Isolation and Characterization of Lactobacillus paracasei 85 and Lactobacillus buchneri 93 to Absorb and Biotransform Zearalenone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Purification of LAB from Pickles
2.3. Strain Screening and Identification
2.4. Quantification of ZEN and Its Metabolites
2.5. Optimization of LAB Incubation Conditions for ZEN Removal
2.6. ZEN Removal Abilities of Whole Cell Culture, Cell-Free Suspension, Cell Pellets, and Inactivated Cell Pellets
2.7. ZEN Metabolization by L. paracasei 85 and L. buchneri 93
2.8. Volatile Organic Compounds Analysis
2.9. Chemical and Enzymatic Treatment
2.10. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.11. Scanning Electron Microscopy (SEM) Analysis
2.12. Statistical Analysis
3. Results
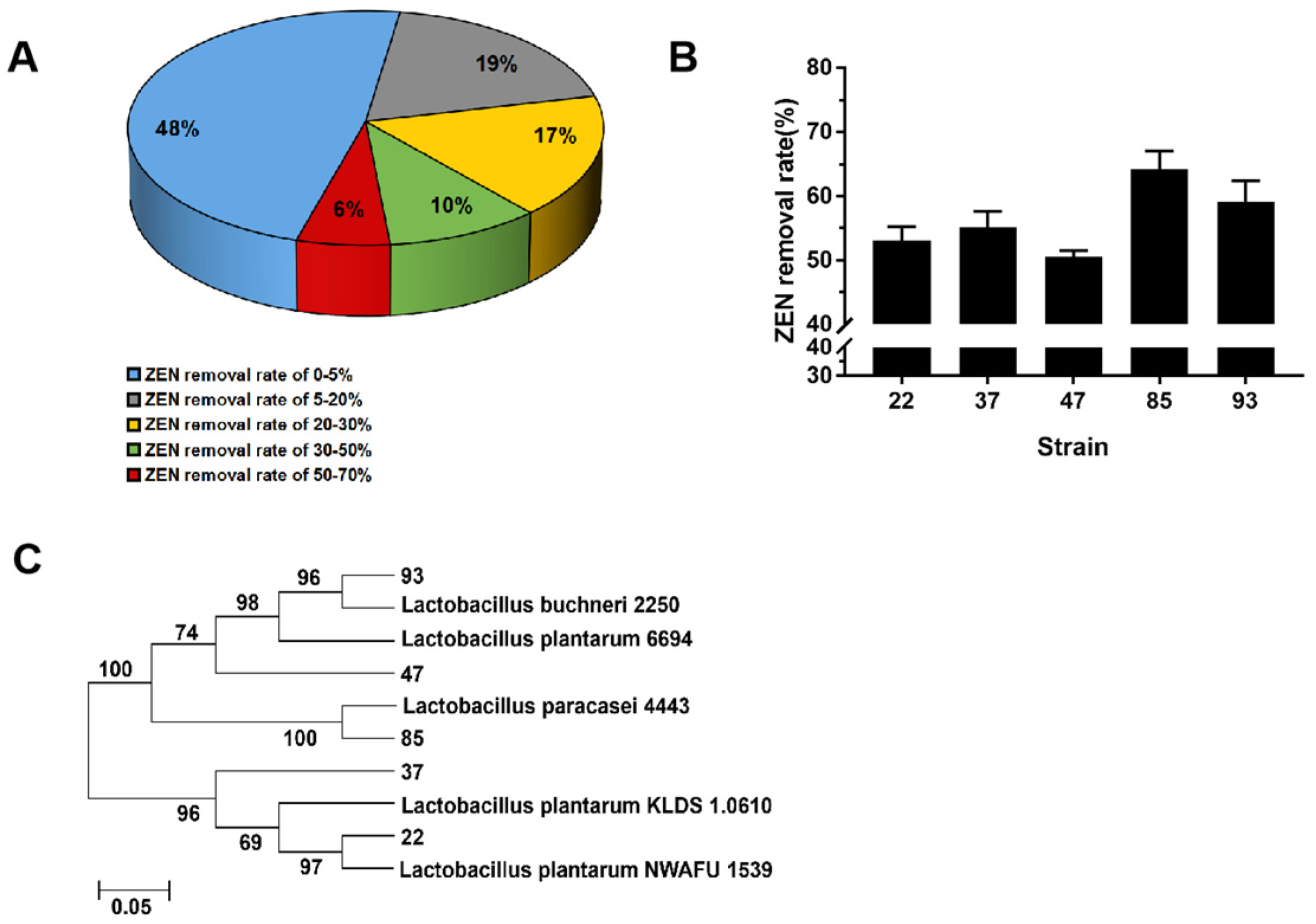
3.1. Screening and Identification of LAB for ZEN Removal
3.2. Condition Optimization of LAB Incubation to Promote the ZEN Removal Potential
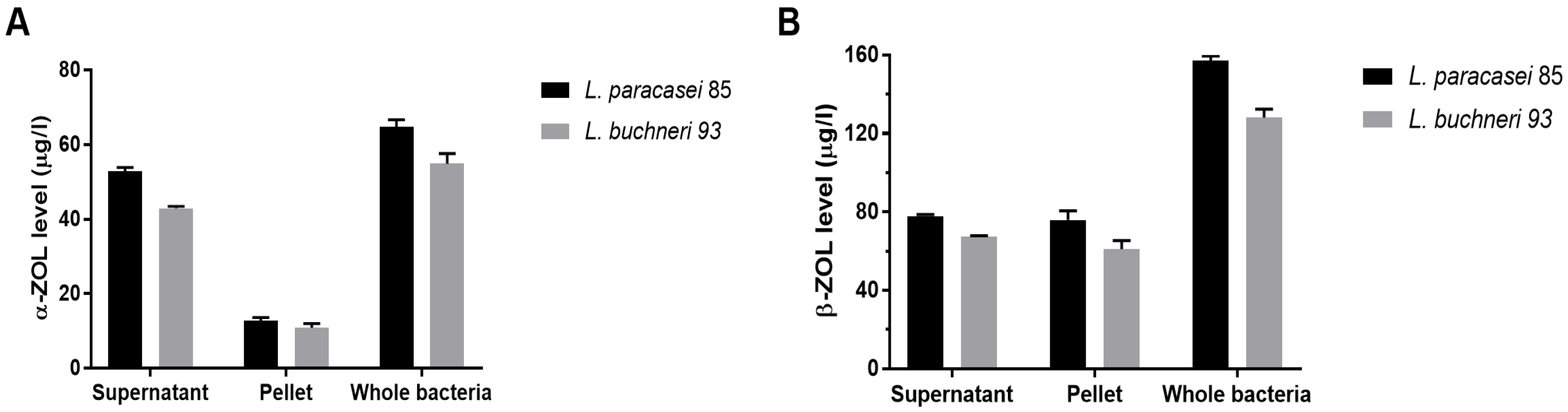
3.3. Adsorption and Biodegradation Participated in ZEN Removal
3.4. Reduction in ZEN Toxicity through LAB Metabolization
3.5. Influence of ZEN Degradation on LAB Volatile Organic Compounds (VOCs) Profile
3.6. The Effect of Chemical and Enzymatic Treatments on ZEN Removal
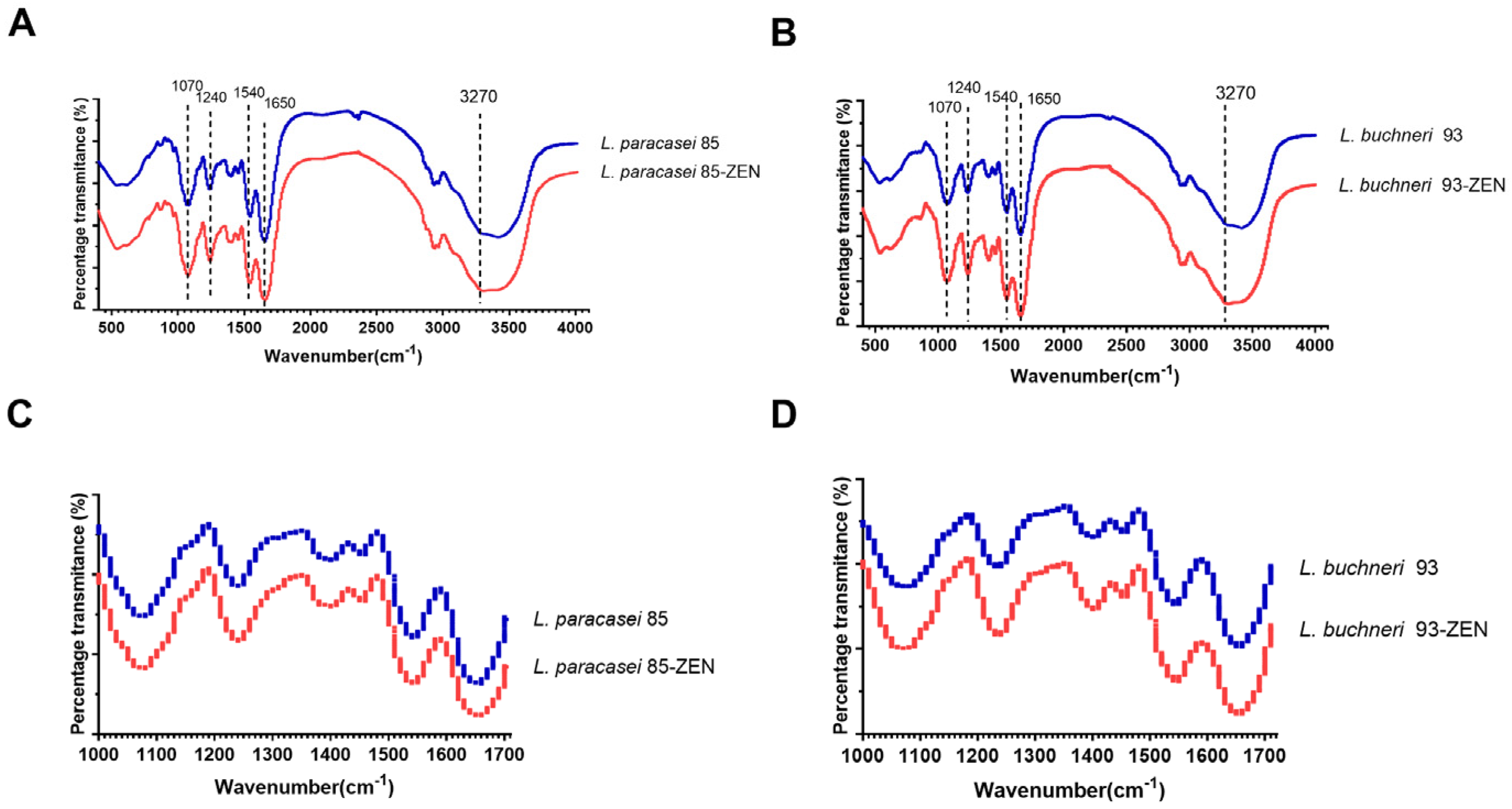
3.7. FTIR Analysis of LAB after ZEN Incubation
3.8. The Damage of Cell Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piotrowska, M. Microbiological Decontamination of Mycotoxins, Opportunities and Limitations. Toxins 2021, 13, 819. [Google Scholar] [CrossRef] [PubMed]
- Złoch, M.; Rogowska, A.; Pomastowski, P.; Railean-Plugaru, V.; Walczak-Skierska, J.; Rudnicka, J.; Buszewski, B. Use of Lactobacillus paracasei strain for zearalenone binding and metabolization. Toxicon 2020, 181, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, H.; Lan, J.; Zhang, R.; Ren, H.; Zhang, X.; Yu, G. Detoxification of zearalenone by three strains of Lactobacillus plantarum from fermented food in vitro. Food Control 2015, 54, 158–164. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone, An oestrogenic mycotoxin. Food. Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Salah-Abbès, J.B.; Belgacem, H.; Ezzdini, K.; Abdel-Wahhab, M.A.; Abbès, S. Zearalenone nephrotoxicity, DNA fragmentation, apoptotic gene expression and oxidative stress protected by Lactobacillus plantarum MON03. Toxicon 2020, 175, 28–35. [Google Scholar] [CrossRef]
- Kos, J.; Hajnal, E.J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Poschmaier, B.; Sulyok, M. Mycotoxins in maize harvested in Republic of Serbia in the period 2012–2015. Part 1, Regulated mycotoxins and its derivatives. Food Chem. 2020, 312, 126034. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, L.; Chen, Y.; Gao, H.; Hua, Y.; Yuan, X.; Yang, H. Mycotoxins in Maize Silage from China in 2019. Toxins 2022, 14, 241. [Google Scholar] [CrossRef]
- Matsuura, Y.; Yoshizawa, T.; Morooka, N. Effect of Food Additives and Heating on the Decomposition of Zearalenone in Wheat Flour. Food. Hyg. Safe. Sci. 1981, 22, 293–298. [Google Scholar] [CrossRef]
- Ryu, D.; Hanna, M.A.; Bullerman, L.B. Stability of zearalenone during extrusion of corn grits. J. Food Protect. 1999, 1482–1484. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Zhai, C.; Yu, Y.; Han, J.; Hu, J.; He, D.; Zhang, H.; Shi, J.; Mohamed, S.R.; Dawood, D.H.; Wang, G.; et al. Isolation, Characterization, and Application of Clostridium sporogenes F39 to Degrade Zearalenone under Anaerobic Conditions. Foods 2022, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Kakeya, H.; Takahashi-Ando, N.; Kimura, M.; Onose, R.; Yamaguchi, I.; Osada, H. Biotransformation of the mycotoxin, zearalenone, to a non-estrogenic compound by a fungal strain of Clonostachys sp. Biosci. Biotechnol. Biochem. 2002, 66, 2723–2726. [Google Scholar] [CrossRef]
- Utermark, J.; Karlovsky, P. Role of Zearalenone Lactonase in Protection of Gliocladium roseum from Fungitoxic Effects of the Mycotoxin Zearalenone. Appl. Environ. Microb. 2007, 73, 637–642. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Evaluation of Health and Nutritional Properties of Powder Milk and Live Lactic Acid Bacteria; Expert Consultation Report; Food and Agriculture Organization of the United Nations and World Health Organization: Rome, Italy, 2001; pp. 1–34. [Google Scholar]
- Fredua-Agyeman, M.; Stapleton, P.; Basit, A.W.; Gaisford, S. Microcalorimetric evaluation of a multi-strain probiotic, Interspecies inhibition between probiotic strains. J. Funct. Foods 2017, 36, 357–361. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, C.; Yang, J.; Tang, Y. Conversion of zearalenone to β-zearalenol and zearalenone-14, 16-diglucoside by Candida parapsilosis ATCC 7330. Food Control 2022, 131, 108429. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Bryła, M.; Gientka, I.; Błażejak, S.; Janowicz, M. Candida utilis ATCC 9950 Cell Walls and β(1,3)/(1,6)-Glucan Preparations Produced Using Agro-Waste as a Mycotoxins Trap. Toxins 2019, 11, 192. [Google Scholar] [CrossRef]
- Yang, S.B.; Zheng, H.C.; Xu, J.Y.; Zhao, X.Y.; Shu, W.J.; Li, X.M.; Song, H.; Ma, Y.H. New biotransformation mode of zearalenone identified in Bacillus subtilis Y816 revealing a novel ZEN conjugate. J. Agric. Food Chem. 2021, 69, 7409–7419. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.D.; Ibrahim, S.A. Lactic Acid Bacteria, Food Safety and Human Health Applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Belgacem, H.; Venditti, M.; Salah-Abbès, J.B.; Minucci, S.; Abbès, S. Potential protective effect of lactic acid bacteria against zearalenone causing reprotoxicity in male mice. Toxicon 2022, 209, 56–65. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Chelule, P.K.; Gqaleni, N. Reduction of fumonisin B1 and zearalenone by lactic acid bacteria in fermented maize meal. J. Food Prot. 2005, 68, 2095–2099. [Google Scholar] [CrossRef]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Chen, X.; Guo, S.Y.; Xiang, W.L.; Liu, L.; Du, H.J.; et al. Characterization of the microbial communities and their correlations with chemical profiles in assorted vegetable Sichuan pickles. Food Control 2020, 113, 107174. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Visessanguan, W. In Vitro Mechanism Assessment of Zearalenone Removal by Plant-Derived Lactobacillus plantarum BCC 47723. Toxins 2021, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, B.V.; Poornachandra Rao, K.; Chennapa, G.; Naik, M.K.; Chandrashekara, K.T.; Sreenivasa, M.Y. Antifungal Attributes of Lactobacillus plantarum MYS6 against Fumonisin Producing Fusarium proliferatum Associated with Poultry Feeds. PLoS ONE 2016, 11, e0155122. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Rațiu, I.A.; Milanowski, M.; Pomastowski, P.; Ligor, T. The effect of biosilver nanoparticles on different bacterial strains’ metabolism reflected in their VOCs profiles. J. Breath Res. 2017, 12, 027105. [Google Scholar] [CrossRef] [PubMed]
- Gimel, J.C.; Brown, W. A light scattering investigation of the sodium dodecyl sulfate-lysozyme system. J. Chem. Phys. 1996, 104, 8112–8117. [Google Scholar] [CrossRef]
- Naumann, D.; Keller, S.; Helm, D.; Schultz, C.; Schrader, B. FT-IR spectroscopy and FT-Raman spectroscopy are powerful analytical tools for the non-invasive characterization of intact microbial cells. J. Chem. Phys. 1995, 347, 399–405. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Animal Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Altalhi, A.D. Plasmid-mediated detoxification of mycotoxin zearalenone in Pseudomonas sp. ZEA-1. Am. J. Biochem. Biotechnol. 2007, 3, 150–158. [Google Scholar] [CrossRef]
- El-Nezami, H.S.; Chrevatidis, A.; Auriola, S.; Salminen, S.; Mykkänen, H. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit. Contam. 2002, 19, 680–686. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. In vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef]
- Ezdini, K.; Salah-Abbès, J.B.; Belgacem, H.; Mannai, M.; Abbès, S. Lactobacillus paracasei alleviates genotoxicity, oxidative stress status and histopathological damage induced by Fumonisin B1 in BALB/c mice. Toxicon 2020, 185, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Čvek, D.; Markov, K.; Frece, J.; Friganović, M.; Duraković, L.; Delaš, F. Adhesion of zearalenone to the surface of lactic acid bacteria cells. Croat. J. Food Tech. Biotech. Nutr. 2012, 7, 49–52. [Google Scholar]
- Bejaoui, H.; Mathieu, F.; Taillandier, P.; Lebrihi, A. Ochratoxin A removal in synthetic and natural grape juices by selected oenological Saccharomyces strains. J. Appl. Microbiol. 2004, 97, 1038–1044. [Google Scholar] [CrossRef]
- Wu, K.; Ren, C.; Gong, Y.; Gao, X.; Rajput, S.A.; Qi, D.; Wang, S. The insensitive mechanism of poultry to zearalenone: A review. Anim. Nutr. 2021, 7, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, X.; Xiao, R.; Dudu, O.E.; Yang, L.; Ma, Y. Impact of Lactobacillus paracasei IMC502 in coculture with traditional starters on volatile and non-volatile metabolite profiles in yogurt. Process Biochem. 2020, 99, 61–69. [Google Scholar] [CrossRef]
- Chen, S.W.; Hsu, J.T.; Chou, Y.A.; Wang, H.T. The application of digestive tract lactic acid bacteria with high esterase activity for zearalenone detoxification. J. Sci. Food Agric. 2018, 98, 3870–3879. [Google Scholar] [CrossRef] [PubMed]
- Haskard, C.; Binnion, C.; Ahokas, J. Factors affecting the sequestration of aflatoxin by Lactobacillus rhamnosus strain GG. Chem. Biol. Interact. 2000, 128, 39–49. [Google Scholar] [CrossRef]
- Lim, W.K.; Rösgen, J.; Englander, S.W. Urea, but not guanidinium, destabilizes proteins by forming hydrogen bonds to the peptide group. Proc. Natl. Acad. Sci. USA 2009, 106, 2595–2600. [Google Scholar] [CrossRef]




| 22 | 37 | 47 | 85 | 93 | |
|---|---|---|---|---|---|
| Esculin | + | + | + | + | + |
| Cellobiose | + | + | + | + | + |
| Maltose | + | + | + | + | + |
| Mannitol | + | + | + | + | − |
| Salicin | + | + | + | + | + |
| Sorbierite | + | + | + | − | − |
| Sucrose | + | + | + | + | + |
| Raffinose | + | + | + | − | − |
| pH | 3.30 | 3.41 | 3.34 | 3.45 | 2.82 |
| Logarithmic period | 6–9 h | 9–12 h | 6–8 h | 4–6 h | 8–12 h |
| Isolate | ZEN Removal Rate (%) | |||
|---|---|---|---|---|
| Whole Cell Culture | Cell-Free Supernatant | Cell Pellet | Inactivated Cell Pellet | |
| L. plantarum 22 | 65.7 ± 2.4 Abc | 33.5 ± 0.7 Ccd | 46.7 ± 3.6 Bb | 70.2 ± 3.8 Ac |
| L. plantarum 37 | 67.8 ± 4.4 Ab | 32.7 ± 2.1 Cd | 54.1 ± 2.9 Bb | 68.2 ± 2.0 Ab |
| L. plantarum 47 | 60.6 ± 3.9 Ac | 36.9 ± 1.5 Cc | 50.4 ± 2.0 Bb | 65.6 ± 2.4 Abc |
| L. paracasei 85 | 78.4 ± 2.2 Aa | 49.0 ± 3.2 Ca | 66.1 ± 3.0 Ba | 80.8 ± 4.3 Aa |
| L. buchneri 93 | 73.0 ± 1.6 Aa | 43.1 ± 1.1 Cb | 60.9 ± 2.4 Ba | 76.4 ± 3.5 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, M.; Hu, J.; Wan, K.; Liu, X.; Chen, P.; Zeng, R.; Wang, F.; Zhao, Y. Isolation and Characterization of Lactobacillus paracasei 85 and Lactobacillus buchneri 93 to Absorb and Biotransform Zearalenone. Toxics 2022, 10, 680. https://doi.org/10.3390/toxics10110680
Gan M, Hu J, Wan K, Liu X, Chen P, Zeng R, Wang F, Zhao Y. Isolation and Characterization of Lactobacillus paracasei 85 and Lactobacillus buchneri 93 to Absorb and Biotransform Zearalenone. Toxics. 2022; 10(11):680. https://doi.org/10.3390/toxics10110680
Chicago/Turabian StyleGan, Min, Jian Hu, Kai Wan, Xiangxiang Liu, Peirong Chen, Rui Zeng, Fuhua Wang, and Yarong Zhao. 2022. "Isolation and Characterization of Lactobacillus paracasei 85 and Lactobacillus buchneri 93 to Absorb and Biotransform Zearalenone" Toxics 10, no. 11: 680. https://doi.org/10.3390/toxics10110680
APA StyleGan, M., Hu, J., Wan, K., Liu, X., Chen, P., Zeng, R., Wang, F., & Zhao, Y. (2022). Isolation and Characterization of Lactobacillus paracasei 85 and Lactobacillus buchneri 93 to Absorb and Biotransform Zearalenone. Toxics, 10(11), 680. https://doi.org/10.3390/toxics10110680





