Leaching and Geochemical Modelling of an Electric Arc Furnace (EAF) and Ladle Slag Heap
Abstract
:1. Introduction
2. Materials and Methods
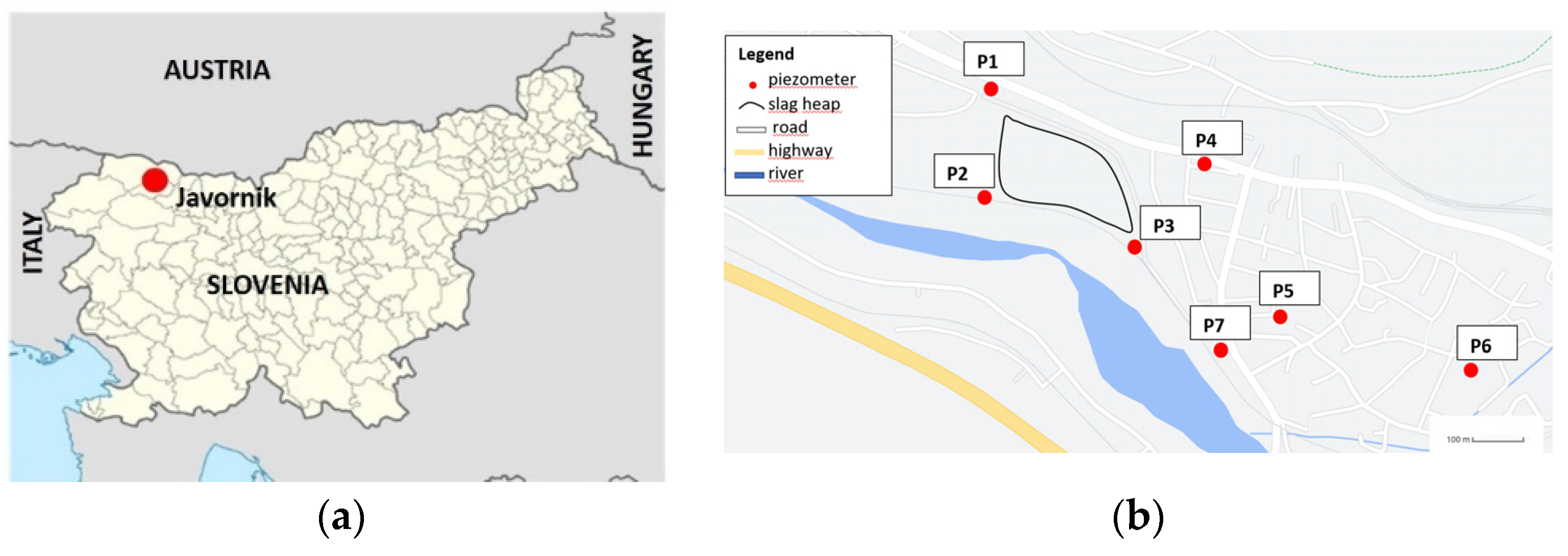
2.1. Sampling Slag from the Metallurgical Slag Heap
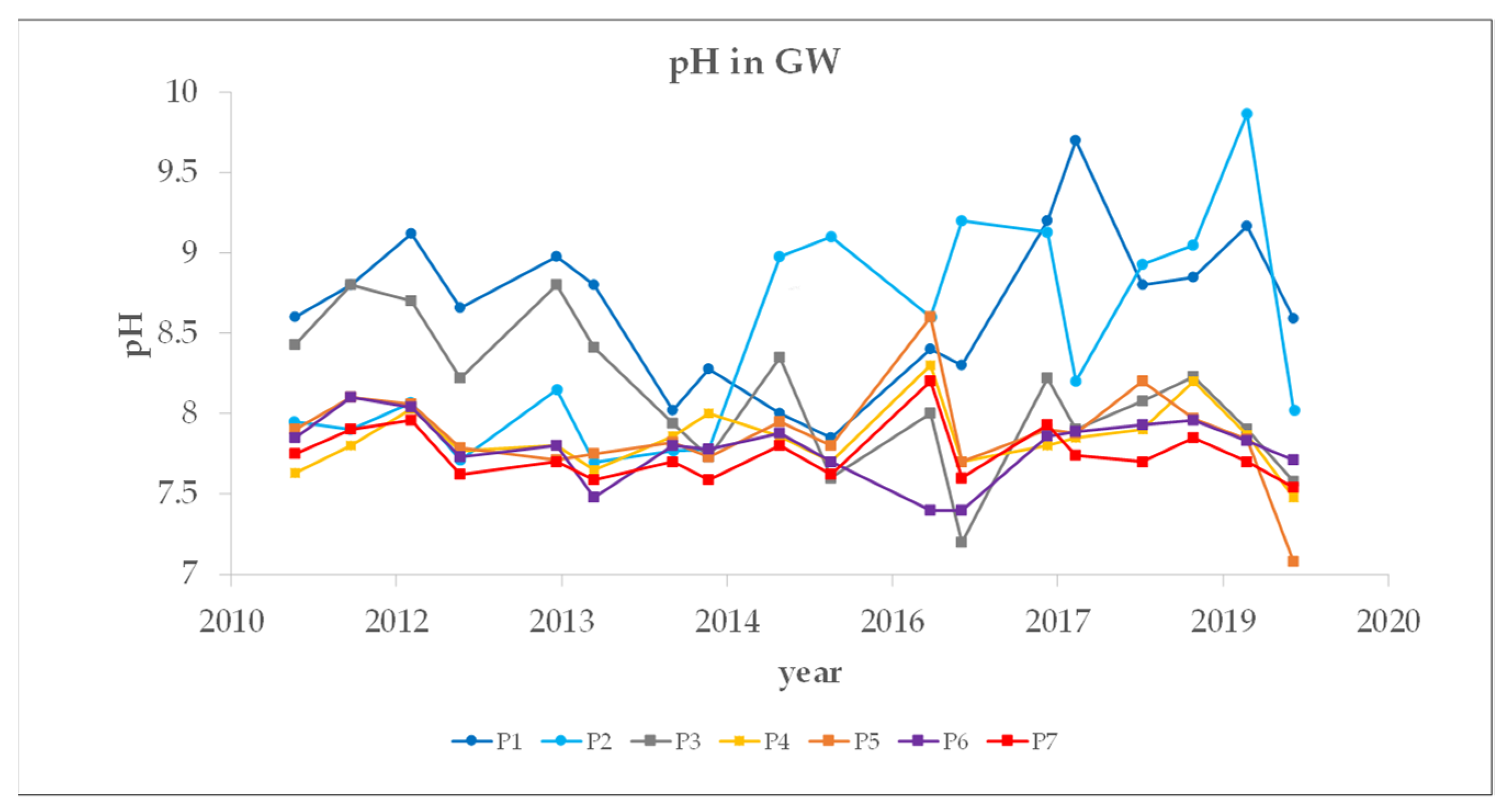
2.2. Groundwater (GW) Monitoring (2011–2019)
2.3. Chemical Analysis
2.3.1. Bulk Chemical Composition
2.3.2. Characterization Leaching Tests
2.3.3. Leachates Analysis
2.3.4. GW Analysis
2.4. Mineralogical Characterization
2.4.1. X-ray Diffraction (XRD) Analysis
2.4.2. Scanning Electron Microscopy (SEM) Analysis
2.5. Data Comparison and Geochemical Modelling
3. Results
3.1. Chemical Characterization of the Landfill Samples
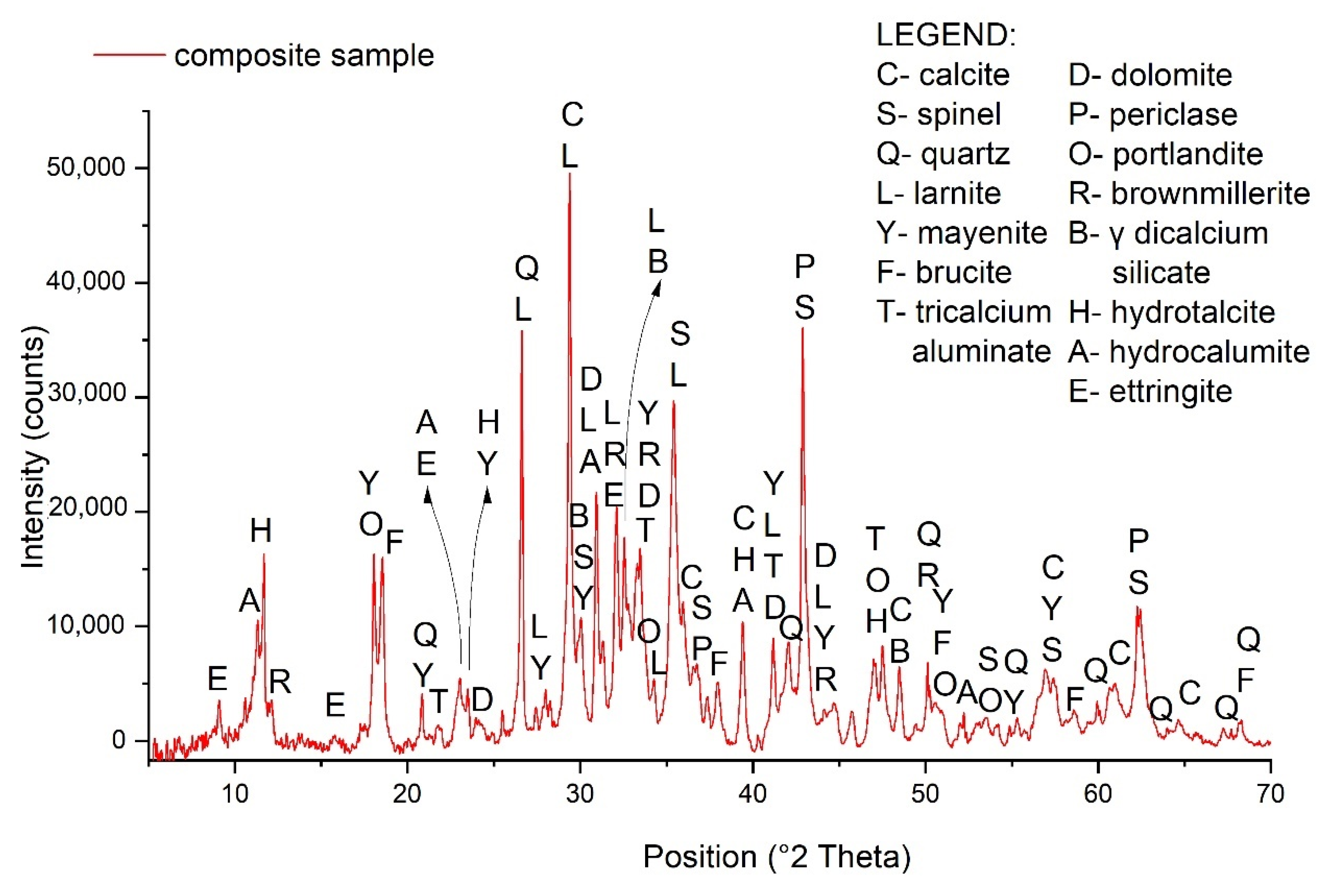
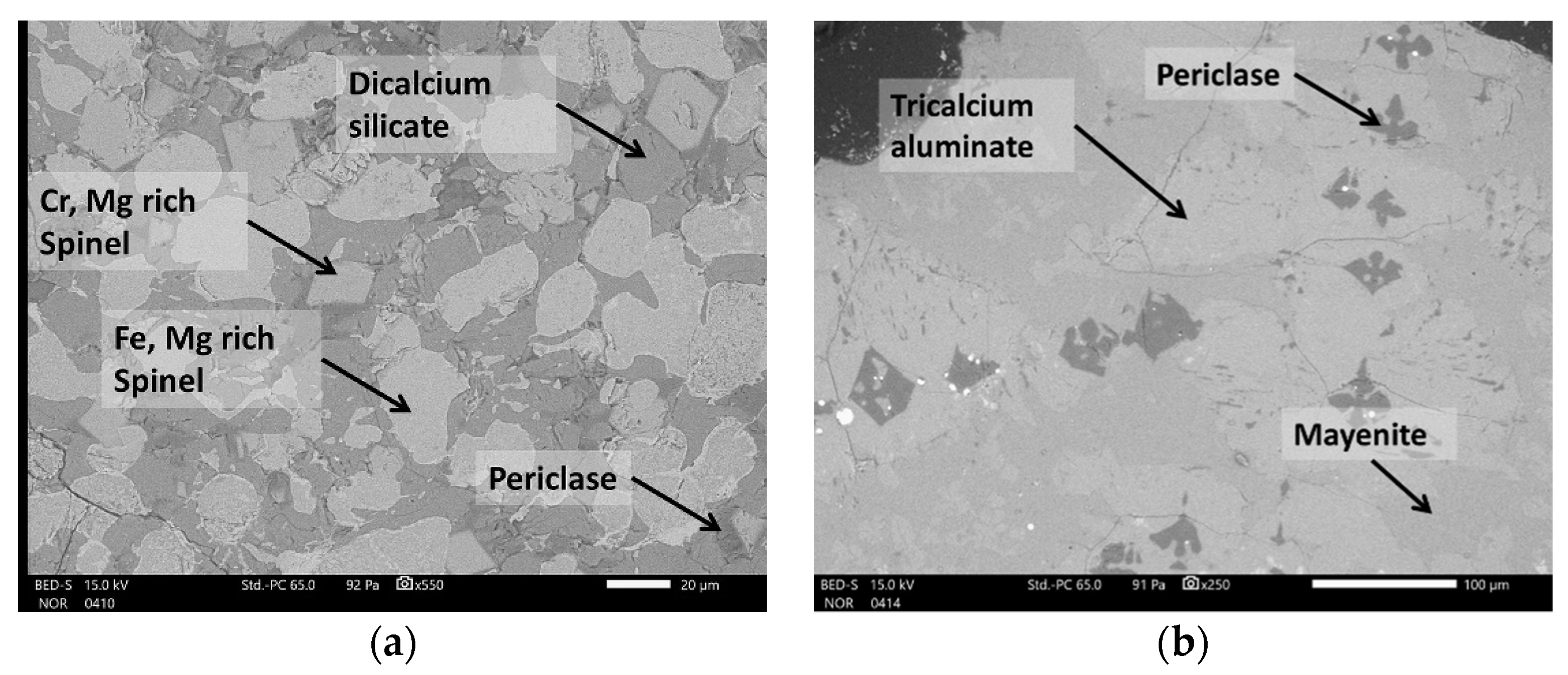
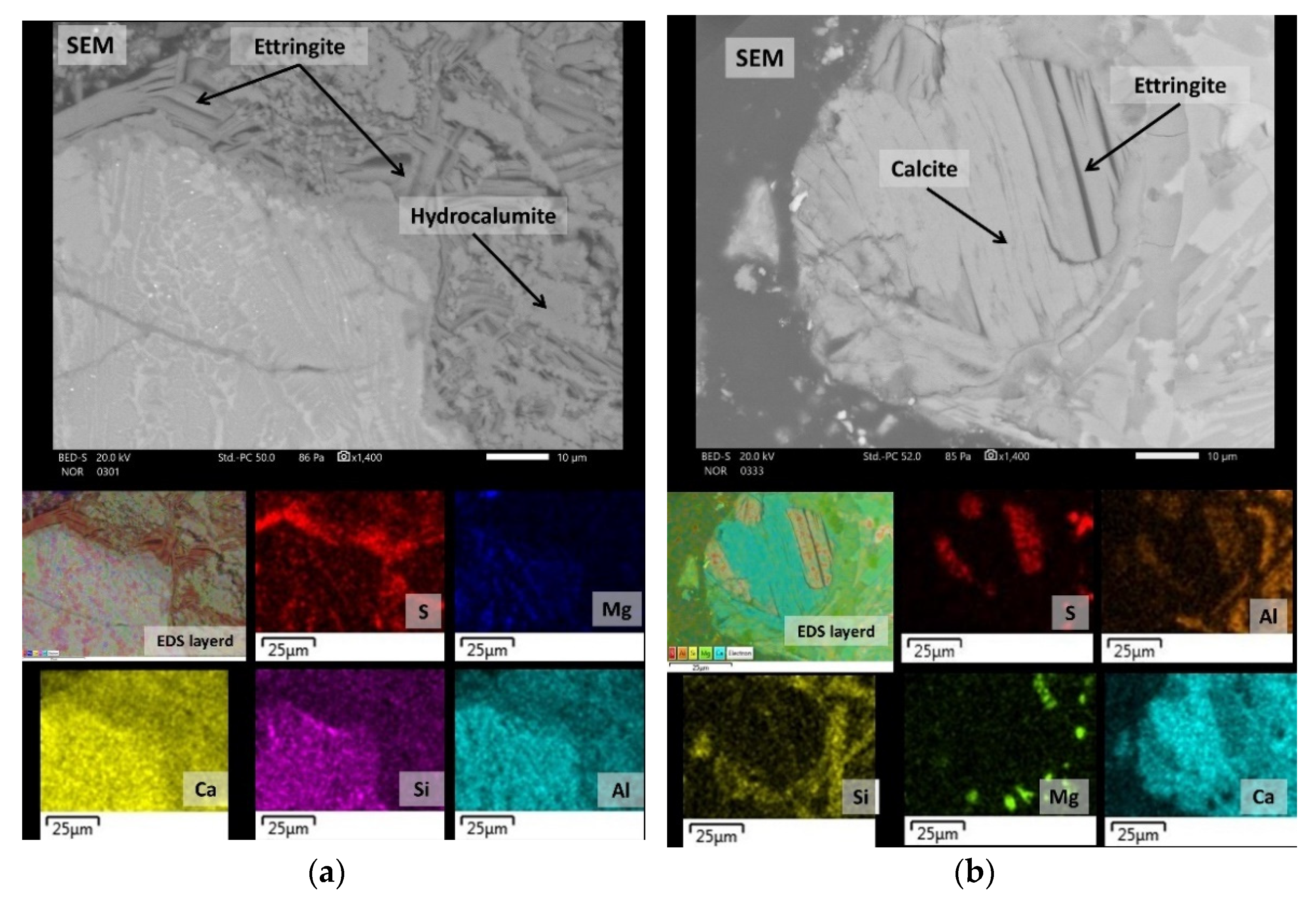
3.2. Mineralogical Characterization
3.3. Groundwater (GW) Monitoring
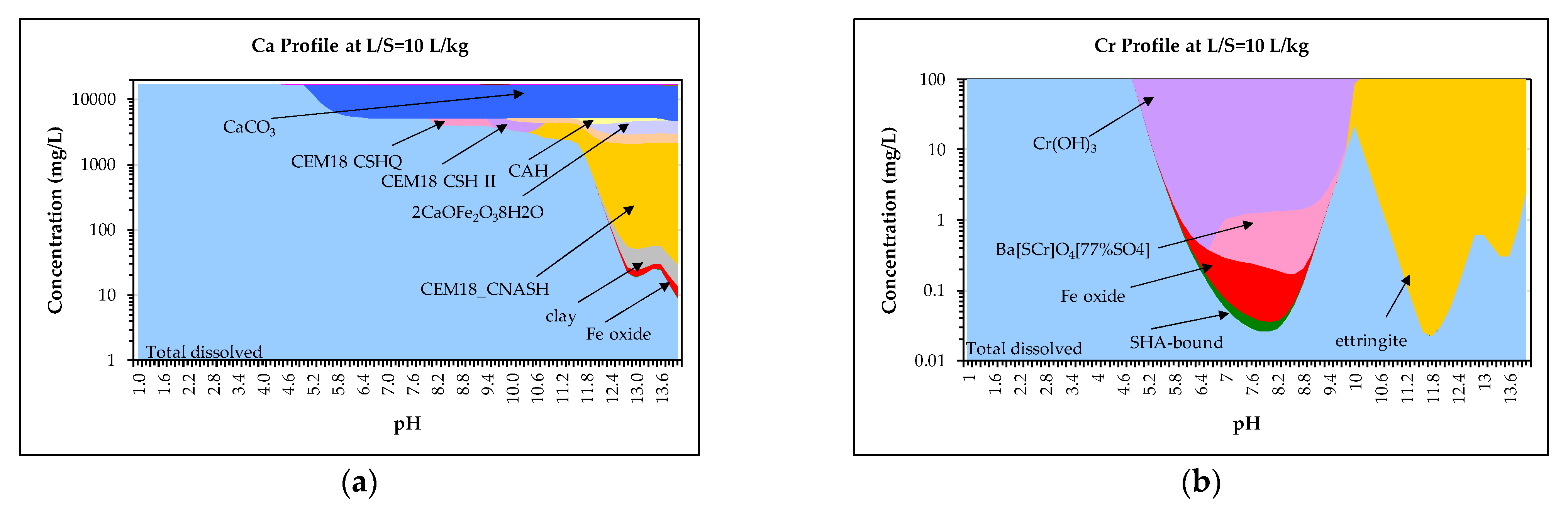
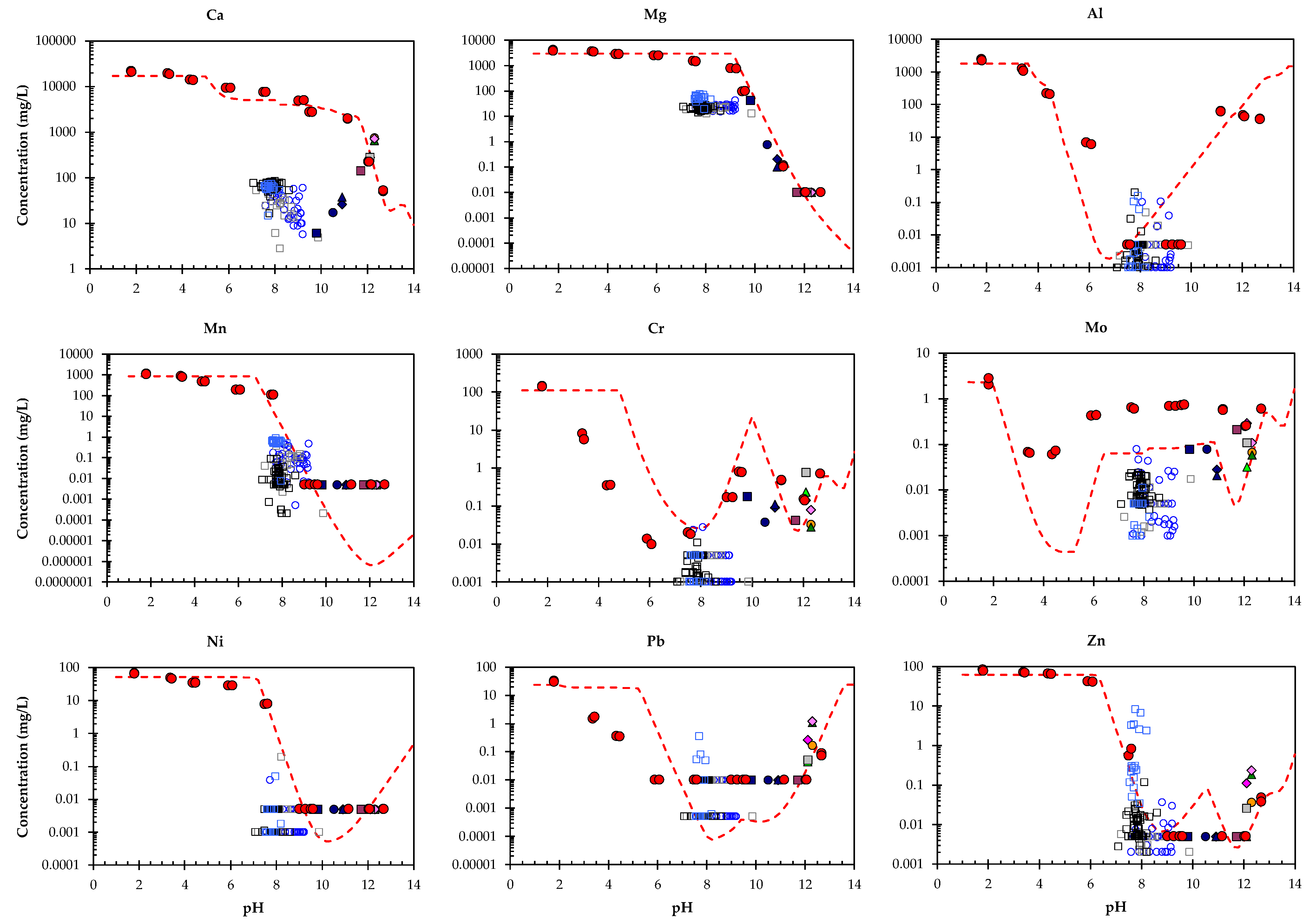
3.4. Geochemical Modelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaverková, M.D. Landfill Impacts on the Environment—Review. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- Burlakovs, J.; Kriipsalu, M.; Klavins, M.; Bhatnagar, A.; Vincevica-Gaile, Z.; Stenis, J.; Jani, Y.; Mykhaylenko, V.; Denafas, G.; Turkadze, T.; et al. Paradigms on landfill mining: From dump site scavenging to ecosystem services revitalization. Resour. Conserv. Recycl. 2017, 123, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.T.; Geysen, D.; Tielemans, Y.; Van Passel, S.; Pontikes, Y.; Blanpain, B.; Quaghebeur, M.; Hoekstra, N. Enhanced Landfill Mining in view of multiple resource recovery: A critical review. J. Clean. Prod. 2013, 55, 45–55. [Google Scholar] [CrossRef]
- Žibret, G.; Lemiere, B.; Mendez, A.-M.; Cormio, C.; Sinnett, D.; Cleall, P.; Szabo, K.; Carvalho, M.T. National Mineral Waste Databases as an Information Source for Assessing Material Recovery Potential from Mine Waste, Tailings and Metallurgical Waste. Minerals 2020, 10, 446. [Google Scholar] [CrossRef]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Gomes, H.I.; Mayes, W.A.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef] [Green Version]
- Mayes, W.M.; Younger, P.L.; Aumônier, J. Hydrogeochemistry of alkaline steel slag leachates in the UK. Water Air Soil Pollut. 2008, 195, 35–50. [Google Scholar] [CrossRef]
- Mayes, W.M.; Younfer, P.L.; Aumôier, J. Buffering of Alkaline Steel Slag Leachate across a Natural Wetland. Environ. Sci. Technol. 2006, 40, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- The European Green Deal, EU. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1576150542719&uri=COM%3A2019%3A640%3AFIN (accessed on 10 December 2021).
- Circular Economy Action Plan, for a Cleaner and more Competitive Europe. European Union. 2020. Available online: https://ec.europa.eu/environment/pdf/circular-economy/new_circular_economy_action_plan.pdf (accessed on 10 December 2021).
- Hogland, M.; Hogland, W.; Marques, M. Enhanced Landfill Mining: Material recovery, energy utilisation and economics in the EU (Directive) perspective. In Proceedings of the International Academic Symposium on Enhanced Landfill Mining, Houthalen-Helchteren, Belgium, 4–6 January 2010; pp. 209–222. [Google Scholar]
- Kosson, D.A.; Garrabrants, A.C.; DeLapp, R.; van der Sloot, H.A. pH-dependent leaching of constituents of potential concern from concrete materials containing coal combustion fly ash. Chemosphere 2014, 103, 140–147. [Google Scholar] [CrossRef]
- Arnold, J.; Kosson, D.S.; Brown, K.G.; Garrabrants, A.C.; Meeussen, J.C.L.; van der Sloot, H.A. Characterization and modeling of major constituent equilibrium chemistry of a blended cement mortar. In Proceedings of the International Workshop NUCPERF 2012: Long-Term Performance of Cementitious Barriers and Reinforced Concrete in Nuclear Power Plant and Radioactive Waste Storage and Disposal (RILEM Event TC 226-CNM and EFC Event 351), Cadarache, France, 12–15 November 2012; L’Hostis, V., Gens, R., Eds.; EDP Sciences: Les Ulis, France, 2013. [Google Scholar]
- Flyhammar, P.; Bendz, D. Leaching of different elements from subbase layers of alternative aggregates in pavement constructions. J. Hazard. Mater. 2006, 137, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Kosson, D.; van der Sloot, H.A.; Garrabrants, A.; Seignette, P. Leaching Test Relationships, Laboratory-to-Field Comparisons and Recommendations for Leaching Evaluation using the Leaching Environmental Assessment Framework (LEAF); US Environmental Protection Agency: Cincinnati, OH, USA, 2014. [Google Scholar]
- Bayless, E.R.; Greeman, T.K.; Harvey, C.C. Hydrology and geochemistry of a slag-affected aquifer and chemical characteristics of slag-affected ground water, northwestern Indiana and northeastern Illinois. Water-Resour. Investig. Rep. 1998, 97, 4198. [Google Scholar]
- Bayless, E.R.; Schulz, M.S. Mineral precipitation and dissolution at two slag-disposal sites in northwestern Indiana, USA. Environ. Earth Sci. 2003, 45, 252–261. [Google Scholar] [CrossRef]
- Roadcap, G.S.; Kelly, W.R.; Bethke, C.M. Geochemistry of extremely alkaline (pH > 12) ground water in slag-fill aquifers. Ground Water 2005, 43, 806–816. [Google Scholar] [CrossRef]
- Riley, A.L.; Mayes, W.M. Long-term evolution of highly alkaline steel slag drainage waters. Environ. Monit. Assess. 2015, 187, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, F.; Fantucci, H.; Nunes, E.; Santos, R.M. Geochemical Modeling Applied in Waste Disposal, and Its Relevance for Municipal Solid Waste Management. Minerals 2020, 10, 846. [Google Scholar] [CrossRef]
- Van der Sloot, H.A.; Seignette, P.; Comans, R.N.J.; van Zomeren, A.; Dijkstra, J.J.; Meeussen, J.C.L.; Kosson, D.S.; Hjelmar, O. Evaluation of environmental aspects of alternative materials using an integrated approach assisted by a database/expert system. In Proceedings of the International Symposium on Recycling and Reuse of Materials, Dundee, Scotland, 9–11 September 2003. [Google Scholar]
- Cappuyns, V.; Alian, V.; Vassilieva, E.; Swennen, R. pH Dependent Leaching Behavior of Zn, Cd, Pb, Cu and As from Mining Wastes and Slags: Kinetics and Mineralogical Control. Waste Biomass Valorization 2014, 5, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Loncnar, M.; van der Sloot, H.A.; Mladenovič, A.; Zupančič, M.; Kobal, L.; Bukovec, P. Study of the leaching behaviour of ladle slags by means of leaching test combined with geochemical modelling and mineralogical investigations. J. Hazard. Mater. 2016, 317, 145–157. [Google Scholar] [CrossRef]
- Loncnar, M. Effect of the Cooling Mode on the Leaching Behaviour of Slags. Ph.D. Thesis, University of Ljubljana, Ljubljana, Slovenia, 2014. [Google Scholar]
- Neuhold, S.; Van Zomeren, A.; Dijkstra, J.J.; Van Der Sloot, H.A.; Drissen, P.; Algermissen, D.; Mudersbach, D.; Schüler, S.; Griessacher, T.; Raith, J.G.; et al. Investigation of Possible Leaching Control Mechanisms for Chromium and Vanadium in Electric Arc Furnace (EAF) Slags Using Combined Experimental and Modeling Approaches. Minerals 2019, 9, 525. [Google Scholar] [CrossRef] [Green Version]
- LeachXS. Available online: http://leachxs.vueinnovations.com/leachxs/leachxs/ (accessed on 29 October 2021).
- Meeussen, J.C. ORCHESTRA: An object-oriented framework for implementing chemical equilibrium models. Environ. Sci. Technol. 2003, 37, 1175–1182. [Google Scholar] [CrossRef]
- Van der Sloot, H.A.; Seignettte, P.F.A.B.; Meeussen, J.C.L.; Hjelmar, O.; Kosson, D.S. A database, speciation modelling and decision support tool for soil, sludge, sediments, wastes and construction products: LeachXS™-Orchestra. In Proceedings of the Second International Symposium on Energy from Biomass and Waste, Venice, Italy, 17 November 2008. [Google Scholar]
- Kinniburgh, D.G.; van Riemsdijk, W.H.; Koopal, L.; Borkovec, M.; Benedetti, M.F.; Avena, M.J. Ion binding to natural organic matter: Competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf. A Physicochem. Eng. Asp. 1999, 151, 147–166. [Google Scholar] [CrossRef]
- Milne, C.J.; Kinniburgh, D.G.; Tipping, E. Generic NICA-Donnan model parameters for proton binding by humic substances. Environ. Sci. Technol. 2001, 35, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Milne, C.J.; Kinniburgh, D.G.; van Riemsdijk, W.H.; Tipping, E. Generic NICA-Donnan model parameters for metal-ion binding by humic substances. Environ. Sci. Technol 2003, 37, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modelling: Hydrous Ferric Oxide; John Wiley & Sons, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Loncnar, M.; van der Sloot, H.A.; Zupančič, M.; Mladenovič, A. Leaching and geochemical modelling of a metallurgical slag heap. In Proceedings of the 5th International Slag Valorisation Symposium, Leuven, Belgium, 3–5 April 2017. [Google Scholar]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef] [Green Version]
- Mombelli, D.; Gruttadauria, A.; Barella, S.; Mapelli, C. The Influence of Slag Tapping Method on the Efficiency of Stabilization Treatment of Electric Arc Furnace Carbon Steel Slag (EAF-C). Minerals 2019, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Strandkvist, I.; Pålsson, K.; Andersson, A.; Olofsson, J.; Lennartsson, A.; Caisa Samuelsson, C.; Engström, F. Minimizing Chromium Leaching from Low-AlloyElectric Arc Furnace (EAF) Slag by Adjusting the Basicity and Cooling Rate to Control Brownmillerite Formation. Appl. Sci. 2020, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.-E.; Larsson, M.L.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Luxán, M.; Sotolongo, R.; Dorrego, F.; Herrero, E. Characteristics of the slags produced in the fusion of scrap steel by electric arc furnace. Cem. Concr. Res. 2000, 30, 517–519. [Google Scholar] [CrossRef]
- Shi, C. Characteristics and cementitious properties of ladle slag fines from steel production. Cem. Concr. Res. 2002, 32, 459–462. [Google Scholar] [CrossRef]
- Zalar Serjun, V.; Mirtič, B.; Mladenovič, A. Evaluation of ladle slag as a potential material for building and civile engineering. Mater. Technol. 2013, 47, 543–550. [Google Scholar]
- Rađenović, A.; Malina, J.; Sofilić, T. Characterization of Ladle Furnace Slag from Carbon Steel Production as a Potential Adsorbent. Adv. Mater. Sci. Eng. 2013, 2013, 198240. [Google Scholar] [CrossRef] [Green Version]
- Faleschini, F.; Brunelli, K.; Zanini, M.A.; Dabala, M.; Pellegrino, C. Electric Arc Furnace Slag as Coarse Recycled Aggregate for Concrete Production. J. Sustain. Metall. 2016, 2, 44–50. [Google Scholar] [CrossRef]
- Herbelin, M.; Bascou, J.; Lavastre, V.; Guillaume, D.; Benbakkar, M.; Peuble, S.; Baron, J.-P. Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques. Minerals 2020, 10, 705. [Google Scholar] [CrossRef]
- Loncnar, M.; Mladenovič, A.; Zupančič, M.; Bukovec, P. Comparision of the Mineralogy and Microstructure of EAF stainless steel slags with reference to cooling treatment. J. Min. Metall. Sect. B Metall. 2017, 53, 19–29. [Google Scholar] [CrossRef]
- Van Zomeren, A.; van der Laan, S.R.; Kobsen, H.A.B.; Huijgen, W.J.J. Changes in mineralogical and leaching properties of converter steel slag resulting from accelerated carbonation at low CO2 pressure. Waste Manag. 2001, 21, 2236–2244. [Google Scholar] [CrossRef] [Green Version]
- Diener, S.; Andreas, L.; Herrmann, I.; Ecke, H.; Lagerkvist, A. Accelerated carbonation of steel slags in landfill cover construction. Waste Manag. 2010, 30, 132–139. [Google Scholar] [CrossRef]
- Juckes, L.M. The volume stability of modern steelmaking slags. Miner. Processing Extr. Metall. 2003, 112, 177–197. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R.N.J. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef]
- Diamond, S. The microstructure of cement paste and concrete––A visual primer. Cem. Concr. Compos. 2004, 26, 919–933. [Google Scholar] [CrossRef]
- Zhang, M.; Reardon, E.J. Removal of B, Cr, Mo and Se from wastewater by incorporation into hydrocalumite and ettringite. Environ. Sci. Technol. 2003, 37, 2947–2952. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: Literature review and experimental study. J. Hazard. Mater. 2006, 136, 20–33. [Google Scholar] [CrossRef]
- Pöllman, H.; Auer, S.; Kuzel, H.J. Solid solution of ettringites. Part II. Incorporation of B(OH)4− and Cr2O42− in 3CaO·Al2O3·3CaSO4·32H2O. Cem. Concr. Res 1993, 23, 422–430. [Google Scholar]
- Perkins, R.B.; Palmer, C.D. Solubility of Ca6[Al(OH)6]2(CrO4)3·26H2O, the chromate analog of ettringite, 5–75 °C. Appl. Geochem. 2000, 15, 1203–1218. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, C.A.; Van Gerven, T.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Ochs, M.; Lothenbach, B.; Giffaut, E. Uptake of oxo-anions by cement through solid-solution formation: Experiment evidence and modelling. Radioch. Acta 2002, 90, 639–646. [Google Scholar] [CrossRef]
- Segni, R.; Vieille, L.; Leroux, F.; Taviot-Guèho, C. Hydrocalumite-type materials: 1. Interest in hazardous waste immobilization. J. Phys. Chem. Solids 2006, 67, 1037–1042. [Google Scholar] [CrossRef]
- Rybka, K.; Matusik, J.; Slaný, M. Technical aspects of selected minerals transformation to LDH-containing materials: The structure, chemistry and affinity towards As(V). J. Environ. Chem. Eng. 2021, 9, 106792. [Google Scholar] [CrossRef]
- Van der Sloot, H.A.; van Zomeren, A.; Meeussen, J.C.L.; Seignette, P.; Bleijerveld, R. Test method selected, validation against field data and predictive modelling for impact evaluation of stabilized waste disposal. J. Hazard. Mater. 2007, 141, 354–369. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 1; World Health Organisation: Geneva, Switzerland, 1997. [Google Scholar]
- Van der Sloot, H.A.; Heasman, L.; Quevauviller, P. Studies in Environmental Science. In Harmonization of Leaching/Extraction Tests, 1st ed.; Elsevier: Amsterdam, The Netherland, 1997; Volume 70. [Google Scholar]
- Christensen, J.B.; Botma, J.J.; Christensen, T.H. Complexation of Cu and Pb by DOC in polluted groundwater: A comparison of experimental data and predictions by computer speciation models (WHAM and MINTEQA2). Water Res. 1999, 33, 3231–3238. [Google Scholar] [CrossRef]
- Christensen, J.B.; Christensen, T.H. Complexation of Cd, Ni, and Zn by DOC in Polluted Groundwater: A Comparison of Approaches Using Resin Exchange, Aquifer Material Sorption, and Computer Speciation Models (WHAM and MINTEQA2). Environ. Sci. Technol. 1999, 33, 3857–3863. [Google Scholar] [CrossRef]
- Van der Sloot, H.A.; Kosson, D.S. Use of characterisation leaching tests and associated modelling tools in assessing the hazardous nature of wastes. J. Hazard. Mater. 2012, 207–208, 36–43. [Google Scholar] [CrossRef]
- Van der Sloot, H.A.; Kosson, D.S.; van Zomeren, A. Leaching, geochemical modelling and field verification of a municipal solid waste and a predominantly non-degradable waste landfill. Waste Manag. 2017, 63, 74–95. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef] [Green Version]
- Huijgen, W.J.J.; Comans, R.N.J. Carbonation of steel slag for CO2 sequestration: Leaching of products and reaction mechanisms. Environ. Sci. Technol. 2006, 40, 2790–2796. [Google Scholar] [CrossRef]




| Individual Sample | L1 | L2 | L3 | L4 | L5 | L6 |
| Sample depth | 200 mm | 200 mm | 200 mm | 200 mm | 200 mm | 200 mm |
| Individual sample | L7 | L8 | L9 | L10 | L11 | |
| Sample depth | 6000 mm | 6000 m | 6000 mm | 6000 mm | 6000 mm | |
| Composite sample | PTO | mix (L2-L11) |
| CaO | SiO2 | Al2O3 | MgO | FeO | Cr2O3 | MnO | |
|---|---|---|---|---|---|---|---|
| PTO | 26.7 ± 0.3 | 14.6 ± 0.2 | 7.0 ± 0.1 | 8.5 ± 0.1 | 20.6 * ± 1 | 2.2 ± 0.3 | 3.7 ± 0.1 |
| EAF slag | 24.5–60 | 9–20 | 2–12.2 | 5–15 | 5.6–34.4 | - | 2.5–8 |
| ladle slag | 30–60 | 2–35 | 5–35 | 1–12.6 | 0–15 | - | 0.5–5 |
| Samples | CaO | SiO2 | Al2O3 | MgO | Fetot | Cr2O3 | MnO | S | C |
|---|---|---|---|---|---|---|---|---|---|
| L1–L6 | 35 ± 5 | 14 ± 2 | 10 ± 3 | 13 ± 3 | 13 ± 3 | 2.9 ± 0.5 | 2.6 ± 0.9 | 0.5 ± 0.2 | 2.7 ± 0.6 |
| L7–L11 | 23 ± 4 | 20 ± 6 | 8 ± 3 | 9 ± 4 | 14 ± 2 | 1.5 ± 0.8 | 3.5 ± 1.9 | 0.28 ± 0.06 | 1.6 ± 0.4 |
| Ca | K | Mg | Na | SO42− | Cl– | HCO3− | |
|---|---|---|---|---|---|---|---|
| Min–Max Average | Min–Max Average | Min–Max Average | Min–Max Average | Min–Max Average | Min–Max Average | Min–Max Average | |
| P1 | 5.70–69.7 23 ± 18 | 2.50–6.36 3.6 ± 1.2 | 16.0–42.3 26 ± 5 | 1.70–26.3 11 ± 4 | 17.4–125 50 ± 36 | 17.1–28.3 21 ± 3 | 12 –363 192 ± 56 |
| P2 | 4.90–77.9 42 ± 23 | 1.50–11.5 5 ± 3 | 13.0–29.1 20 ± 5 | 9.50–20.3 14 ± 3 | 1.72–78.6 41 ± 23 | 14.9–24.9 19 ± 3 | 101–313 194 ± 56 |
| P3 | 2.80–71.3 37 ± 17 | 1.30–6.10 2.4 ± 1.3 | 18.5–26.3 22 ± 2 | 8.70–15.1 12 ± 2 | 5.19–74.2 32 ± 17 | 13.8–25.0 18 ± 3 | 138 –296 202 ± 47 |
| P4 | 16.7–84.9 69 ± 17 | 2.70–8.04 5 ± 2 | 17.3–30.2 25 ± 3 | 1.70–16.4 12 ± 4 | 14.0–130 82 ± 29 | 13.2–25.2 19 ± 3 | 147–311 229 ± 47 |
| P5 | 9.66–49.2 65 ± 10 | 1.40–4.88 3 ± 1 | 14.0–26.4 20 ± 3 | 5.10–15.4 11 ± 3 | 8.00–73.5 45 ± 18 | 7.54–23.8 15 ± 5 | 149–301 219 ± 44 |
| P6 | 61.7–81.4 71 ± 5 | 4.60–11.5 6.8 ± 1.6 | 19.8–24.1 22 ± 2 | 7.30–18.9 15 ± 3 | 9.00–95.8 64 ± 22 | 15.8–29.3 21 ± 3 | 134–2.340 359 ± 505 |
| P7 | 14.7–74.3 60 ± 13 | 1.35–6.10 8.13 ± 1.4 | 19.5–77.1 54 ± 1 | 1.42–15.5 8.4 ± 3.6 | 4.50–28.8 14 ± 8 | 4.05–14.3 7.7 ± 3.3 | 181–1.151 486 ± 194 |
| Cr | Ni | Pb | Zn | Mo | |
|---|---|---|---|---|---|
| Min–Max Average | Min–Max Average | Min–Max Average | Min–Max Average | Min–Max Average | |
| P1 | <1 <1 | <1 <1 | <0.5 <0.50 | <2– 36 6.27 ± 10.3 | <1–6.7 1.53 ± 1.52 |
| P2 | <1–27.4 3.3 ± 8.1 | <1 * <1 | <0.5 <0.5 | <2–10.3 2.4 ± 2.6 | <1–78.9 21.3 ± 19.2 |
| P3 | <1 <1 | <1 * <1 | <0.5 <0.5 | <2–5.7 2.09 ± 2.1 | <1–17.4 0.83 ± 0.57 |
| P4 | <1–10.9 1.9 ± 2.5 | <1–1.1 0.53 ± 0.32 | <0.5 <0.5 | <2–23.6 8.9 ± 7.1 | <1–11 5.87 ± 3.34 |
| P5 | <1–1.5 0.74 ± 0.37 | <1 <1 | <0.5 <0.5 | <2–33.1 9.42 | <1–14 6.34 ± 3.89 |
| P6 | <1–5.1 1.15 ± 1.14 | <1 <1 | <0.5 <0.5 | <2–29.9 9.35 ± 8.0 | 13.0–23.7 18.5 ± 3.62 |
| P7 | <1 <1 | <1–1.8 * 0.58 ± 0.32 | <0.5–0.6 ** 0.28 ± 0.09 | 34.5–308 *** 186 ± 100 | <1–11.1 1.45 ± 2.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loncnar, M.; Mladenovič, A.; Zalar Serjun, V.; Zupančič, M.; van der Sloot, H.A. Leaching and Geochemical Modelling of an Electric Arc Furnace (EAF) and Ladle Slag Heap. Toxics 2022, 10, 10. https://doi.org/10.3390/toxics10010010
Loncnar M, Mladenovič A, Zalar Serjun V, Zupančič M, van der Sloot HA. Leaching and Geochemical Modelling of an Electric Arc Furnace (EAF) and Ladle Slag Heap. Toxics. 2022; 10(1):10. https://doi.org/10.3390/toxics10010010
Chicago/Turabian StyleLoncnar, Mojca, Ana Mladenovič, Vesna Zalar Serjun, Marija Zupančič, and Hans A. van der Sloot. 2022. "Leaching and Geochemical Modelling of an Electric Arc Furnace (EAF) and Ladle Slag Heap" Toxics 10, no. 1: 10. https://doi.org/10.3390/toxics10010010
APA StyleLoncnar, M., Mladenovič, A., Zalar Serjun, V., Zupančič, M., & van der Sloot, H. A. (2022). Leaching and Geochemical Modelling of an Electric Arc Furnace (EAF) and Ladle Slag Heap. Toxics, 10(1), 10. https://doi.org/10.3390/toxics10010010







