 | 0.121 | 0.642 | 0.045 |
| PCDD/Fs TEQ | −0.028 | 0.854 | 0.705 | 0.023 |
| Dioxin in Breast Milk (β) | Gender (β) | Duration of Breast Feeding (β) | R2 | |
|---|---|---|---|---|
| PCDD congeners | ||||
| 1,2,3,7,8-PeCDD | −0.034 | 0.174 | 0.189 | 0.063 |
| 1,2,3,6,7,8-HxCDD | 0.197 | 0.178 | 0.298 | 0.106 |
| 1,2,3,4,6,7,8-HpCDD | 0.321 * | 0.054 | 0.209 | 0.143 |
| OCDD | 0.133 | 0.003 | 0.147 | 0.042 |
| PCDF congeners | ||||
| 2,3,4,7,8-PeCDF | 0.035 | 0.102 | 0.378 * | 0.170 |
| 1,2,3,4,7,8-HxCDF | 0.460 ** | 0.115 | 0.261 | 0.226 |
| 1,2,3,6,7,8-HxCDF | 0.372 * | −0.007 | 0.381 ** | 0.280 |
| 1,2,3,4,6,7,8-HpCDF | 0.426 ** | 0.173 | 0.257 | 0.209 |
| TEQ PCDDs | −0.106 | 0.104 | 0.214 | 0.072 |
| TEQ PCDFs | 0.221 | −0.006 | 0.339 * | 0.158 |
| TEQ PCDD/Fs | −0.083 | −0.021 | 0.278 | 0.086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manh, H.D.; Kido, T.; Takasuga, T.; Yamashita, M.; Giang, L.M.; Nakagawa, H. The Relationship of Dioxin Levels in Serum of 9-Year-Old Vietnamese Children and Their Mothers’ Breast Milk. Toxics 2022, 10, 155. https://doi.org/10.3390/toxics10040155
Manh HD, Kido T, Takasuga T, Yamashita M, Giang LM, Nakagawa H. The Relationship of Dioxin Levels in Serum of 9-Year-Old Vietnamese Children and Their Mothers’ Breast Milk. Toxics. 2022; 10(4):155. https://doi.org/10.3390/toxics10040155
Chicago/Turabian StyleManh, Ho Dung, Teruhiko Kido, Takumi Takasuga, Michiko Yamashita, Le Minh Giang, and Hideaki Nakagawa. 2022. "The Relationship of Dioxin Levels in Serum of 9-Year-Old Vietnamese Children and Their Mothers’ Breast Milk" Toxics 10, no. 4: 155. https://doi.org/10.3390/toxics10040155
APA StyleManh, H. D., Kido, T., Takasuga, T., Yamashita, M., Giang, L. M., & Nakagawa, H. (2022). The Relationship of Dioxin Levels in Serum of 9-Year-Old Vietnamese Children and Their Mothers’ Breast Milk. Toxics, 10(4), 155. https://doi.org/10.3390/toxics10040155






