Exposure of Midge Larvae (Chironomus riparius) to Graphene Oxide Leads to Development Alterations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Graphene Oxide (GO)
2.2. GO Physical Dispersion before Contamination
2.3. Chironomus Rearing
2.4. Experimental Design and Exposure Conditions
2.5. Data Acquisition
2.5.1. Survival Rate
2.5.2. Growth Measurement
2.5.3. Determination of Development Delay
2.5.4. Teratogenicity Assessment
2.6. Integrated Biomarker Response (IBR)
2.7. Statistical Analysis
3. Results and Discussion
3.1. GO Exposure and Survival Rate
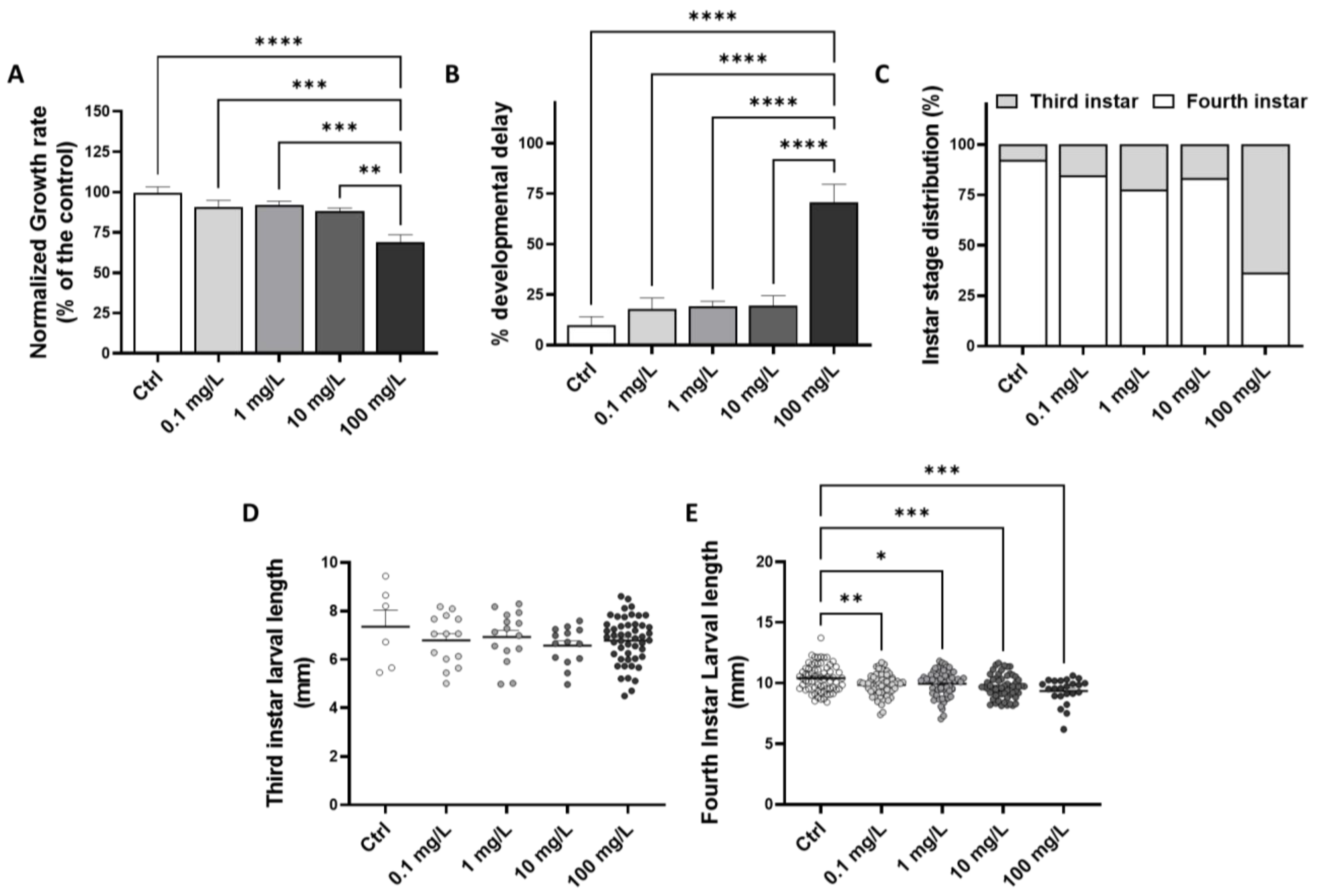
3.2. Effects of GO Exposure on Larval Growth and Development
3.3. Effects of GO Exposure on the Induction of Mentum Deformities
3.4. Integration of Biological Responses into the IBR Index
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dideikin, A.T.; Vul’, A.Y. Graphene Oxide and Derivatives: The Place in Graphene Family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Piner, R.D.; Jung, I.; Yang, D.; Velamakanni, A.; Nguyen, S.T.; Ruoff, R.S. Aqueous Suspension and Characterization of Chemically Modified Graphene Sheets. Chem. Mater. 2008, 20, 6592–6594. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascon, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Fan, M.; Hui, D. Graphene Oxide Incorporated Functional Materials: A Review. Compos. Part B Eng. 2018, 145, 270–280. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.M.; Tadé, M.O. Adsorptive Remediation of Environmental Pollutants Using Novel Graphene-Based Nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A Review on Heavy Metal Ions Adsorption from Water by Graphene Oxide and Its Composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Paramasivan, T.; Sivarajasekar, N.; Muthusaravanan, S.; Subashini, R.; Prakashmaran, J.; Sivamani, S.; Ajmal Koya, P. Graphene Family Materials for the Removal of Pesticides from Water. In A New Generation Material Graphene: Applications in Water Technology; Naushad, M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 309–327. ISBN 978-3-319-75484-0. [Google Scholar]
- Gopinath, K.P.; Vo, D.-V.N.; Gnana Prakash, D.; Adithya Joseph, A.; Viswanathan, S.; Arun, J. Environmental Applications of Carbon-Based Materials: A Review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Liu, Z. Synthesis Challenges for Graphene Industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, H.; Cheng, H.-M.; Ruoff, R.S. Mass Production and Industrial Applications of Graphene Materials. Natl. Sci. Rev. 2018, 5, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Pu, Y.; Tang, M.; Zhang, T. Environmental and Health Effects of Graphene-Family Nanomaterials: Potential Release Pathways, Transformation, Environmental Fate and Health Risks. Nano Today 2022, 42, 101379. [Google Scholar] [CrossRef]
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological Effects of Carbon Based Nanomaterials in Aquatic Organisms. Sci. Total Environ. 2018, 619–620, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Mottier, A.; Mouchet, F.; Pinelli, É.; Gauthier, L.; Flahaut, E. Environmental Impact of Engineered Carbon Nanoparticles: From Releases to Effects on the Aquatic Biota. Curr. Opin. Biotechnol. 2017, 46, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scown, T.M.; van Aerle, R.; Tyler, C.R. Review: Do Engineered Nanoparticles Pose a Significant Threat to the Aquatic Environment? Crit. Rev. Toxicol. 2010, 40, 653–670. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Adeleye, A.S.; Sung, L.; Ho, K.T.; Burgess, R.M.; Petersen, E.J. Detection and Quantification of Graphene-Family Nanomaterials in the Environment. Environ. Sci. Technol. 2018, 52, 4491–4513. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, L.; Pretti, C.; Gabriel, B.; Marques, P.A.A.P.; Freitas, R.; Neto, V. An Overview of Graphene Materials: Properties, Applications and Toxicity on Aquatic Environments. Sci. Total Environ. 2018, 631–632, 1440–1456. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastrzębska, A.M.; Olszyna, A.R. The Ecotoxicity of Graphene Family Materials: Current Status, Knowledge Gaps and Future Needs. J. Nanoparticle Res. 2015, 17, 40. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal Effect of Graphene Oxide and Reduced Graphene Oxide: Influence of Shape of Bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Saxena, P.; Sangela, V.; Ranjan, S.; Dutta, V.; Dasgupta, N.; Phulwaria, M.; Rathore, D.S. Harish Aquatic Nanotoxicology: Impact of Carbon Nanomaterials on Algal Flora. Energ. Ecol. Environ. 2020, 5, 240–252. [Google Scholar] [CrossRef]
- Yin, J.; Fan, W.; Du, J.; Feng, W.; Dong, Z.; Liu, Y.; Zhou, T. The Toxicity of Graphene Oxide Affected by Algal Physiological Characteristics: A Comparative Study in Cyanobacterial, Green Algae, Diatom. Environ. Pollut. 2020, 260, 113847. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Cai, Z.; Li, B. A Mechanism Study on Toxicity of Graphene Oxide to Daphnia Magna: Direct Link between Bioaccumulation and Oxidative Stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Fekete-Kertész, I.; László, K.; Terebesi, C.; Gyarmati, B.S.; Farah, S.; Márton, R.; Molnár, M. Ecotoxicity Assessment of Graphene Oxide by Daphnia Magna through a Multimarker Approach from the Molecular to the Physiological Level Including Behavioral Changes. Nanomaterials 2020, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.L.; Clemente, Z.; Jonsson, C.; Silva, M.; Vallim, J.H.; de Medeiros, A.M.Z.; Martinez, D.S.T. Nanoecotoxicity Assessment of Graphene Oxide and Its Relationship with Humic Acid: Nanoecotoxicity of Graphene Oxide and Humic Acid. Environ. Toxicol. Chem. 2018, 37, 1998–2012. [Google Scholar] [CrossRef]
- Martin-Folgar, R.; Esteban-Arranz, A.; Negri, V.; Morales, M. Toxicological Effects of Three Different Types of Highly Pure Graphene Oxide in the Midge Chironomus Riparius. Sci. Total Environ. 2022, 815, 152465. [Google Scholar] [CrossRef]
- Nicacio, G.; Juen, L. Chironomids as Indicators in Freshwater Ecosystems: An Assessment of the Literature. Insect Conserv. Divers. 2015, 8, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Molineri, C.; Tejerina, E.G.; Torrejón, S.E.; Pero, E.J.I.; Hankel, G.E. Indicative Value of Different Taxonomic Levels of Chironomidae for Assessing the Water Quality. Ecol. Indic. 2020, 108, 105703. [Google Scholar] [CrossRef]
- Watts, M.M.; Pascoe, D. A Comparative Study of Chironomus Riparius Meigen and Chironomus Tentans Fabricius (Diptera:Chironomidae) in Aquatic Toxicity Tests. Arch. Environ. Contam. Toxicol. 2000, 39, 299–306. [Google Scholar] [CrossRef]
- de Souza Beghelli, F.G.; Lopez-Dovál, J.C.; Rosa, A.H.; Pompêo, M.; Carlos, V.M. Lethal and Sublethal Effects of Metal-Polluted Sediments on Chironomus Sancticaroli Strixino and Strixino, 1981. Ecotoxicology 2018, 27, 286–299. [Google Scholar] [CrossRef]
- Arambourou, H.; Planelló, R.; Llorente, L.; Fuertes, I.; Barata, C.; Delorme, N.; Noury, P.; Herrero, Ó.; Villeneuve, A.; Bonnineau, C. Chironomus Riparius Exposure to Field-Collected Contaminated Sediments: From Subcellular Effect to Whole-Organism Response. Sci. Total Environ. 2019, 671, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Park, S.Y.; Choi, J. Evaluation of the Effect of Silver Nanoparticles and Silver Ions Using Stress Responsive Gene Expression in Chironomus Riparius. Chemosphere 2013, 92, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Bour, A.; Mouchet, F.; Cadarsi, S.; Silvestre, J.; Chauvet, E.; Bonzom, J.-M.; Pagnout, C.; Clivot, H.; Gauthier, L.; Pinelli, E. Impact of CeO 2 Nanoparticles on the Functions of Freshwater Ecosystems: A Microcosm Study. Environ. Sci. Nano 2016, 3, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Bour, A.; Mouchet, F.; Verneuil, L.; Evariste, L.; Silvestre, J.; Pinelli, E.; Gauthier, L. Toxicity of CeO2 Nanoparticles at Different Trophic Levels—Effects on Diatoms, Chironomids and Amphibians. Chemosphere 2015, 120, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Evariste, L.; Mottier, A.; Lagier, L.; Cadarsi, S.; Barret, M.; Sarrieu, C.; Soula, B.; Mouchet, F.; Flahaut, E.; Pinelli, E.; et al. Assessment of Graphene Oxide Ecotoxicity at Several Trophic Levels Using Aquatic Microcosms. Carbon 2020, 156, 261–271. [Google Scholar] [CrossRef]
- Mouchet, F.; Rowenczyk, L.; Minet, A.; Clergeaud, F.; Silvestre, J.; Pinelli, E.; Ferriol, J.; Leflaive, J.; Ten-Hage, L.; Gigault, J.; et al. Ecotoxicity of Heteroaggregates of Polystyrene Nanospheres in Chironomidae and Amphibian. Nanomaterials 2022, 12, 2730. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Wotton, R.S. Organic Matter Processing by Chironomid Larvae (Diptera: Chironomidae). Hydrobiologia 1998, 382, 151–159. [Google Scholar] [CrossRef]
- Stief, P.; Beer, D. de Bioturbation Effects of Chironomus Riparius on the Benthic N-Cycle as Measured Using Microsensors and Microbiological Assays. Aquat. Microb. Ecol. 2002, 27, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Samuiloviene, A.; Bartoli, M.; Bonaglia, S.; Cardini, U.; Vybernaite-Lubiene, I.; Marzocchi, U.; Petkuviene, J.; Politi, T.; Zaiko, A.; Zilius, M. The Effect of Chironomid Larvae on Nitrogen Cycling and Microbial Communities in Soft Sediments. Water 2019, 11, 1931. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.; Volkmann, S.; Dettinger-Klemm, P.M.A. Benthic–Pelagic Coupling in Lake Ecosystems: The Key Role of Chironomid Pupae as Prey of Pelagic Fish. Ecosphere 2012, 3, art14. [Google Scholar] [CrossRef]
- Sánchez, M.I.; Green, A.J.; Alejandre, R. Shorebird Predation Affects Density, Biomass, and Size Distribution of Benthic Chironomids in Salt Pans: An Exclosure Experiment. J. N. Am. Benthol. Soc. 2006, 25, 9–18. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lobato, B.; Merino, C.; Barranco, V.; Centeno, T.A. Large-Scale Conversion of Helical-Ribbon Carbon Nanofibers to a Variety of Graphene-Related Materials. RSC Adv. 2016, 6, 57514–57520. [Google Scholar] [CrossRef] [Green Version]
- Lagier, L.; Mouchet, F.; Laplanche, C.; Mottier, A.; Cadarsi, S.; Evariste, L.; Sarrieu, C.; Lonchambon, P.; Pinelli, E.; Flahaut, E.; et al. Surface Area of Carbon-Based Nanoparticles Prevails on Dispersion for Growth Inhibition in Amphibians. Carbon 2017, 119, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Government of Canada, P.S. and P.C. Biological Test Method. Test for Survival and Growth in Sediment Using Larvae of Freshwater Midges (Chironomus Tentans or Chironomus Riparius)/[Issued by] Method Development and Application Section, Environmental Technology Centre: En49-24/1-32E-PDF—Government of Canada Publications—Canada. Available online: https://publications.gc.ca/site/eng/460451/publication.html (accessed on 16 August 2022).
- AFNOR NF T90-339-1 Qualité de l’eau—Détermination de la Toxicité des Sédiments d’eau Douce vis-à-vis de Chironomus riparius. Available online: https://m.boutique.afnor.org/fr-fr/norme/nf-t903391/qualite-de-leau-determination-de-la-toxicite-des-sediments-deau-douce-visav/fa165302/34939 (accessed on 16 August 2022).
- Egeler, P.; Henry, K.S.; Riedhammer, C. Potential Effects of Food Addition to Sediment on Test Conditions in Sediment Toxicity Tests. J. Soils Sediments 2010, 10, 377–388. [Google Scholar] [CrossRef]
- Mottier, A.; Mouchet, F.; Laplanche, C.; Cadarsi, S.; Lagier, L.; Arnault, J.-C.; Girard, H.A.; León, V.; Vázquez, E.; Sarrieu, C.; et al. Surface Area of Carbon Nanoparticles: A Dose Metric for a More Realistic Ecotoxicological Assessment. Nano Lett. 2016, 16, 3514–3518. [Google Scholar] [CrossRef] [Green Version]
- Warwick, W.F.; Tisdale, N.A. Morphological Deformities in Chironomus, Cryptochironomus, and Procladius Larvae (Diptera: Chironomidae) from Two Differentially Stressed Sites in Tobin Lake, Saskatchewan. Can. J. Fish. Aquat. Sci. 1988, 45, 1123–1144. [Google Scholar] [CrossRef]
- Salmelin, J.; Vuori, K.-M.; Hämäläinen, H. Inconsistency in the Analysis of Morphological Deformities in Chironomidae (Insecta: Diptera) Larvae. Environ. Toxicol. Chem. 2015, 34, 1891–1898. [Google Scholar] [CrossRef]
- Vermeulen, A.C.; Dall, P.C.; Lindegaard, C.; Ollevier, F.; Goddeeris, B. Improving the Methodology of Chironomid Deformation Analysis for Sediment Toxicity Assessment: A Case Study in Three Danish Lowland Streams. Arch. Für Hydrobiol. 1998, 144, 103–125. [Google Scholar] [CrossRef]
- Beliaeff, B.; Burgeot, T. Integrated Biomarker Response: A Useful Tool for Ecological Risk Assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar] [CrossRef]
- Devin, S.; Burgeot, T.; Giambérini, L.; Minguez, L.; Pain-Devin, S. The Integrated Biomarker Response Revisited: Optimization to Avoid Misuse. Environ. Sci. Pollut. Res. 2014, 21, 2448–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion Behaviour of Graphene Oxide and Reduced Graphene Oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Mansukhani, N.D.; Guiney, L.M.; Hersam, M.C.; Bouchard, D. Aggregation and Stability of Reduced Graphene Oxide: Complex Roles of Divalent Cations, PH, and Natural Organic Matter. Environ. Sci. Technol. 2015, 49, 10886–10893. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aqueous Aggregation and Stability of Graphene Nanoplatelets, Graphene Oxide, and Reduced Graphene Oxide in Simulated Natural Environmental Conditions: Complex Roles of Surface and Solution Chemistry. Environ. Sci. Pollut. Res. 2018, 25, 10956–10965. [Google Scholar] [CrossRef]
- Avant, B.; Bouchard, D.; Chang, X.; Hsieh, H.-S.; Acrey, B.; Han, Y.; Spear, J.; Zepp, R.; Knightes, C.D. Environmental Fate of Multiwalled Carbon Nanotubes and Graphene Oxide across Different Aquatic Ecosystems. NanoImpact 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Petersen, E.J.; Niu, J.; Chang, X.; Wang, P.; Lin, S.; Gao, S.; Mao, L. Biological Uptake, Distribution, and Depuration of Radio-Labeled Graphene in Adult Zebrafish: Effects of Graphene Size and Natural Organic Matter. ACS Nano 2017, 11, 2872–2885. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Zha, Y.; Dong, S.; Zhu, Z.; Lv, Z.; Gu, Y.; Deng, R.; Wang, M.; Gao, S.; Mao, L. Uptake Route Altered the Bioavailability of Graphene in Misgurnus Anguillicaudatus: Comparing Waterborne and Sediment Exposures. Environ. Sci. Technol. 2022, 56, 9435–9445. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, X.; Tian, S.; Lv, X.; Chen, Z.; Jiang, Y.; Liao, X.; Cai, Z.; Chen, B. Graphene Oxide in the Marine Environment: Toxicity to Artemia Salina with and without the Presence of Phe and Cd2+. Chemosphere 2018, 211, 390–396. [Google Scholar] [CrossRef]
- Cavion, F.; Fusco, L.; Sosa, S.; Manfrin, C.; Alonso, B.; Zurutuza, A.; Loggia, R.D.; Tubaro, A.; Prato, M.; Pelin, M. Ecotoxicological Impact of Graphene Oxide: Toxic Effects on the Model Organism Artemia Franciscana. Environ. Sci. Nano 2020, 7, 3605–3615. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, F.; Chen, W.; Zhu, B.; Wang, G. Toxicity Evaluation of Graphene Oxide on Cysts and Three Larval Stages of Artemia Salina. Sci. Total Environ. 2017, 595, 101–109. [Google Scholar] [CrossRef]
- Méndez-Fernández, L.; Martínez-Madrid, M.; Rodriguez, P. Toxicity and Critical Body Residues of Cd, Cu and Cr in the Aquatic Oligochaete Tubifextubifex (Müller) Based on Lethal and Sublethal Effects. Ecotoxicology 2013, 22, 1445–1460. [Google Scholar] [CrossRef] [PubMed]
- Pirow, R.; Wollinger, F.; Paul, R.J. The Importance of the Feeding Current for Oxygen Uptake in the Water Flea Daphnia Magna. J. Exp. Biol. 1999, 202, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.N.; Wang, N.; Ingersoll, C.G.; Hardesty, D.K.; Brunson, E.L.; Li, H.; Deng, B. Toxicity of Carbon Nanotubes to Freshwater Aquatic Invertebrates. Environ. Toxicol. Chem. 2012, 31, 1823–1830. [Google Scholar] [CrossRef]
- Waissi-Leinonen, G.C.; Nybom, I.; Pakarinen, K.; Akkanen, J.; Leppänen, M.T.; Kukkonen, J.V.K. Fullerenes(NC60) Affect the Growth and Development of the Sediment-Dwelling Invertebrate Chironomus Riparius Larvae. Environ. Pollut. 2015, 206, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, Q.; Zhao, H.; Wang, L.; Guo, S.; Li, X. Ecotoxicological Effects of Graphene Oxide on the Protozoan Euglena Gracilis. Chemosphere 2015, 128, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Walshe, B.M. Feeding Mechanisms of Chironomus Larvæ. Nature 1947, 160, 474. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [Green Version]
- Bantun, F.; Singh, R.; Alkhanani, M.F.; Almalki, A.H.; Alshammary, F.; Khan, S.; Haque, S.; Srivastava, M. Gut Microbiome Interactions with Graphene Based Nanomaterials: Challenges and Opportunities. Sci. Total Environ. 2022, 830, 154789. [Google Scholar] [CrossRef]
- Senderovich, Y.; Halpern, M. The Protective Role of Endogenous Bacterial Communities in Chironomid Egg Masses and Larvae. ISME J. 2013, 7, 2147–2158. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Shu, Y.; Tian, C.; Wang, C.; Fang, T.; Xiao, B.; Wu, X. Effects of Chronic Exposure to Microcystin-LR on Life-History Traits, Intestinal Microbiota and Transcriptomic Responses in Chironomus Pallidivittatus. Sci. Total Environ. 2022, 823, 153624. [Google Scholar] [CrossRef]
- Sela, R.; Halpern, M. The Chironomid Microbiome Plays a Role in Protecting Its Host From Toxicants. Front. Ecol. Evol. 2022, 10, 42. [Google Scholar] [CrossRef]
- Guo, L.; Von Dem Bussche, A.; Buechner, M.; Yan, A.; Kane, A.B.; Hurt, R.H. Adsorption of Essential Micronutrients by Carbon Nanotubes and the Implications for Nanotoxicity Testing. Small 2008, 4, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, H.L.; Sibly, R.M.; Hutchinson, T.H.; Maund, S.J. The Influence of Larval Density, Food Availability and Habitat Longevity on the Life History and Population Growth Rate of the Midge Chironomus Riparius. Oikos 2003, 102, 515–524. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy Homeostasis as an Integrative Tool for Assessing Limits of Environmental Stress Tolerance in Aquatic Invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Saraiva, A.S.; Sarmento, R.A.; Gravato, C.; Rodrigues, A.C.M.; Campos, D.; Simão, F.C.P.; Soares, A.M.V.M. Strategies of Cellular Energy Allocation to Cope with Paraquat-Induced Oxidative Stress: Chironomids vs Planarians and the Importance of Using Different Species. Sci. Total Environ. 2020, 741, 140443. [Google Scholar] [CrossRef] [PubMed]
- Sibley, P.K.; Benoit, D.A.; Ankley, G.T. The Significance of Growth in Chironomus Tentans Sediment Toxicity Tests: Relationship to Reproduction and Demographic Endpoints. Environ. Toxicol. Chem. 1997, 16, 336–345. [Google Scholar] [CrossRef]
- Ball, S.L.; Baker, R.L. The Non-Lethal Effects of Predators and the Influence of Food Availability on Life History of Adult Chironomus Tentans (Diptera: Chironomidae). Freshw. Biol. 1995, 34, 1–12. [Google Scholar] [CrossRef]
- Stanko-Mishic, S.; And, J.K.C.; Silver, P. Manipulation of Habitat Quality: Effects on Chironomid Life History Traits. Freshw. Biol. 1999, 41, 719–727. [Google Scholar] [CrossRef]
- Frelik, A.; Koszałka, J.; Pakulnicka, J. Trophic Relations between Adult Water Beetles from the Dytiscidae Family and Non-Biting Midges (Diptera: Chironomidae). Biologia 2016, 71, 931–940. [Google Scholar] [CrossRef]
- van Geert, U.; Kerkurm, F.C.M.; Smit, H. Life Cycle Patterns, Density, and Frequency of Deformities in Chironomus Larvae (Diptera: Chironomidae) over a Contaminated Sediment Gradient. Can. J. Fish. Aquat. Sci. 1992, 49, 2291–2299. [Google Scholar] [CrossRef]
- Warwick, W.F. Morphological Deformities in Chironomidae (Diptera) Larvae from the Lac St. Louis and Laprairie Basins of the St. Lawrence River. J. Great Lakes Res. 1990, 16, 185–208. [Google Scholar] [CrossRef]
- Vedamanikam, V.J.; Shazili, N.A.M. Observations of Mouthpart Deformities in the Chironomus Larvae Exposed to Different Concentrations of Nine Heavy Metals. Toxicol. Environ. Chem. 2009, 91, 57–63. [Google Scholar] [CrossRef]
- Warwick, W.F. Chironomidae (Diptera) Responses to 2800 Years of Cultura Influence; A Palaeolimnological Study with Special Reference to Sedimentation, Eutrophication and Contamination Processes. Can. Entomol. 1980, 112, 1193–1238. [Google Scholar] [CrossRef]
- Vermeulen, A.C. Elaborating Chironomid Deformities as Bioindicators of Toxic Sediment Stress: The Potential Application of Mixture Toxicity Concepts. Ann. Zool. Fenn. 1995, 32, 265–285. [Google Scholar]
- Meregalli, G.; Pluymers, L.; Ollevier, F. Induction of Mouthpart Deformities in Chironomus Riparius Larvae Exposed to 4-n-Nonylphenol. Environ. Pollut. 2001, 111, 241–246. [Google Scholar] [CrossRef]
- Dias, V.; Vasseur, C.; Bonzom, J.-M. Exposure of Chironomus Riparius Larvae to Uranium: Effects on Survival, Development Time, Growth, and Mouthpart Deformities. Chemosphere 2008, 71, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Di Veroli, A.; Santoro, F.; Pallottini, M.; Selvaggi, R.; Scardazza, F.; Cappelletti, D.; Goretti, E. Deformities of Chironomid Larvae and Heavy Metal Pollution: From Laboratory to Field Studies. Chemosphere 2014, 112, 9–17. [Google Scholar] [CrossRef]
- Devin, S.; Buffet, P.E.; Châtel, A.; Perrein-Ettajani, H.; Valsami-Jones, E.; Mouneyrac, C. The Integrated Biomarker Response: A Suitable Tool to Evaluate Toxicity of Metal-Based Nanoparticles. Nanotoxicology 2017, 11, 1–6. [Google Scholar] [CrossRef]
- Aouini, F.; Trombini, C.; Sendra, M.; Blasco, J. Biochemical Response of the Clam Ruditapes Philippinarum to Silver (AgD and AgNPs) Exposure and Application of an Integrated Biomarker Response Approach. Mar. Environ. Res. 2019, 152, 104783. [Google Scholar] [CrossRef] [PubMed]
- García-Medina, S.; Galar-Martínez, M.; Cano-Viveros, S.; Ruiz-Lara, K.; Gómez-Oliván, L.M.; Islas-Flores, H.; Gasca-Pérez, E.; Pérez-Pastén-Borja, R.; Arredondo-Tamayo, B.; Hernández-Varela, J.; et al. Bioaccumulation and Oxidative Stress Caused by Aluminium Nanoparticles and the Integrated Biomarker Responses in the Common Carp (Cyprinus Carpio). Chemosphere 2022, 288, 132462. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evariste, L.; Lagier, L.; Chary, C.; Mottier, A.; Cadarsi, S.; Pinelli, E.; Flahaut, E.; Gauthier, L.; Mouchet, F. Exposure of Midge Larvae (Chironomus riparius) to Graphene Oxide Leads to Development Alterations. Toxics 2022, 10, 588. https://doi.org/10.3390/toxics10100588
Evariste L, Lagier L, Chary C, Mottier A, Cadarsi S, Pinelli E, Flahaut E, Gauthier L, Mouchet F. Exposure of Midge Larvae (Chironomus riparius) to Graphene Oxide Leads to Development Alterations. Toxics. 2022; 10(10):588. https://doi.org/10.3390/toxics10100588
Chicago/Turabian StyleEvariste, Lauris, Laura Lagier, Chloé Chary, Antoine Mottier, Stéphanie Cadarsi, Eric Pinelli, Emmanuel Flahaut, Laury Gauthier, and Florence Mouchet. 2022. "Exposure of Midge Larvae (Chironomus riparius) to Graphene Oxide Leads to Development Alterations" Toxics 10, no. 10: 588. https://doi.org/10.3390/toxics10100588
APA StyleEvariste, L., Lagier, L., Chary, C., Mottier, A., Cadarsi, S., Pinelli, E., Flahaut, E., Gauthier, L., & Mouchet, F. (2022). Exposure of Midge Larvae (Chironomus riparius) to Graphene Oxide Leads to Development Alterations. Toxics, 10(10), 588. https://doi.org/10.3390/toxics10100588







