Cytotoxicity of Thiopurine Drugs in Patients with Inflammatory Bowel Disease
Abstract
:1. Introduction
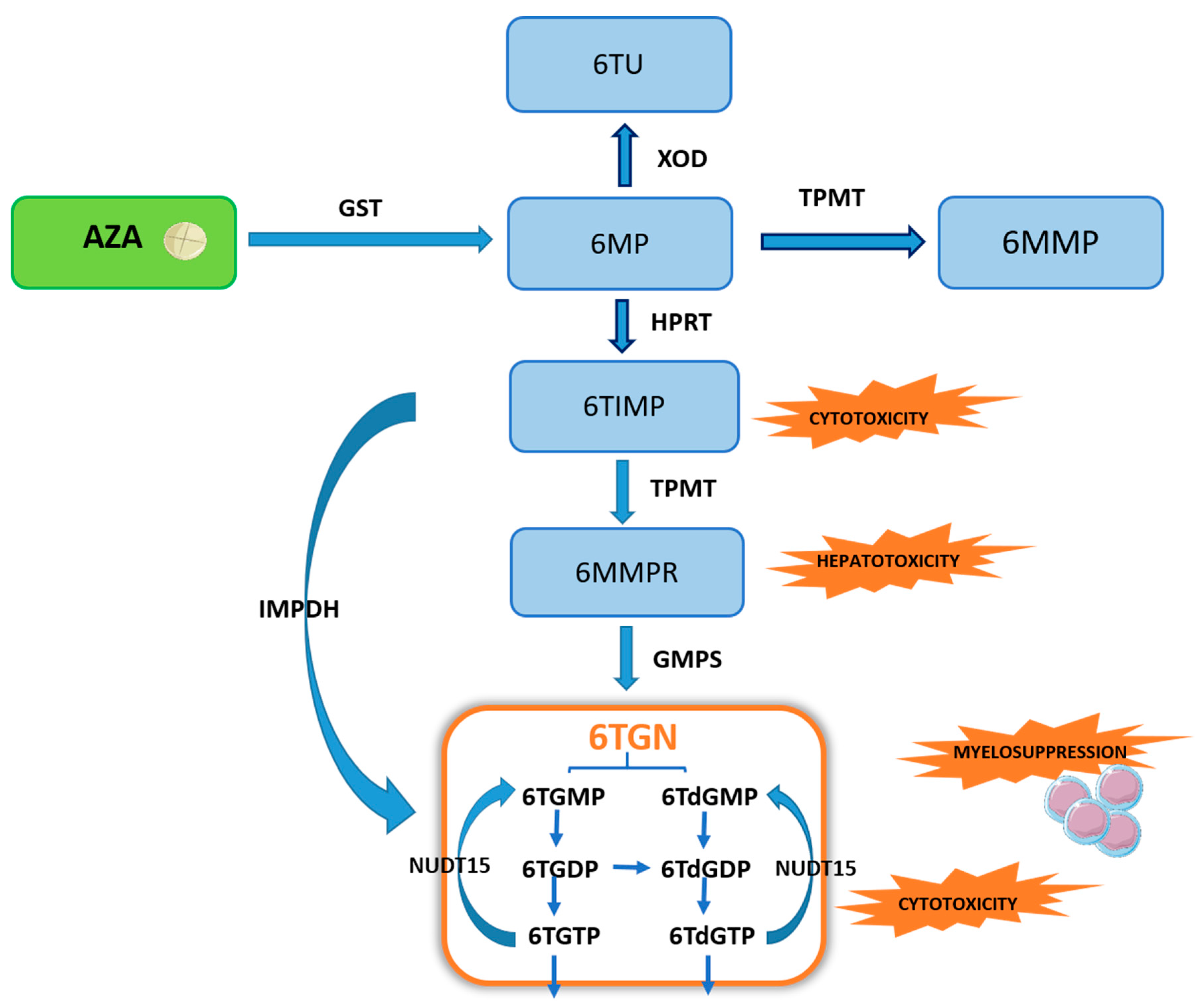
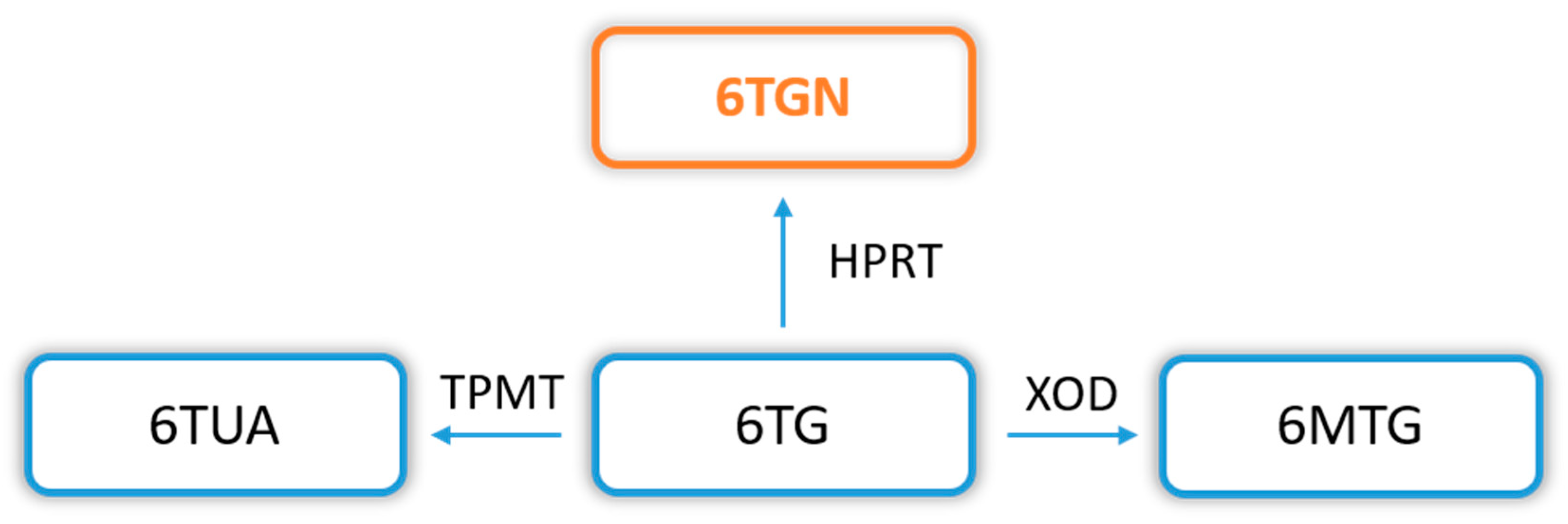
2. Metabolism of Thiopurine Drugs
3. Cytotoxic Properties and the Mechanism of Action of Thiopurines
3.1. Induction of Cell Apoptosis
3.2. Inhibition of DNA Replication and RNA Transcription
3.3. Inhibition of De Novo Purine Synthesis
4. Pharmacological Aspects and Clinical Characteristics of IBD Patients Treated with Thiopurines
4.1. Thiopurines in Crohn’s Disease (CD) Treatment
4.2. Thiopurines in Ulcerative Colitis (UC) Treatment
4.3. Combination Therapy of Thiopurines and Infliximab in IBD
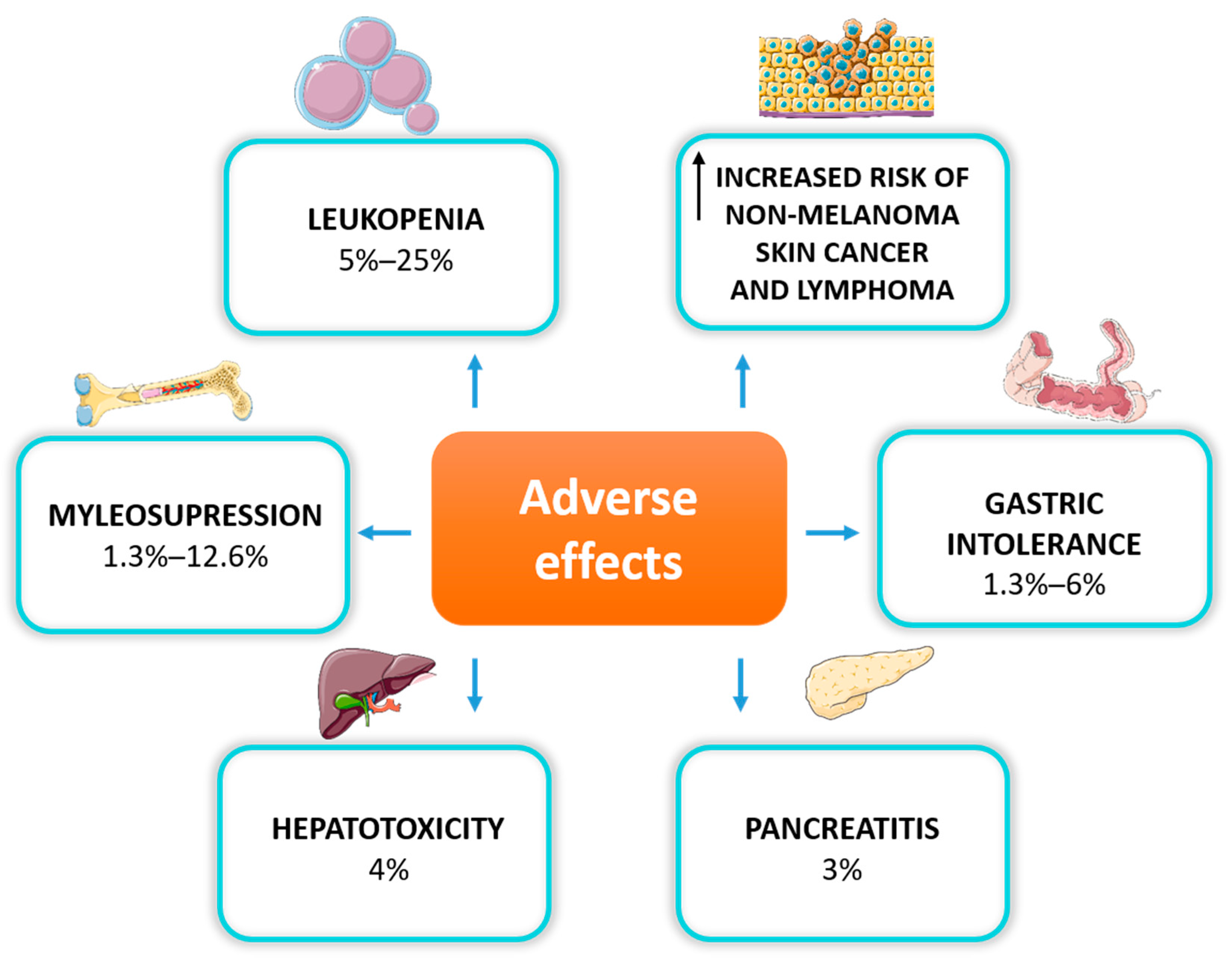
4.4. Safety and Adverse Effects of Thiopurine Treatment
4.5. Thiopurine Cytotoxicity and Pregnancy in IBD
4.6. Solutions to Cytotoxicity and Resistance to Thiopurines
5. Assessment of the Cytotoxicity of Thiopurine Drugs in IBD Patients
5.1. Thiopurine Metabolite Levels
5.2. Enzyme Activity
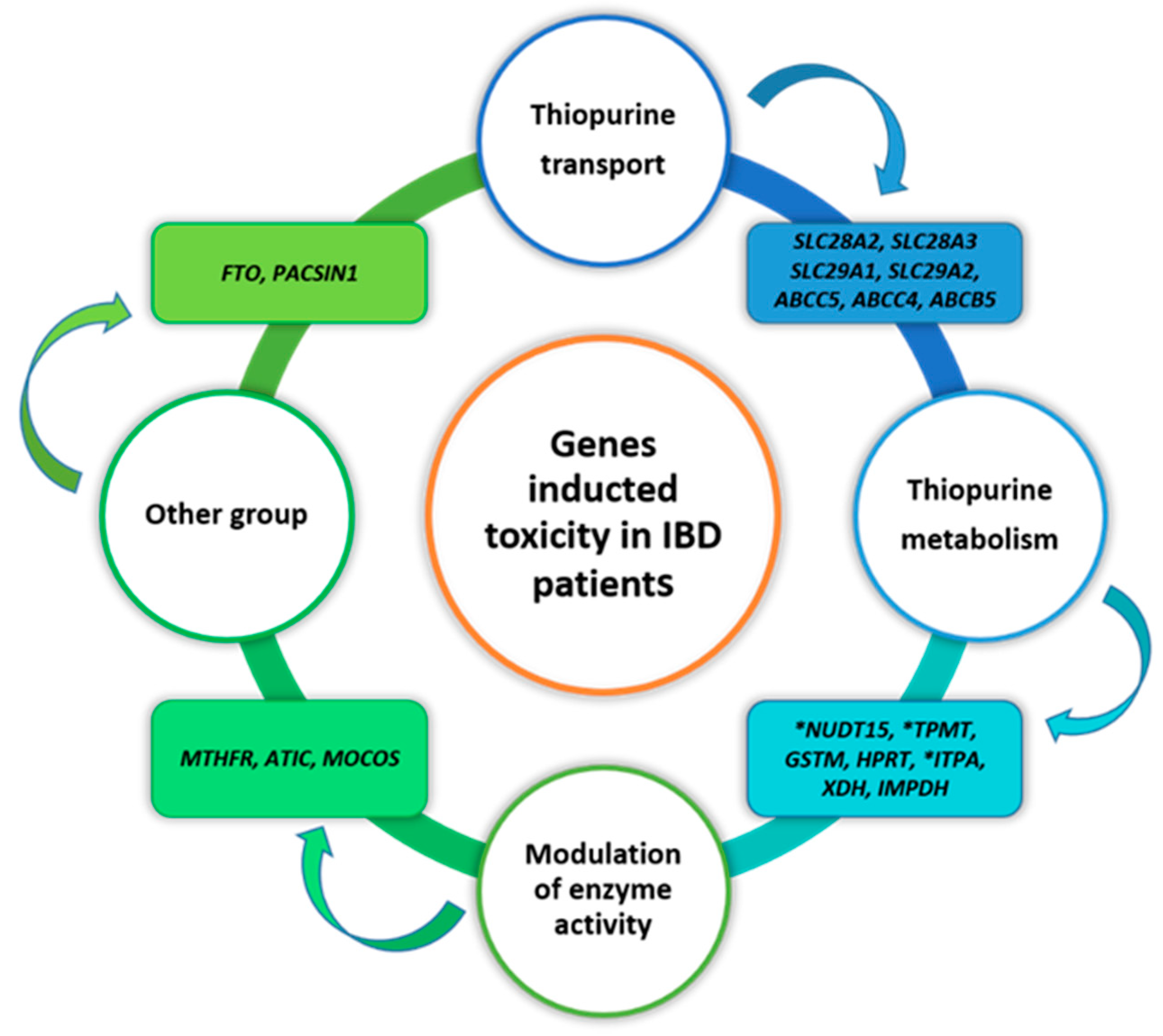
5.3. Genes Implicated in Thiopurine-Induced Toxicity in IBD Patients
6. Future Perspective in Cytotoxicity Research of Thiopurine Drugs in IBD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brooke, B.; Hoffmann, D.C.; Swarbrick, E.T. Azathioprine For Crohn’s Disease. Lancet 1969, 294, 612–614. [Google Scholar] [CrossRef]
- Warner, B.; Johnston, E.; Arenas-Hernandez, M.; Marinaki, A.; Irving, P.; Sanderson, J. A Practical Guide to Thiopurine Prescribing and Monitoring in IBD. Frontline Gastroenterol. 2018, 9, 10–15. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, M.; Ordás, I.; Cabré, E.; Garcia-Sanchez, V.; Bastida, G.; Peñalva, M.; Gomollón, F.; García-Planella, E.; Merino, O.; Gutiérrez, A.; et al. Safety of Thiopurine Therapy in Inflammatory Bowel Disease: Long-Term Follow-up Study of 3931 Patients. Inflamm. Bowel Dis. 2013, 19, 1404–1410. [Google Scholar] [CrossRef]
- Jharap, B.; Seinen, M.L.; de Boer, N.K.H.; van Ginkel, J.R.; Linskens, R.K.; Kneppelhout, J.C.; Mulder, C.J.J.; van Bodegraven, A.A. Thiopurine Therapy in Inflammatory Bowel Disease Patients: Analyses of Two 8-Year Intercept Cohorts. Inflamm. Bowel Dis. 2010, 16, 1541–1549. [Google Scholar] [CrossRef]
- Sahasranaman, S.; Howard, D.; Roy, S. Clinical Pharmacology and Pharmacogenetics of Thiopurines. Eur. J. Clin. Pharmacol. 2008, 64, 753–767. [Google Scholar] [CrossRef]
- Amin, J.; Huang, B.; Yoon, J.; Shih, D.Q. Update 2014: Advances to Optimize 6-Mercaptopurine and Azathioprine to Reduce Toxicity and Improve Efficacy in the Management of IBD. Inflamm. Bowel Dis. 2015, 21, 445–452. [Google Scholar] [CrossRef]
- Lucafò, M.; Stocco, G.; Martelossi, S.; Favretto, D.; Franca, R.; Malusà, N.; Lora, A.; Bramuzzo, M.; Naviglio, S.; Cecchin, E.; et al. Azathioprine Biotransformation in Young Patients with Inflammatory Bowel Disease: Contribution of Glutathione-S Transferase M1 and A1 Variants. Genes 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Stocco, G.; Cuzzoni, E.; De Iudicibus, S.; Franca, R.; Favretto, D.; Malusà, N.; Londero, M.; Cont, G.; Bartoli, F.; Martelossi, S.; et al. Deletion of Glutathione-S-Transferase M1 Reduces Azathioprine Metabolite Concentrations in Young Patients with Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2014, 48, 43–51. [Google Scholar] [CrossRef]
- Wong, D.R.; Coenen, M.J.H.; Derijks, L.J.J.; Vermeulen, S.H.; van Marrewijk, C.J.; Klungel, O.H.; Scheffer, H.; Franke, B.; Guchelaar, H.-J.; de Jong, D.J.; et al. The TOPIC Recruitment Team. Early Prediction of Thiopurine-Induced Hepatotoxicity in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2017, 45, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Dubinsky, M.C.; Lamothe, S.; Yang, H.Y.; Targan, S.R.; Sinnett, D.; Theoret, Y.; Seidman, E.G. Pharmacogenomics and Metabolite Measurement for 6-Mercaptopurine Therapy in Inflammatory Bowel Disease. Gastroenterology 2000, 118, 705–713. [Google Scholar] [CrossRef]
- Cuffari, C. Thiopurine Methyltransferase Activity Influences Clinical Response to Azathioprine in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2004, 2, 410–417. [Google Scholar] [CrossRef]
- Sayani, F.A.; Prosser, C.; Bailey, R.J.; Jacobs, P.; Fedorak, R.N. Thiopurine Methyltransferase Enzyme Activity Determination before Treatment of Inflammatory Bowel Disease with Azathioprine: Effect on Cost and Adverse Events. Can. J. Gastroenterol. 2005, 19, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Gisbert, J.P.; Niño, P.; Rodrigo, L.; Cara, C.; Guijarro, L.G. Thiopurine Methyltransferase (TPMT) Activity and Adverse Effects of Azathioprine in Inflammatory Bowel Disease: Long-Term Follow-Up Study of 394 Patients. Am. J. Gastroenterol. 2006, 101, 2769–2776. [Google Scholar] [CrossRef]
- Derijks, L.J.J.; Gilissen, L.P.L.; Engels, L.G.J.B.; Bos, L.P.; Bus, P.J.; Lohman, J.J.H.M.; van Deventer, S.J.H.; Hommes, D.W.; Hooymans, P.M. Pharmacokinetics of 6-Thioguanine in Patients with Inflammatory Bowel Disease. Ther. Drug Monit. 2006, 28, 45–50. [Google Scholar] [CrossRef]
- Haglund, S.; Vikingsson, S.; Söderman, J.; Hindorf, U.; Grännö, C.; Danelius, M.; Coulthard, S.; Peterson, C.; Almer, S. The Role of Inosine-5′-Monophosphate Dehydrogenase in Thiopurine Metabolism in Patients with Inflammatory Bowel Disease. Ther. Drug Monit. 2011, 33, 200–208. [Google Scholar] [CrossRef]
- Kudo, M.; Moteki, T.; Sasaki, T.; Konno, Y.; Ujiie, S.; Onose, A.; Mizugaki, M.; Ishikawa, M.; Hiratsuka, M. Functional Characterization of Human Xanthine Oxidase Allelic Variants. Pharmacogenet. Genom. 2008, 18, 243–251. [Google Scholar] [CrossRef]
- Wong, D.R.; Derijks, L.J.J.; den Dulk, M.O.; Gemmeke, E.H.K.M.; Hooymans, P.M. The Role of Xanthine Oxidase in Thiopurine Metabolism: A Case Report. Ther. Drug Monit. 2007, 29, 845–848. [Google Scholar] [CrossRef] [Green Version]
- Valerie, N.C.K.; Hagenkort, A.; Page, B.D.G.; Masuyer, G.; Rehling, D.; Carter, M.; Bevc, L.; Herr, P.; Homan, E.; Sheppard, N.G.; et al. NUDT15 Hydrolyzes 6-Thio-DeoxyGTP to Mediate the Anticancer Efficacy of 6-Thioguanine. Cancer Res. 2016, 76, 5501–5511. [Google Scholar] [CrossRef] [Green Version]
- Toyonaga, T.; Kobayashi, T.; Kuronuma, S.; Ueno, A.; Kiyohara, H.; Okabayashi, S.; Takeuchi, O.; Redfern, C.P.F.; Terai, H.; Ozaki, R.; et al. Increased DNA-Incorporated Thiopurine Metabolite as a Possible Mechanism for Leukocytopenia through Cell Apoptosis in Inflammatory Bowel Disease Patients with NUDT15 Mutation. J. Gastroenterol. 2021, 56, 999–1007. [Google Scholar] [CrossRef]
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Lehr, H.A.; Wirtz, S.; Becker, C.; Atreya, R.; et al. CD28-Dependent Rac1 Activation Is the Molecular Target of Azathioprine in Primary Human CD4+ T Lymphocytes. J. Clin. Investig. 2003, 111, 1133–1145. [Google Scholar] [CrossRef] [Green Version]
- Poppe, D.; Tiede, I.; Fritz, G.; Becker, C.; Bartsch, B.; Wirtz, S.; Strand, D.; Tanaka, S.; Galle, P.R.; Bustelo, X.R.; et al. Azathioprine Suppresses Ezrin-Radixin-Moesin-Dependent T Cell-APC Conjugation through Inhibition of Vav Guanosine Exchange Activity on Rac Proteins. J. Immunol. 2006, 176, 640–651. [Google Scholar] [CrossRef]
- Daehn, I.; Brem, R.; Barkauskaite, E.; Karran, P. 6-Thioguanine Damages Mitochondrial DNA and Causes Mitochondrial Dysfunction in Human Cells. FEBS Lett. 2011, 585, 3941–3946. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.J.; Andersen, A.; Slørdal, L. Quantitation of 6-Thioguanine Residues in Peripheral Blood Leukocyte DNA Obtained from Patients Receiving 6-Mercaptopurine-Based Maintenance Therapy. Cancer Res. 1995, 55, 1670–1674. [Google Scholar]
- Rappaport, H.P. Replication of the Base Pair 6-Thioguanine/5-Methyl-2-Pyrimidinone with the Large Klenow Fragment of Escherichia Coli DNA Polymerase I. Biochemistry 1993, 32, 3047–3057. [Google Scholar] [CrossRef]
- You, C.; Dai, X.; Yuan, B.; Wang, Y. Effects of 6-Thioguanine and S6-Methylthioguanine on Transcription in Vitro and in Human Cells. J. Biol. Chem. 2012, 287, 40915–40923. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Zhang, J.; Wang, H.; Xiong, L.; Cai, Q.; Wang, T.; Jacobsen, S.; Pradhan, S.; Wang, Y. 6-Thioguanine Reactivates Epigenetically Silenced Genes in Acute Lymphoblastic Leukemia Cells by Facilitating Proteasome-Mediated Degradation of DNMT1. Cancer Res. 2011, 71, 1904–1911. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Nishii, R.; Perez-Andreu, V.; Yang, W.; Klussmann, F.A.; Zhao, X.; Lin, T.-N.; Hoshitsuki, K.; Nersting, J.; Kihira, K.; et al. NUDT15 Polymorphisms Alter Thiopurine Metabolism and Hematopoietic Toxicity. Nat. Genet. 2016, 48, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Coulthard, S.A.; Hogarth, L.A.; Little, M.; Matheson, E.C.; Redfern, C.P.F.; Minto, L.; Hall, A.G. The Effect of Thiopurine Methyltransferase Expression on Sensitivity to Thiopurine Drugs. Mol. Pharmacol. 2002, 62, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Coulthard, S.A.; Berry, P.; McGarrity, S.; Ansari, A.; Redfern, C.P.F. Liquid Chromatography–Mass Spectrometry for Measuring Deoxythioguanosine in DNA from Thiopurine-Treated Patients. J. Chromatogr. B 2016, 1028, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Reinisch, W.; Panés, J.; Lémann, M.; Schreiber, S.; Feagan, B.; Schmidt, S.; Sturniolo, G.C.; Mikhailova, T.; Alexeeva, O.; Sanna, L.; et al. A Multicenter, Randomized, Double-Blind Trial of Everolimus Versus Azathioprine and Placebo to Maintain Steroid-Induced Remission in Patients with Moderate-to-Severe Active Crohn’s Disease. Am. J. Gastroenterol. 2008, 103, 2284–2292. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.L.; Kaplan, G.G.; Peyrin-Biroulet, L.; Baidoo, L.; Devlin, S.; Melmed, G.Y.; Tanyingoh, D.; Raffals, L.; Irving, P.; Kozuch, P.; et al. Effects of Concomitant Immunomodulator Therapy on Efficacy and Safety of Anti–Tumor Necrosis Factor Therapy for Crohn’s Disease: A Meta-Analysis of Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2015, 13, 2233–2240.e2. [Google Scholar] [CrossRef] [Green Version]
- Chande, N.; Patton, P.H.; Tsoulis, D.J.; Thomas, B.S.; MacDonald, J.K. Azathioprine or 6-Mercaptopurine for Maintenance of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2015, 10, CD000067. [Google Scholar] [CrossRef]
- Panés, J.; López–SanRomán, A.; Bermejo, F.; García–Sánchez, V.; Esteve, M.; Torres, Y.; Domènech, E.; Piqueras, M.; Gomez–García, M.; Gutiérrez, A.; et al. Early Azathioprine Therapy Is No More Effective Than Placebo for Newly Diagnosed Crohn’s Disease. Gastroenterology 2013, 145, 766–774.e1. [Google Scholar] [CrossRef]
- Boyapati, R.K.; Torres, J.; Palmela, C.; Parker, C.E.; Silverberg, O.M.; Upadhyaya, S.D.; Nguyen, T.M.; Colombel, J.-F. withdrawal of Immunosuppressant or Biologic Therapy for Patients with Quiescent Crohn’s Disease. Cochrane Database Syst. Rev. 2018, 5, CD012540. [Google Scholar] [CrossRef]
- Ardizzone, S. Randomised Controlled Trial of Azathioprine and 5-Aminosalicylic Acid for Treatment of Steroid Dependent Ulcerative Colitis. Gut 2006, 55, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Christophorou, D.; Funakoshi, N.; Duny, Y.; Valats, J.-C.; Bismuth, M.; Pineton De Chambrun, G.; Daures, J.-P.; Blanc, P. Systematic Review with Meta-Analysis: Infliximab and Immunosuppressant Therapy vs. Infliximab Alone for Active Ulcerative Colitis. Aliment. Pharmacol. Ther. 2015, 41, 603–612. [Google Scholar] [CrossRef]
- Fraser, A.G. The Efficacy of Azathioprine for the Treatment of Inflammatory Bowel Disease: A 30 Year Review. Gut 2002, 50, 485–489. [Google Scholar] [CrossRef]
- Privitera, G.; Pugliese, D.; Onali, S.; Petito, V.; Scaldaferri, F.; Gasbarrini, A.; Danese, S.; Armuzzi, A. Combination Therapy in Inflammatory Bowel Disease—From Traditional Immunosuppressors towards the New Paradigm of Dual Targeted Therapy. Autoimmun. Rev. 2021, 20, 102832. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mathôt, R.A.; van der Kleij, D.; Rispens, T.; Ashruf, Y.; Jansen, J.M.; Rietdijk, S.; Löwenberg, M.; Ponsioen, C.Y.; Singh, S.; et al. Pharmacokinetic Features and Presence of Antidrug Antibodies Associate with Response to Infliximab Induction Therapy in Patients with Moderate to Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2016, 14, 251–258.e2. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European Evidence-Based Consensus on the Prevention, Diagnosis and Management of Opportunistic Infections in Inflammatory Bowel Disease. J. Crohns Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Schaffeler, E.; Marx, C.; Fischer, C.; Lang, T.; Behrens, C.; Gregor, M.; Eichelbaum, M.; Zanger, U.; Kaskas, B. Azathioprine Therapy and Adverse Drug Reactions in Patients with Inflammatory Bowel Disease: Impact of Thiopurine S-Methyltransferase Polymorphism. Pharmacogenetics 2002, 12, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Abramson, O.; Pascua, M.; Liu, L.; Asakura, L.M.; Velayos, F.S.; Hutfless, S.M.; Alison, J.E.; Herrinton, L.J. Timing of Myelosuppression During Thiopurine Therapy for Inflammatory Bowel Disease: Implications for Monitoring Recommendations. Clin. Gastroenterol. Hepatol. 2009, 7, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, D.Q.; Nguyen, M.; Zheng, L.; Ibanez, P.; Mei, L.; Kwan, L.Y.; Bradford, K.; Ting, C.; Targan, S.R.; Vasiliauskas, E.A. Split-Dose Administration of Thiopurine Drugs: A Novel and Effective Strategy for Managing Preferential 6-MMP Metabolism. Aliment. Pharmacol. Ther. 2012, 36, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Teich, N.; Mohl, W.; Bokemeyer, B.; Bündgens, B.; Büning, J.; Miehlke, S.; Hüppe, D.; Maaser, C.; Klugmann, T.; Kruis, W.; et al. Azathioprine-Induced Acute Pancreatitis in Patients with Inflammatory Bowel Diseases—A Prospective Study on Incidence and Severity. J. Crohns Colitis 2016, 10, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.; Arenas, M.; Greenfield, S.M.; Morris, D.; Lindsay, J.; Gilshenan, K.; Smith, M.; Lewis, C.; Marinaki, A.; Duley, J.; et al. Prospective Evaluation of the Pharmacogenetics of Azathioprine in the Treatment of Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2008, 28, 973–983. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Rutgeerts, P.; Sandborn, W.J.; Sands, B.E.; Diamond, R.H.; Blank, M.; Montello, J.; Tang, L.; Cornillie, F.; Colombel, J.-F. A Pooled Analysis of Infections, Malignancy, and Mortality in Infliximab- and Immunomodulator-Treated Adult Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2012, 107, 1051–1063. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Langholff, W.; Londhe, A.; Sandborn, W.J. Drug Therapies and the Risk of Malignancy in Crohn’s Disease: Results From the TREATTM Registry. Inflamm. BOWEL Dis. 2014, 109, 12. [Google Scholar] [CrossRef]
- D’Haens, G.; Reinisch, W.; Panaccione, R.; Satsangi, J.; Petersson, J.; Bereswill, M.; Arikan, D.; Perotti, E.; Robinson, A.M.; Kalabic, J.; et al. Open: Lymphoma Risk and Overall Safety Profile of Adalimumab in Patients with Crohn’s Disease with up to 6 Years of Follow-up in the PYRAMID Registry. Am. J. Gastroenterol. 2018, 113, 872–882. [Google Scholar] [CrossRef]
- D’Haens, G.; Reinisch, W.; Colombel, J.-F.; Panes, J.; Ghosh, S.; Prantera, C.; Lindgren, S.; Hommes, D.W.; Huang, Z.; Boice, J.; et al. Five-Year Safety Data From ENCORE, a European Observational Safety Registry for Adults with Crohn’s Disease Treated with Infliximab [Remicade®] or Conventional Therapy. J. Crohns Colitis 2016, 11, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Ungaro, R.C.; Brenner, E.J.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Steinwurz, F.; et al. Effect of IBD Medications on COVID-19 Outcomes: Results from an International Registry. Gut 2021, 70, 725–732. [Google Scholar] [CrossRef]
- De Boer, N.K.H.; Jarbandhan, S.V.A.; de Graaf, P.; Mulder, C.J.J.; van Elburg, R.M.; van Bodegraven, A.A. Azathioprine Use During Pregnancy: Unexpected Intrauterine Exposure to Metabolites. Am. J. Gastroenterol. 2006, 101, 1390–1392. [Google Scholar] [CrossRef]
- Flanagan, E.; Wright, E.K.; Hardikar, W.; Sparrow, M.P.; Connell, W.R.; Kamm, M.A.; De Cruz, P.; Brown, S.J.; Thompson, A.; Greenway, A.; et al. Maternal Thiopurine Metabolism during Pregnancy in Inflammatory Bowel Disease and Clearance of Thiopurine Metabolites and Outcomes in Exposed Neonates. Aliment. Pharmacol. Ther. 2021, 53, 810–820. [Google Scholar] [CrossRef]
- Kanis, S.L.; de Lima-Karagiannis, A.; de Boer, N.K.H.; van der Woude, C.J. Use of Thiopurines During Conception and Pregnancy Is Not Associated with Adverse Pregnancy Outcomes or Health of Infants at One Year in a Prospective Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1232–1241.e1. [Google Scholar] [CrossRef]
- Van der Woude, C.J.; Ardizzone, S.; Bengtson, M.B.; Fiorino, G.; Fraser, G.; Katsanos, K.; Kolacek, S.; Juillerat, P.; Mulders, A.G.M.G.J.; Pedersen, N.; et al. The Second European Evidenced-Based Consensus on Reproduction and Pregnancy in Inflammatory Bowel Disease. J. Crohns Colitis 2015, 9, 107–124. [Google Scholar] [CrossRef]
- Mahadevan, U.; Long, M.D.; Kane, S.V.; Roy, A.; Dubinsky, M.C.; Sands, B.E.; Cohen, R.D.; Chambers, C.D.; Sandborn, W.J. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women with Inflammatory Bowel Disease. Gastroenterology 2021, 160, 1131–1139. [Google Scholar] [CrossRef]
- Nguyen, G.C. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016, 150, 734–757.e1. [Google Scholar] [CrossRef] [Green Version]
- Andoh, A.; Kawahara, M.; Imai, T.; Tatsumi, G.; Inatomi, O.; Kakuta, Y. Thiopurine Pharmacogenomics and Pregnancy in Inflammatory Bowel Disease. J. Gastroenterol. 2021, 56, 881–890. [Google Scholar] [CrossRef]
- Govani, S.M.; Higgins, P.D.R. Combination of Thiopurines and Allopurinol: Adverse Events and Clinical Benefit in IBD. J. Crohns Colitis 2010, 4, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Hoentjen, F.; Seinen, M.L.; Hanauer, S.B.; de Boer, N.K.H.; Rubin, D.T.; Bouma, G.; Harrell, L.E.; van Bodegraven, A.A. Safety and Effectiveness of Long-Term Allopurinol–Thiopurine Maintenance Treatment in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 363–369. [Google Scholar] [CrossRef]
- Moreau, B.; Clement, P.; Theoret, Y.; Seidman, E.G. Allopurinol in Combination with Thiopurine Induces Mucosal Healing and Improves Clinical and Metabolic Outcomes in IBD. Ther. Adv. Gastroenterol. 2017, 10, 819–827. [Google Scholar] [CrossRef]
- Seinen, M.L.; van Asseldonk, D.P.; de Boer, N.K.H.; Losekoot, N.; Smid, K.; Mulder, C.J.J.; Bouma, G.; Peters, G.J.; van Bodegraven, A.A. The Effect of Allopurinol and Low-Dose Thiopurine Combination Therapy on the Activity of Three Pivotal Thiopurine Metabolizing Enzymes: Results from a Prospective Pharmacological Study. J. Crohns Colitis 2013, 7, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.Z.; Chua, E.W. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front. Pharmacol. 2018, 9, 1107. [Google Scholar] [CrossRef]
- Crouwel, F.; Simsek, M.; Mulder, C.J.J.; Buiter, H.J.C.; De Boer, N.K. Thioguanine Therapy in Inflammatory Bowel Diseases. A Practical Guide. J. Gastrointestin. Liver Dis. 2020, 29, 637–645. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Vasiliauskas, E.A.; Singh, H.; Abreu, M.T.; Papadakis, K.A.; Tran, T.; Martin, P.; Vierling, J.M.; Geller, S.A.; Targan, S.R.; et al. 6-Thioguanine Can Cause Serious Liver Injury in Inflammatory Bowel Disease Patients. Gastroenterology 2003, 125, 298–303. [Google Scholar] [CrossRef]
- Biemans, V.B.C.; Savelkoul, E.; Gabriëls, R.Y.; Simsek, M.; Dijkstra, G.; Pierik, M.J.; West, R.L.; de Boer, N.K.H.; Hoentjen, F. A Comparative Analysis of Tioguanine versus Low-Dose Thiopurines Combined with Allopurinol in Inflammatory Bowel Disease Patients. Aliment. Pharmacol. Ther. 2020, 51, 1076–1086. [Google Scholar] [CrossRef]
- Simsek, M.; Deben, D.S.; Horjus, C.S.; Bénard, M.V.; Lissenberg-Witte, B.I.; Buiter, H.J.C.; van Luin, M.; Seinen, M.L.; Mulder, C.J.J.; Wong, D.R.; et al. Sustained Effectiveness, Safety and Therapeutic Drug Monitoring of Tioguanine in a Cohort of 274 IBD Patients Intolerant for Conventional Therapies. Aliment. Pharmacol. Ther. 2019, 50, 54–65. [Google Scholar] [CrossRef]
- Movva, R.; Lobb, M.; Ó Cuív, P.; Florin, T.H.J.; Duley, J.A.; Oancea, I. Microbial Metabolism of Thiopurines: A Method to Measure Thioguanine Nucleotides. J. Microbiol. Methods 2016, 128, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Oancea, I.; Movva, R.; Das, I.; Aguirre de Cárcer, D.; Schreiber, V.; Yang, Y.; Purdon, A.; Harrington, B.; Proctor, M.; Wang, R.; et al. Colonic Microbiota Can Promote Rapid Local Improvement of Murine Colitis by Thioguanine Independently of T Lymphocytes and Host Metabolism. Gut 2017, 66, 59–69. [Google Scholar] [CrossRef]
- Relling, M.V.; Schwab, M.; Whirl-Carrillo, M.; Suarez-Kurtz, G.; Pui, C.; Stein, C.M.; Moyer, A.M.; Evans, W.E.; Klein, T.E.; Antillon-Klussmann, F.G.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT 15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 2019, 105, 1095–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, P.; Estevinho, M.M.; Dias, C.C.; Ministro, P.; Kopylov, U.; Danese, S.; Peyrin-Biroulet, L.; Magro, F. Thiopurines’ Metabolites and Drug Toxicity: A Meta-Analysis. J. Clin. Med. 2020, 9, 2216. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.S.; Wirthgen, E.; Däbritz, J. Biomarkers Predictive of Response to Thiopurine Therapy in Inflammatory Bowel Disease. Front. Med. 2020, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Gilissen, L.P.L.; Wong, D.R.; Engels, L.G.J.B.; Bierau, J.; Bakker, J.A.; Paulussen, A.D.C.; Romberg-Camps, M.J.; Stronkhorst, A.; Bus, P.; Bos, L.P.; et al. Therapeutic Drug Monitoring of Thiopurine Metabolites in Adult Thiopurine Tolerant IBD Patients on Maintenance Therapy. J. Crohns Colitis 2012, 6, 698–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estevinho, M.M.; Afonso, J.; Rosa, I.; Lago, P.; Trindade, E.; Correia, L.; Dias, C.C.; Magro, F.; on behalf GEDII [Portuguese IBD Group]. A Systematic Review and Meta-Analysis of 6-Thioguanine Nucleotide Levels and Clinical Remission in Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1381–1392. [Google Scholar] [CrossRef] [Green Version]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S.; Gerson, L.; Hirano, I.; Nguyen, G.C.; Rubenstein, J.H.; Smalley, W.E.; et al. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Dujardin, R.W.G.; Meijer, B.; de Boer, N.K.H.; D’Haens, G.R.; Löwenberg, M. Usefulness of Mean Corpuscular Volume as a Surrogate Marker for Monitoring Thiopurine Treatment in Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2016, 28, 991–996. [Google Scholar] [CrossRef]
- Kopylov, U.; Battat, R.; Benmassaoud, A.; Paradis-Surprenant, L.; Seidman, E.G. Hematologic Indices as Surrogate Markers for Monitoring Thiopurine Therapy in IBD. Dig. Dis. Sci. 2015, 60, 478–484. [Google Scholar] [CrossRef]
- Lennard, L.; Singleton, H.J. High-Performance Liquid Chromatographic Assay of the Methyl and Nucleotide Metabolites of 6-Mercaptopurine: Quantitation of Red Blood Cell 6-Thioguanine Nucleotide, 6-Thioinosinic Acid and 6-Methylmercaptopurine Metabolites in a Single Sample. J. Chromatogr. B. Biomed. Sci. App. 1992, 583, 83–90. [Google Scholar] [CrossRef]
- Vikingsson, S.; Carlsson, B.; Almer, S.H.; Peterson, C. Monitoring of Thiopurine Metabolites in Patients with Inflammatory Bowel Disease-What Is Actually Measured? Ther. Drug Monit. 2009, 31, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Genova, E.; Fusco, L.; Marisat, M.; Hofmann, U.; Favretto, D.; Lucafò, M.; Taddio, A.; Martelossi, S.; Ventura, A.; et al. Pharmacokinetics and Pharmacodynamics of Thiopurines in an in Vitro Model of Human Hepatocytes: Insights from an Innovative Mass Spectrometry Assay. Chem. Biol. Interact. 2017, 275, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Genova, E. Insights into the Cellular Pharmacokinetics and Pharmacodynamics of Thiopurine Antimetabolites in a Model of Human Intestinal Cells. Chem. Interact. 2021, 9, 109624. [Google Scholar] [CrossRef] [PubMed]
- González-Lama, Y.; Bermejo, F.; López-Sanromán, A.; García-Sánchez, V.; Esteve, M.; Cabriada, J.L.; McNicholl, A.G.; Pajares, R.; Casellas, F.; Merino, O.; et al. Thiopurine Methyl-Transferase Activity and Azathioprine Metabolite Concentrations Do Not Predict Clinical Outcome in Thiopurine-Treated Inflammatory Bowel Disease Patients: Clinical Usefulness of Thiopurine Methyl-Transferase and Azathioprine Metabolites. Aliment. Pharmacol. Ther. 2011, 34, 544–554. [Google Scholar] [CrossRef]
- Weinshilboum, R.M.; Sladek, S.L. Mercaptopurine Pharmacogenetics: Monogenic Inheritance of Erythrocyte Thiopurine Methyltransferase Activity. Am. J. Hum. Genet. 1980, 32, 651–662. [Google Scholar]
- Kwan, L.Y.; Devlin, S.M.; Mirocha, J.M.; Papadakis, K.A. Thiopurine Methyltransferase Activity Combined with 6-Thioguanine Metabolite Levels Predicts Clinical Response to Thiopurines in Patients with Inflammatory Bowel Disease. Dig. Liver Dis. 2008, 40, 425–432. [Google Scholar] [CrossRef]
- Larussa, T.; Suraci, E.; Lentini, M.; Nazionale, I.; Gallo, L.; Abenavoli, L.; Imeneo, M.; Costanzo, F.S.; Cuda, G.; Luzza, F. High Prevalence of Polymorphism and Low Activity of Thiopurine Methyltransferase in Patients with Inflammatory Bowel Disease. Eur. J. Intern. Med. 2012, 23, 273–277. [Google Scholar] [CrossRef]
- Booth, R.A.; Ansari, M.T.; Loit, E.; Tricco, A.C.; Weeks, L.; Doucette, S.; Skidmore, B.; Sears, M.; Sy, R.; Karsh, J. Assessment of Thiopurine S-Methyltransferase Activity in Patients Prescribed Thiopurines: A Systematic Review. Ann. Intern. Med. 2011, 154, 814. [Google Scholar] [CrossRef] [Green Version]
- Coelho, T.; Andreoletti, G.; Ashton, J.J.; Batra, A.; Afzal, N.A.; Gao, Y.; Williams, A.P.; Beattie, R.M.; Ennis, S. Genes Implicated in Thiopurine-Induced Toxicity: Comparing TPMT Enzyme Activity with Clinical Phenotype and Exome Data in a Paediatric IBD Cohort. Sci. Rep. 2016, 6, 34658. [Google Scholar] [CrossRef] [Green Version]
- Harmand, P.-O.; Solassol, J. Thiopurine Drugs in the Treatment of Ulcerative Colitis: Identification of a Novel Deleterious Mutation in TPMT. Genes 2020, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Lee, S.H.; Lee, M.-N.; Kim, J.-W.; Kim, Y.-H.; Kim, M.J.; Lee, Y.M.; Kang, B.; Choe, Y.H.; Lee, N.H.; et al. Complete Sequence-Based Screening of TPMT Variants in the Korean Population. Pharmacogenet. Genom. 2015, 25, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Wu, H.-Y.; Yang, X.; Xu, H.-Q.; Li, Y.-C.; Shi, D.-C.; Huang, J.-F.; Huang, Q.; Fu, W.-L. Association between Thiopurine S-Methyltransferase Polymorphisms and Thiopurine-Induced Adverse Drug Reactions in Patients with Inflammatory Bowel Disease: A Meta-Analysis. PLoS ONE 2015, 10, e0121745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatsu, N.; Matsui, T.; Murakami, Y.; Ishihara, H.; Hisabe, T.; Nagahama, T.; Maki, S.; Beppu, T.; Takaki, Y.; Hirai, F.; et al. Adverse Reactions to Azathioprine Cannot Be Predicted by Thiopurine S-Methyltransferase Genotype in Japanese Patients with Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2009, 24, 1258–1264. [Google Scholar] [CrossRef]
- Banerjee, R.; Ravikanth, V.V.; Pal, P.; Bale, G.; Avanthi, U.S.; Goren, I.; Girish, B.G.; Mitnala, S.; Reddy, D.N. NUDT15 C415T Variant Compared with TPMT Genotyping in Predicting Azathioprine-Induced Leucopenia: Prospective Analysis of 1014 Inflammatory Bowel Disease Patients in India. Aliment. Pharmacol. Ther. 2020, 52, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; He, Y.; Wang, H.-X.; Liao, C.-L.; Peng, Y.; Tao, L.-J.; Zhang, W.; Yang, H.-X. Comparison of TPMT and NUDT15 Polymorphisms in Chinese Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2018, 24, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Voskuil, M.D.; Bangma, A.; Weersma, R.K.; Festen, E.A.M. Predicting (Side) Effects for Patients with Inflammatory Bowel Disease: The Promise of Pharmacogenetics. World J. Gastroenterol. 2019, 25, 2539–2548. [Google Scholar] [CrossRef]
- Nishii, R.; Moriyama, T.; Janke, L.J.; Yang, W.; Suiter, C.C.; Lin, T.-N.; Li, L.; Kihira, K.; Toyoda, H.; Hofmann, U.; et al. Preclinical Evaluation of NUDT15-Guided Thiopurine Therapy and Its Effects on Toxicity and Antileukemic Efficacy. Blood 2018, 131, 2466–2474. [Google Scholar] [CrossRef]
- Schaeffeler, E.; Jaeger, S.U.; Klumpp, V.; Yang, J.J.; Igel, S.; Hinze, L.; Stanulla, M.; Schwab, M. Impact of NUDT15 Genetics on Severe Thiopurine-Related Hematotoxicity in Patients with European Ancestry. Genet. Med. 2019, 21, 2145–2150. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.J.; Harrison, J.W.; Heap, G.A.; Voskuil, M.D.; Andersen, V.; Anderson, C.A.; Ananthakrishnan, A.N.; Barrett, J.C.; Beaugerie, L.; Bewshea, C.M.; et al. Association of Genetic Variants in NUDT15 with Thiopurine-Induced Myelosuppression in Patients with Inflammatory Bowel Disease. JAMA 2019, 321, 773. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Yang, Y.-L.; Nishii, R.; Ariffin, H.; Liu, C.; Lin, T.-N.; Yang, W.; Lin, D.-T.; Yu, C.-H.; Kham, S.; et al. Novel Variants in NUDT15 and Thiopurine Intolerance in Children with Acute Lymphoblastic Leukemia from Diverse Ancestry. Blood 2017, 130, 1209–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.J.; Whirl-Carrillo, M.; Scott, S.A.; Turner, A.J.; Schwab, M.; Tanaka, Y.; Suarez-Kurtz, G.; Schaeffeler, E.; Klein, T.E.; Miller, N.A.; et al. Pharmacogene Variation Consortium Gene Introduction: NUDT15. Clin. Pharmacol. Ther. 2019, 105, 1091–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, R.; Lee, M.; Kim, K.; Baek, S.; Kim, T.J.; Hong, S.N.; Kim, Y.; Lee, S. Effects of Various Genetic Polymorphisms on Thiopurine Treatment-associated Outcomes for Korean Patients with Crohn’s Disease. Br. J. Clin. Pharmacol. 2020, 86, 2302–2313. [Google Scholar] [CrossRef] [PubMed]
- Duley, J.A.; Somogyi, A.A.; Martin, J.H. The Future of Thiopurine Pharmacogenomics. Pharmacogenomics 2012, 13, 1549–1552. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-N.; Kang, B.; Choi, S.Y.; Kim, M.J.; Woo, S.Y.; Kim, J.-W.; Choe, Y.H.; Lee, S.-Y. Relationship Between Azathioprine Dosage, 6-Thioguanine Nucleotide Levels, and Therapeutic Response in Pediatric Patients with IBD Treated with Azathioprine. Inflamm. Bowel Dis. 2015, 21, 1054–1062. [Google Scholar] [CrossRef]
- Citterio-Quentin, A.; Moulsma, M.; Gustin, M.-P.; Boulieu, R. ITPA Activity in Adults and Children Treated with or without Azathioprine: Relationship Between TPMT Activity, Thiopurine Metabolites, and Co-Medications. Ther. Drug Monit. 2017, 39, 483–491. [Google Scholar] [CrossRef]
- Chang, J.Y.; Park, S.J.; Jung, E.S.; Jung, S.-A.; Moon, C.M.; Chun, J.; Park, J.J.; Kim, E.S.; Park, Y.; Kim, T.-I.; et al. Genotype-Based Treatment with Thiopurine Reduces Incidence of Myelosuppression in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 2010–2018.e2. [Google Scholar] [CrossRef]
- Chen, S.; Tan, W.Z.; Sutiman, N.; Lim, C.; Lee, S.S.; Leong, W.F.; Tjai, M.; Wang, C.; Kong, C.S.C.; Chuah, S.W.; et al. An Intronic FTO Variant Rs16952570 Confers Protection against Thiopurine-Induced Myelotoxicities in Multiethnic Asian IBD Patients. Pharm. J. 2020, 20, 505–515. [Google Scholar] [CrossRef]
- Wilson, A.; Jansen, L.E.; Rose, R.V.; Gregor, J.C.; Ponich, T.; Chande, N.; Khanna, R.; Yan, B.; Jairath, V.; Khanna, N.; et al. HLA-DQA1-HLA-DRB1 Polymorphism Is a Major Predictor of Azathioprine-Induced Pancreatitis in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2018, 47, 615–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangma, A.; Voskuil, M.D.; Uniken Venema, W.T.C.; Brugge, H.; Hu, S.; Lanting, P.; Franke, L.; Dijkstra, G.; Festen, E.A.M.; Weersma, R.K. Predicted Efficacy of a Pharmacogenetic Passport for Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 51, 1105–1115. [Google Scholar] [CrossRef]
- Van den Bosch, B.J.; Coenen, M.J. Pharmacogenetics of Inflammatory Bowel Disease. Pharmacogenomics 2021, 22, 55–66. [Google Scholar] [CrossRef]
- Effenberger, M.; Reider, S.; Waschina, S.; Bronowski, C.; Enrich, B.; Adolph, T.E.; Koch, R.; Moschen, A.R.; Rosenstiel, P.; Aden, K.; et al. Microbial Butyrate Synthesis Indicates Therapeutic Efficacy of Azathioprine in IBD Patients. J. Crohns Colitis 2021, 15, 88–98. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakerska-Banaszak, O.; Łykowska-Szuber, L.; Walczak, M.; Żuraszek, J.; Zielińska, A.; Skrzypczak-Zielińska, M. Cytotoxicity of Thiopurine Drugs in Patients with Inflammatory Bowel Disease. Toxics 2022, 10, 151. https://doi.org/10.3390/toxics10040151
Zakerska-Banaszak O, Łykowska-Szuber L, Walczak M, Żuraszek J, Zielińska A, Skrzypczak-Zielińska M. Cytotoxicity of Thiopurine Drugs in Patients with Inflammatory Bowel Disease. Toxics. 2022; 10(4):151. https://doi.org/10.3390/toxics10040151
Chicago/Turabian StyleZakerska-Banaszak, Oliwia, Liliana Łykowska-Szuber, Michał Walczak, Joanna Żuraszek, Aleksandra Zielińska, and Marzena Skrzypczak-Zielińska. 2022. "Cytotoxicity of Thiopurine Drugs in Patients with Inflammatory Bowel Disease" Toxics 10, no. 4: 151. https://doi.org/10.3390/toxics10040151
APA StyleZakerska-Banaszak, O., Łykowska-Szuber, L., Walczak, M., Żuraszek, J., Zielińska, A., & Skrzypczak-Zielińska, M. (2022). Cytotoxicity of Thiopurine Drugs in Patients with Inflammatory Bowel Disease. Toxics, 10(4), 151. https://doi.org/10.3390/toxics10040151





