Free Monoterpene Isomer Profiles of Vitis Vinifera L. cv. White Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Sample Preparation
2.4. Head Space Solid Phase Micro-Extraction Coupled with Multidimensional Gas Chromatography Mass Spectrometery (HS-SPME-MDGC-MS)
2.5. Statistical Analysis
3. Results
3.1. Varietal Wines
3.2. Monoterpene Content in Varietal Wines
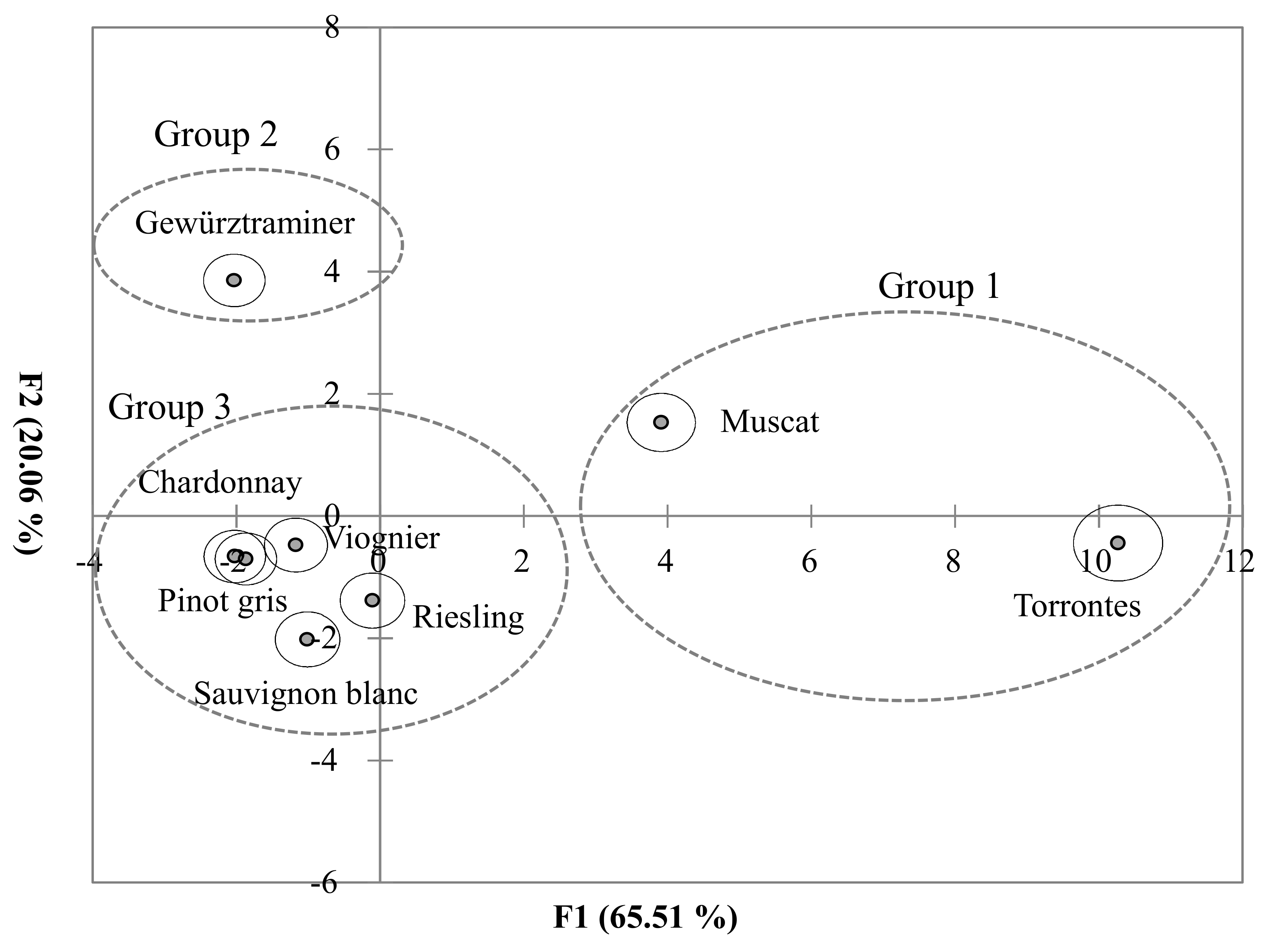
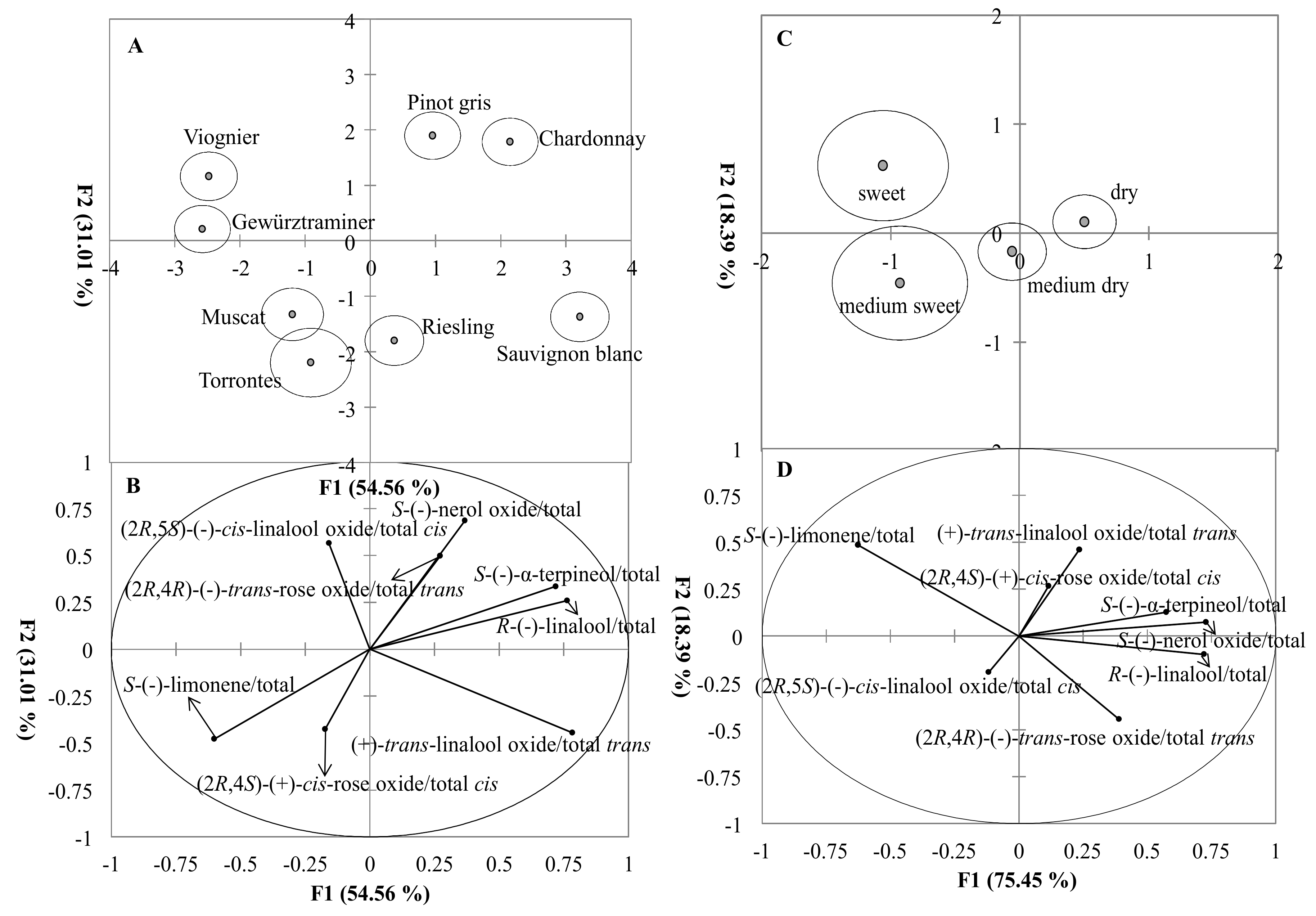
3.3. Classification of the Grape Variety and Wine Style Based on Monoterpene Isomer Concentrations
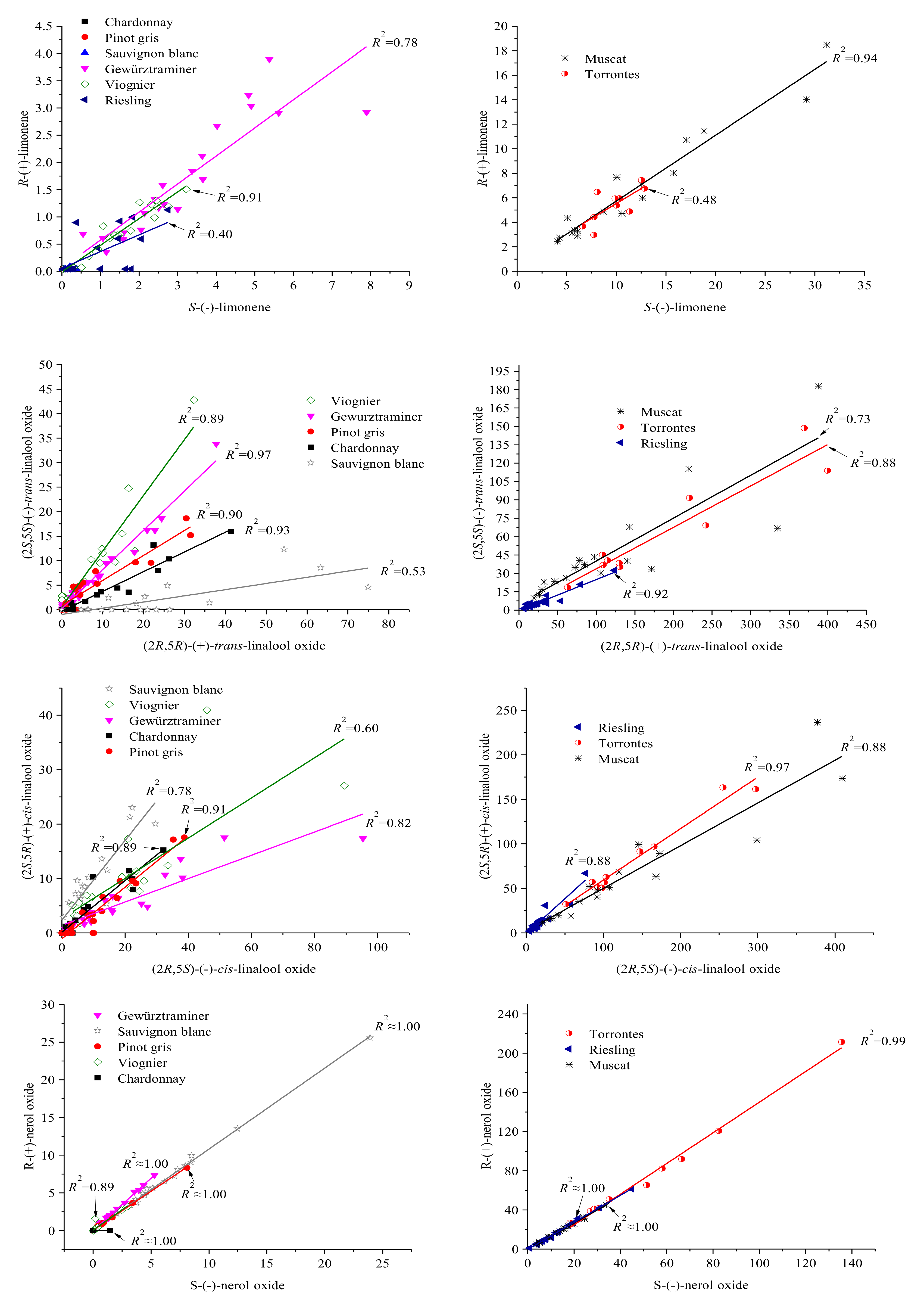
3.4. Enantiomer Fraction (EF) in Varietal Wines
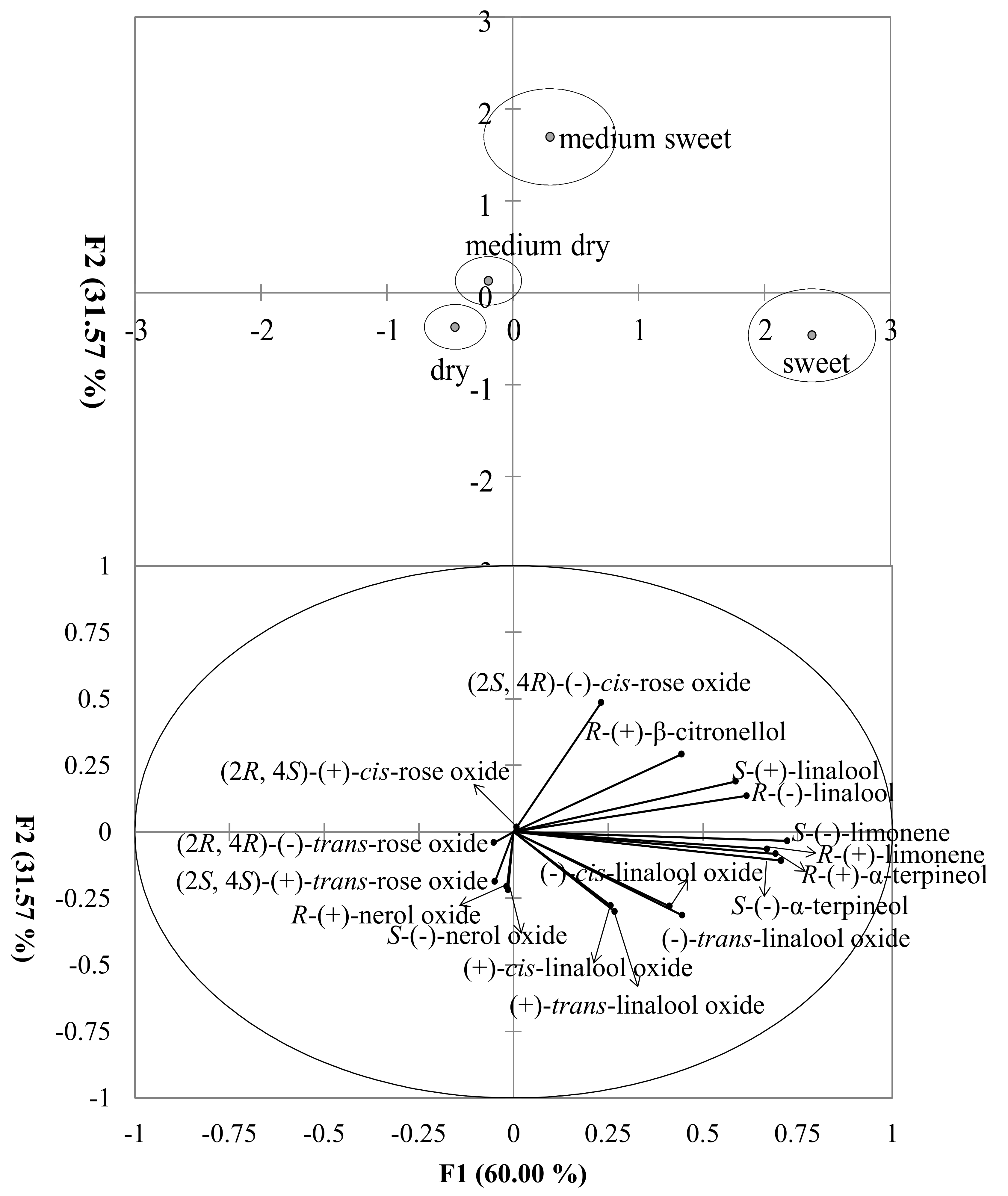
3.5. Classification of the Grape Variety and Wine Style Based on Enantiomer Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–60. [Google Scholar] [CrossRef]
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111. [Google Scholar] [CrossRef]
- Rapp, A. Natural flavours of wine: Correlation between instrumental analysis and sensory perception. Fresenius J. Anal. Chem. 1990, 337, 777–785. [Google Scholar] [CrossRef]
- Calleja, A.; Falqué, E. Volatile composition of Mencıa wines. Food Chem. 2005, 90, 357–363. [Google Scholar] [CrossRef]
- Zamuz, S.; Vilanova, M. Volatile composition of the Vitis vinifera Albarino musts according to geographic areas from Rias Baixas DO (Spain). Italian J. Food Sci. 2006, 18, 323. [Google Scholar]
- Williams, P.; Strauss, C.; Wilson, B. Classification of the monoterpenoid composition of Muscat grapes. Am. J. Enol. Vitic. 1981, 32, 230–235. [Google Scholar]
- Wagner, R.; Dirninger, N.; Fuchs, V.; Bronner, A. In Study of the intervarietal differences in the concentration of volatile constituents (linalool and geraniol) in the aroma of the grape. Interest of such analyses for the appreciation of the quality of the harvest. Int. Symp. Qual. Vintage. Cape Town 1977, 137–142. [Google Scholar]
- Vilanova, M.; Genisheva, Z.A.; Graña, M.; Oliveira, J. Determination of odorants in varietal wines from international grape cultivars (Vitis vinifera) grown in NW Spain. S. Afr. J. Enol. Vitic. 2013, 34, 212–222. [Google Scholar] [CrossRef]
- Versini, G.; Rapp, A.; Volkmann, C.; Scienza, A. Flavour compounds of clones from different grape varieties. Vitis 1990, Special Issue, 513–524. [Google Scholar]
- Rapp, A.; Hastrich, H.; Engel, L.; Knipser, W. Possibilities of Characterizing Wine Quality and Vine Varieties by Means of Capillary Chromatography. In Flavor of Foods and Beverages: Chemistry and Technology; Charalambous, G., Inglett, G.E., Eds.; Academic Press: New York, NY, USA, 1978; p. 391. [Google Scholar]
- Armstrong, D.W.; Chang, C.D.; Li, W.Y. Relevance of enantiomeric separations in food and beverage analyses. J. Agric. Food Chem. 1990, 38, 1674–1677. [Google Scholar] [CrossRef]
- Marchelli, R.; Dossena, A.; Palla, G. The potential of enantioselective analysis as a quality control tool. Trends Food Sci. Technol. 1996, 7, 113–119. [Google Scholar] [CrossRef]
- Lehmann, D.; Dietrich, A.; Hener, U.; Mosandl, A. Stereoisomeric flavour compounds. LXX: 1-p-menthene-8-thiol: Separation and sensory evaluation of the enantiomers by enantioselective gas chromatography-olfactometry. Phytochem. Anal. 1995, 6, 255–257. [Google Scholar] [CrossRef]
- Peña, R.M.; Barciela, J.; Herrero, C.; García-Martín, S. Optimization of solid-phase microextraction methods for GC-MS determination of terpenes in wine. J. Sci. Food Agric. 2005, 85, 1227–1234. [Google Scholar] [CrossRef]
- Padrayuttawat, A.; Yoshizawa, T.; Tamura, H.; Tokunaga, T. Optical Isomers and Odor Thresholds of Volatile Constituents in Citrus sudachi. Food Sci. Technol. Int. Tokyo 1997, 3, 402–408. [Google Scholar] [CrossRef]
- Song, M.; Xia, Y.; Tomasino, E. Investigation of a quantitative method for the analysis of chiral monoterpenes in white wine by HS–SPME–MDGC–MS of different wine matrices. Molecules 2015, 20, 7359–7378. [Google Scholar] [CrossRef] [PubMed]
- Rebelein, H. Rapid method for the determination of the alcohol, sugar and total SO2 contents (by distillation) in wine and fruit juices and also for determining blood alcohol. Chem. Mikrobiol. Technol. Lebensm 1973, 2, 112–121. [Google Scholar]
- Maul, E.; Sudharma, K.; Ganesh, A.; Hundemer, M.; Kecke, S.; Marx, G.; Schreiber, T.; Walk, M.; vom Weg, S.; Mahler-Ries, A. 30 Years VIVC–Vitis International Variety Catalogue. 2014. Available online: www.vivc.de (accessed on 10 September 2017).
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwöhner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS–SPME–GC–MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation. No 1512/2005 Amending Regulation (EC) No 753/2002 Laying Down Certain Rules for Applying Council Regulation (EC) No 1496/1999 as Regards the Description, Designation, Presentation and Protection of Certain Wine Sector Products (OJ L 241, 17.9. 2005); European Commission: Brussels, Belgium, 2002.
- A Guide to the Analysis of Chiral Compounds by GC. Available online: https://www.restek.com/pdfs/59889.pdf (accessed on 2 August 2017).
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective perception of chiral odorants. Tetrahedron: Asymmetry 2003, 14, 1–42. [Google Scholar] [CrossRef]
- Perkins, M.L.; D’Arcy, B.R.; Lisle, A.T.; Deeth, H.C. Solid phase microextraction of stale flavour volatiles from the headspace of UHT milk. J. Sci. Food Agric. 2005, 85, 2421–2428. [Google Scholar] [CrossRef]
- Ohloff, G.; Giersch, W.; Schulte–Elte, K.H.; Enggist, P.; Demole, E. Synthesis of (R)–and (R)–4–methyl–6–2′–methylprop–1′–enyl–5, 6–dihydro–2H–pyran (Nerol oxide) and Natural Occurrence of its Racemate. Helvetica Chimica Acta 1980, 63, 1582–1588. [Google Scholar] [CrossRef]
- Garneau, F.-X.; Collin, G.; Gagnon, H. Chemical composition and stability of the hydrosols obtained during essential oil production. II. The case of Picea glauca (Moench) Voss., Solidago puberula Nutt., and Mentha piperita L. Am. J. Essent. Oils Nat. Prod. 2014, 2, 29–35. [Google Scholar]
- Gunata, Z.; Bitteur, S.; Brillouet, J.-M.; Bayonove, C.; Cordonnier, R. Sequential enzymic hydrolysis of potentially aromatic glycosides from grape. Carbohydr. Res. 1988, 184, 139–149. [Google Scholar] [CrossRef]
- Poole, C. Handbook of Methods and Instrumentation in Separation Science; Academic Press: New York, NY, USA, 2009; Volume 1. [Google Scholar]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Croghan, C.; Egeghy, P. Methods of dealing with values below the limit of detection using SAS. In Proceedings of the Southern SAS User Group, St. Petersburg, FL, USA, 22–24 September 2003; pp. 22–24. [Google Scholar]
- Askari, C.; Hener, U.; Schmarr, H.G.; Rapp, A.; Mosandl, A. Stereodifferentiation of some chiral monoterpenes using multidimensional gas chromatography. Fresenius J. Anal. Chem. 1991, 340, 768–772. [Google Scholar] [CrossRef]
- Tominaga, T.; Niclass, Y.; Frérot, E.; Dubourdieu, D. Stereoisomeric distribution of 3–mercaptohexan–1–ol and 3–mercaptohexyl acetate in dry and sweet white wines made from Vitis vinifera (Var. Sauvignon Blanc and Semillon). J. Agric. Food Chem. 2006, 54, 7251–7255. [Google Scholar] [CrossRef] [PubMed]
- Harner, T.; Wiberg, K.; Norstrom, R. Enantiomer fractions are preferred to enantiomer ratios for describing chiral signatures in environmental analysis. Environ. Sci. Technol. 2000, 34, 218–220. [Google Scholar] [CrossRef]
- Mateo, J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Boidron, J.; Terrier, A. Aroma of Muscat grape varieties. J. Agric. Food Chem. 1975, 23, 1042–1047. [Google Scholar] [CrossRef]
- Guth, H. Identification of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Ruiz-García, L.; Hellín, P.; Flores, P.; Fenoll, J. Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.K.; Acree, T.E. Similarities in the aroma chemistry of Gewürztraminer variety wines and lychee (Litchi chinesis Sonn.) fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Mosandl, A.; Gubesch, M.; Wüst, M. Enantioselective analysis of monoterpenes in different grape varieties during berry ripening using stir bar sorptive extraction-and solid phase extraction-enantioselective-multidimensional gas chromatography-mass spectrometry. J. Chromatogr. 2006, 1112, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Koslitz, S.; Renaud, L.; Kohler, M.; Wüst, M. Stereoselective formation of the varietal aroma compound rose oxide during alcoholic fermentation. J. Agric. Food Chem. 2008, 56, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Sefton, M.; Francis, I.; Williams, P. The volatile composition of Chardonnay juices: A study by flavor precursor analysis. Am. J. Enol. Vitic. 1993, 44, 359–370. [Google Scholar]
- Dimitriadis, E.; Williams, P. The development and use of a rapid analytical technique for estimation of free and potentially volatile monoterpene flavorants of grapes. Am. J. Enol. Vitic. 1984, 35, 66–71. [Google Scholar]
- Arrhenius, S.; McCloskey, L.; Sylvan, M. Chemical markers for aroma of Vitis vinifera var. Chardonnay regional wines. J. Agric. Food Chem. 1996, 44, 1085–1090. [Google Scholar] [CrossRef]
- Swipson, R.; Miller, G. Aroma composition of Chardonnay wine. Vitis 1984, 23, 143–158. [Google Scholar]
- Guchu, E.; Díaz-Maroto, M.; Pérez-Coello, M.; González-Viñas, M.; Ibáñez, M.C. Volatile composition and sensory characteristics of Chardonnay wines treated with American and Hungarian oak chips. Food Chem. 2006, 99, 350–359. [Google Scholar] [CrossRef]
- Salinas, M.; Zalacain, A.; Pardo, F.; Alonso, G.L. Stir bar sorptive extraction applied to volatile constituents evolution during Vitis vinifera ripening. J. Agric. Food Chem. 2004, 52, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Gunata, Y.; Bayonove, C.; Baumes, R.; Cordonnier, R. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Bayonove, C.; Cordonnier, R. Recherches sur l’arome du muscat. II. Profils aromatiques de cepages muscat et non muscat. importance du linalol chez les muscats. Ann. Technol. Agr. 1970, 19, 95–105. [Google Scholar]
- Bayonove, C.; Cordonnier, R. Recherches sur l'arôme du muscat. I. Evolution des constituants volatils au cours de la maturation du Muscat d'Alexandrie. Ann. Technol. Agric 1970, 19, 79–93. [Google Scholar]
- Marais, J. Terpene concentrations and wine quality of Vitis vinifera L. cv. Gewürztraminer as affected by grape maturity and cellar. Vitis 1978, 26, 231–245. [Google Scholar]
- Câmara, J.; Herbert, P.; Marques, J.; Alves, M. Varietal flavour compounds of four grape varieties producing Madeira wines. Anal. Chim. Acta. 2004, 513, 203–207. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Agüero, C.B.; Rodríguez, J.G.; Martínez, L.E.; Dangl, G.S.; Meredith, C.P. Identity and parentage of Torrontés cultivars in Argentina. Am. J. Enol. Vitic. 2003, 54, 318–321. [Google Scholar]
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.E.; Meredith, C.P. The parentage of a classic wine grape, Cabernet Sauvignon. Nat. Genet. 1997, 16, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.; Boursiquot, J.-M.; Chu, K.; Johansson, H.; Meredith, C. Historical genetics: The parentage of Chardonnay, Gamay, and other wine grapes of northeastern France. Science 1999, 285, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.E.; Meredith, C.P. Genetic similarities among wine grape cultivars revealed by restriction fragment–length polymorphism (RFLP) analysis. J. Am. Soc. Hort. Sci. 1996, 121, 620–624. [Google Scholar]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Sefton, M. Hydrolytically-released volatile secondary metabolites from a juice sample of Vitis vinifera grape cvs Merlot and Cabernet Sauvignon. Aust. J. Grape Wine Res. 1998, 4, 30–38. [Google Scholar] [CrossRef]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- De Carvalho, C.C.; da Fonseca, M.M.R. Biotransformation of terpenes. Biotechnol. Adv. 2006, 24, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bock, G.; Benda, I.; Schreier, P. Microbial transformation of geraniol and nerol by Botrytis cinerea. Appl. Microbiol. Biotechnol. 1988, 27, 351–357. [Google Scholar] [CrossRef]
- Hock, R.; Benda, I.; Schreier, P. Formation of terpenes by yeasts during alcoholic fermentation. Zeitschrift für Lebensmittel–Untersuchung und Forschung 1984, 179, 450–452. [Google Scholar] [CrossRef]
- Zoecklein, B.; Marcy, J.; Williams, J.; Jasinski, Y. Effect of Native Yeasts and Selected Strains ofSaccharomyces cerevisiaeon Glycosyl Glucose, Potential Volatile Terpenes, and Selected Aglycones of White Riesling (Vitis vinifera L.) Wines. J. Food Compos. Anal. 1997, 10, 55–65. [Google Scholar] [CrossRef]
- Palomo, E.S.; Díaz-Maroto Hidalgo, M.A.; Gonzalez-Vinas, M.; Pérez-Coello, M. Aroma enhancement in wines from different grape varieties using exogenous glycosidases. Food Chem. 2005, 92, 627–635. [Google Scholar] [CrossRef]
- Peéa-Alvarez, A.; Capella, S.; Juárez, R.; Labastida, C. Determination of terpenes in tequila by solid phase microextraction-gas chromatography-mass spectrometry. J. Chromatogr. 2006, 1134, 291–297. [Google Scholar]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Guntert, M. Changes in aroma substances during the storage of white wines in bottles. Available online: http://agris.fao.org/agris-search/search.do?recordID=US201301396683 (accessed on 18 January 2017).
- Williams, P.J.; Strauss, C.R.; Wilson, B. Hydroxylated linalool derivatives as precursors of volatile monoterpenes of Muscat grapes. J. Agric. Food Chem. 1980, 28, 766–771. [Google Scholar] [CrossRef]
- Finefield, J.M.; Sherman, D.H.; Kreitman, M.; Williams, R.M. Enantiomeric natural products: occurrence and biogenesis. Angew. Chem. Int. Ed. 2012, 51, 4802–4836. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, H.; Vogt, G.; Aaby, K.; Olsen, E. Interaction of terpenes with sweet taste in carrots (Daucus carota L.). In Proceedings of the XXVI International Horticultural Congress: Advances in Vegetable Breeding, Toronto, ON, Canada, 11 August 2002; pp. 377–386. [Google Scholar]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Di Stefano, R. The glycoside nature of terpenes of Muscat grape skins. The β–glycosidase activity of grape skins. Viticultural and Enological Sciences/Die Weinwissenschaft 1989, 44, 158–161. [Google Scholar]

| Variety | Region a | Vintage | Style of Wine (Bottles) | |||
|---|---|---|---|---|---|---|
| Dry | Medium Dry | Medium Sweet | Sweet | |||
| Chardonnay | AU | 2012 | 3 | |||
| 2013 | 2 | 2 | ||||
| OR | 2012 | 4 | ||||
| 2013 | 1 | 2 | 1 | |||
| CAL | 2012 | 3 | ||||
| 2013 | 2 | 1 | ||||
| Gewürztraminer | OR | 2012 | 1 | 1 | 1 | |
| 2013 | 1 | 2 | ||||
| FR | 2012 | 2 | 1 | |||
| 2013 | 1 | 4 | ||||
| NY | 2012 | 1 | 1 | |||
| 2013 | 2 | 1 | ||||
| CAL | 2012 | 1 | ||||
| 2013 | 1 | |||||
| Muscat | FR | 2012 | 2 | 2 | ||
| 2013 | 2 | 1 | ||||
| CAL | 2012 | 2 | ||||
| 2013 | 1 | 2 | ||||
| IT | 2012 | 2 | ||||
| 2013 | 3 | |||||
| Pinot gris | OR | 2012 | 4 | |||
| 2013 | 3 | 1 | ||||
| FR | 2012 | 3 | ||||
| 2013 | 3 | |||||
| IT | 2012 | 2 | 2 | |||
| 2013 | 2 | 1 | ||||
| Riesling | OR | 2012 | 3 | |||
| 2013 | 1 | |||||
| FR | 2012 | 3 | ||||
| 2013 | 1 | 3 | ||||
| GR | 2012 | 1 | 2 | |||
| 2013 | 1 | 1 | 1 | |||
| AU | 2013 | 1 | 1 | |||
| Sauvignon blanc | NZ | 2012 | 3 | 1 | ||
| 2013 | 1 | 3 | ||||
| SA | 2012 | 2 | ||||
| 2013 | 3 | |||||
| FR | 2012 | 3 | ||||
| 2013 | 2 | 1 | ||||
| Torrontes | ARG | 2012 | 4 | 1 | ||
| 2013 | 4 | 1 | ||||
| Viognier | FR | 2012 | 3 | 1 | ||
| 2013 | 3 | |||||
| OR | 2012 | 1 | ||||
| 2013 | 1 | 3 | ||||
| CAL | 2012 | 3 | 1 | |||
| 2013 | 3 | 1 | ||||
| Chardonnay | Gewürztraminer | Muscat | Pinot Gris | Riesling | Sauvignon Blanc | Torrontes | Viognier | |
|---|---|---|---|---|---|---|---|---|
| S-(−)-limonene | 0.03 ± 0.00 a | 3.17 ± 0.39 b | 11.96 ± 2.00 c | 0.03 ± 0.00 a | 0.87 ± 0.20 ab | 0.08 ± 0.02 a | 9.70 ± 0.67 c | 1.53 ± 0.19 ab |
| R-(+)-limonene | 0.04 ± 0.00 a | 1.69 ± 0.23 a | 6.78 ± 1.10 b | 0.04 ± 0.00 a | 0.32 ± 0.09 a | 0.05 ± 0.00 a | 5.39 ± 0.45 b | 0.74 ± 0.10 a |
| Total hydrocarbon | 0.07 ± 0.00 a | 4.86 ± 0.60 b | 18.74 ± 3.08 c | 0.07 ± 0.00 a | 1.19 ± 0.27 ab | 0.13 ± 0.02 a | 15.08 ± 1.05 c | 2.27 ± 0.29 ab |
| (2R, 4S)-(+)-cis-rose oxide | 0.02 ± 0.00 a | 0.20 ± 0.04 bc | 0.34 ± 0.09 c | 0.04 ± 0.01 ab | 0.07 ± 0.02 ab | 0.04 ± 0.01 ab | 0.80 ± 0.11 d | 0.06 ± 0.02 ab |
| (2S, 4R)-(−)-cis-rose oxide | 0.06 ± 0.00 a | 0.70 ± 0.17 bc | 0.50 ± 0.08 b | 0.09 ± 0.02 a | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.85 ± 0.13 c | 0.06 ± 0.00 a |
| (2R, 4R)-(−)-trans-rose oxide | 0.00 ± 0.00 a | 0.25 ± 0.05 a | 0.20 ± 0.07 a | 0.01 ± 0.00 a | 0.04 ± 0.02 a | 0.02 ± 0.02 a | 1.57 ± 0.67 b | 0.00 ± 0.00 a |
| (2S, 4S)-(+)-trans-rose oxide | 0.00 ± 0.00 a | 0.05 ± 0.01 a | 0.11 ± 0.03 a | 0.00 ± 0.00 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.74 ± 0.23 b | 0.00 ± 0.00 a |
| Total rose oxide | 0.08 ± 0.00 a | 1.19 ± 0.22 c | 1.14 ± 0.17 bc | 0.14 ± 0.03 a | 0.19 ± 0.05 ab | 0.15 ± 0.05 a | 3.96 ± 1.01 d | 0.12 ± 0.02 a |
| (2R,5R)-(+)-trans-linalool oxide | 8.53 ± 2.49 a | 9.70 ± 2.15 a | 120.55 ± 25.80 b | 7.11 ± 2.13 a | 32.22 ± 6.47 a | 23.63 ± 4.66 a | 188.60 ± 36.82 c | 7.51 ± 1.84 a |
| (2R,5S)-(−)-cis-linalool oxide | 6.98 ± 2.05 a | 20.79 ± 4.78 a | 136.21 ± 28.73 b | 11.77 ± 2.38 a | 19.78 ± 4.12 a | 8.82 ± 1.88 a | 139.63 ± 25.03 b | 19.32 ± 4.50 a |
| (2S,5S)-(−)-trans-linalool oxide | 3.14 ± 1.04 a | 7.93 ± 1.74 a | 47.32 ± 10.46 b | 4.48 ± 1.14 a | 7.78 ± 1.70 a | 2.02 ± 0.79 a | 63.78 ± 13.16 b | 9.13 ± 2.21 a |
| (2S,5R)-(+)-cis-linalool oxide | 3.48 ± 1.03 a | 5.86 ± 1.13 a | 67.42 ± 14.63 b | 4.53 ± 1.18 a | 14.75 ± 3.43 a | 8.86 ± 1.54 a | 82.39 ± 14.63 b | 9.80 ± 2.11 a |
| S-(−)-nerol oxide | 0.07 ± 0.07 a | 1.83 ± 0.30 ab | 14.41 ± 1.99 c | 0.80 ± 0.40 a | 12.27 ± 2.51 bc | 6.33 ± 1.17 abc | 53.35 ± 11.14 d | 0.33 ± 0.18 a |
| R-(+)-nerol oxide | 0.00 ± 0.00 a | 2.53 ± 0.41 ab | 19.30 ± 2.64 c | 0.88 ± 0.42 ab | 16.76 ± 3.47 bc | 6.86 ± 1.26 abc | 77.12 ± 17.49 d | 0.43 ± 0.21 a |
| Total linalool and nerol oxide | 22.20 ± 6.55 a | 48.65 ± 10.34 a | 405.20 ± 80.48 b | 29.58 ± 7.40 a | 103.55 ± 21.32 a | 56.50 ± 10.48 a | 604.87 ± 114.29 c | 46.51 ± 10.34 a |
| R-(−)-linalool | 0.77 ± 0.34 a | 57.49 ± 4.81 bc | 127.63 ± 36.88 d | 0.14 ± 0.13 a | 2.96 ± 1.02 ab | 0.54 ± 0.30 a | 63.35 ± 10.94 c | 29.85 ± 5.19 abc |
| S-(+)-linalool | 0.68 ± 0.32 a | 55.27 ± 5.97 b | 108.24 ± 31.80 c | 0.08 ± 0.07 a | 2.90 ± 1.05 a | 0.49 ± 0.29 a | 48.26 ± 10.08 ab | 24.90 ± 4.87 ab |
| S-(−)-α-terpineol | 1.80 ± 0.22 a | 56.35 ± 7.06 a | 276.36 ± 39.95 b | 5.63 ± 0.62 a | 30.31 ± 3.33 a | 10.13 ± 1.40 a | 264.04 ± 15.79 b | 49.02 ± 4.75 a |
| R-(+)-α-terpineol | 0.45 ± 0.16 a | 58.89 ± 7.62 b | 252.72 ± 34.37 c | 2.87 ± 0.74 a | 26.19 ± 3.29 ab | 3.28 ± 0.92 a | 248.49 ± 14.22 c | 45.36 ± 4.43 ab |
| R-(+)-β-citronellol | 1.67 ± 0.27 a | 17.55 ± 2.69 c | 14.98 ± 5.78 c | 1.21 ± 0.29 a | 0.44 ± 0.20 a | 0.70 ± 0.23 a | 12.51 ± 1.82 bc | 2.70 ± 0.46 ab |
| Total alcohols | 5.36 ± 0.98 a | 245.56 ± 22.89 b | 779.94 ± 143.43 c | 9.92 ± 1.08 a | 62.80 ± 8.03 ab | 15.13 ± 2.41 a | 636.64 ± 48.91 c | 151.84 ± 16.01 ab |
| Total isomers | 27.71 ± 6.36 a | 300.26 ± 30.65 a | 1205.02 ± 174.02 b | 39.71 ± 7.71 a | 167.73 ± 22.69 a | 71.90 ± 11.19 a | 1260.55 ± 81.84 b | 200.74 ± 20.32 a |
| S-(−)-limonene/total | (2R,4S)-(+)-cis-rose oxide/total cis | (2R,4R)-(−)-trans-rose oxide/total trans | (2R,5R)-(+)-trans-linalool oxide/ total trans | (2R, 5S)-(−)-cis-linalool oxide/ total cis | S-(−)-nerol oxide/total | R-(−)-linalool/total | S-(−)-α-terpineol/total | |
|---|---|---|---|---|---|---|---|---|
| Chardonnay | 0.39 ± 0.00 a | 0.23 ± 0.00 a | 1.00 ± 0.00 c | 0.78 ± 0.02 bc | 0.77 ± 0.04 d | 0.59 ± 0.02 d | 0.79 ± 0.03 bc | 0.86 ± 0.05 b |
| Gewürztraminer | 0.66 ± 0.02 b | 0.25 ± 0.02 ab | 0.86 ± 0.03 bc | 0.49 ± 0.03 a | 0.75 ± 0.01 cd | 0.42 ± 0.00 ab | 0.52 ± 0.01 a | 0.49 ± 0.00 a |
| Muscat | 0.63 ± 0.01 b | 0.41 ± 0.05 cd | 0.76 ± 0.05 b | 0.70 ± 0.02 bc | 0.67 ± 0.01 bcd | 0.43 ± 0.00 ab | 0.54 ± 0.01 a | 0.52 ± 0.00 a |
| Pinot gris | 0.39 ± 0.00 a | 0.26 ± 0.02 ab | 0.98 ± 0.02 c | 0.65 ± 0.04 b | 0.79 ± 0.03 d | 0.52 ± 0.01 c | 0.85 ± 0.01 c | 0.75 ± 0.06 b |
| Riesling | 0.68 ± 0.05 b | 0.39 ± 0.05 bcd | 0.86 ± 0.07 bc | 0.80 ± 0.01 c | 0.59 ± 0.02 ab | 0.42 ± 0.00 ab | 0.71 ± 0.04 b | 0.55 ± 0.01 a |
| Sauvignon blanc | 0.50 ± 0.04 a | 0.28 ± 0.03 abc | 0.98 ± 0.02 c | 0.94 ± 0.02 d | 0.50 ± 0.05 a | 0.48 ± 0.00 bc | 0.81 ± 0.03 bc | 0.82 ± 0.04 b |
| Torrontes | 0.64 ± 0.01 b | 0.49 ± 0.02 d | 0.58 ± 0.07 a | 0.75 ± 0.01 bc | 0.63 ± 0.01 abc | 0.41 ± 0.00 a | 0.59 ± 0.02 a | 0.51 ± 0.00 a |
| Viognier | 0.68 ± 0.02 b | 0.33 ± 0.05 abc | 0.99 ± 0.01 c | 0.37 ± 0.04 a | 0.64 ± 0.02 bc | 0.52 ± 0.03 c | 0.57 ± 0.02 a | 0.52 ± 0.00 a |
| S-(−)-limonene pair | (2R,4S)-(+)-cis-rose oxide pair | (2R,4R)-(−)-trans-rose oxide pair | (2R,5R)-(+)-trans-linalool oxide pair | (2R,5S)-(−)-cis-linalool oxide pair | S-(−)-nerol oxide pair | R-(−)-linalool pair | S-(−)-α-terpineol pair | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Slope | R2 | Slope | R2 | Slope | R2 | Slope | R2 | Slope | R2 | Slope | R2 | Slope | R2 | Slope | |
| Chardonnay | - | - | - | - | - | - | 0.93 | 0.40 | 0.89 | 0.47 | 1.00 | −1.6 × 10−34 | 0.97 | 0.94 | 0.35 | 0.45 |
| Gewürztraminer | 0.78 | 0.51 | 0.27 | 2.66 | 0.02 | 0.06 | 0.97 | 0.80 | 0.82 | 0.21 | 1.00 | 1.39 | 0.92 | 1.19 | 1.00 | 1.08 |
| Muscat | 0.94 | 0.54 | 0.27 | 0.50 | 0.80 | 0.42 | 0.73 | 0.35 | 0.88 | 0.48 | 1.00 | 1.32 | 0.99 | 0.86 | 1.00 | 0.86 |
| Pinot gris | - | - | 0.89 | 1.91 | 1.00 | 0.69 | 0.90 | 0.51 | 0.91 | 0.47 | 1.00 | 1.03 | 1.00 | 0.58 | 0.02 | 0.32 |
| Riesling | 0.40 | 0.31 | 0.32 | 0.29 | 0.51 | 0.31 | 0.92 | 0.25 | 0.88 | 0.78 | 1.00 | 1.37 | 0.97 | 1.02 | 0.86 | 0.92 |
| Sauvignon blanc | - | - | 0.71 | 0.74 | 1.00 | 0.59 | 0.53 | 0.13 | 0.78 | 0.73 | 1.00 | 1.07 | 0.99 | 0.95 | 0.30 | 0.38 |
| Torrontes | 0.48 | 0.49 | 0.61 | 0.97 | 0.92 | 0.33 | 0.88 | 0.34 | 0.97 | 0.58 | 0.99 | 1.56 | 0.85 | 0.86 | 0.97 | 0.88 |
| Viognier | 0.91 | 0.49 | 1.00 | 1.5 × 10−17 | - | - | 0.89 | 1.14 | 0.60 | 0.37 | 0.89 | 1.07 | 0.86 | 0.87 | 0.96 | 0.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Fuentes, C.; Loos, A.; Tomasino, E. Free Monoterpene Isomer Profiles of Vitis Vinifera L. cv. White Wines. Foods 2018, 7, 27. https://doi.org/10.3390/foods7020027
Song M, Fuentes C, Loos A, Tomasino E. Free Monoterpene Isomer Profiles of Vitis Vinifera L. cv. White Wines. Foods. 2018; 7(2):27. https://doi.org/10.3390/foods7020027
Chicago/Turabian StyleSong, Mei, Claudio Fuentes, Athena Loos, and Elizabeth Tomasino. 2018. "Free Monoterpene Isomer Profiles of Vitis Vinifera L. cv. White Wines" Foods 7, no. 2: 27. https://doi.org/10.3390/foods7020027
APA StyleSong, M., Fuentes, C., Loos, A., & Tomasino, E. (2018). Free Monoterpene Isomer Profiles of Vitis Vinifera L. cv. White Wines. Foods, 7(2), 27. https://doi.org/10.3390/foods7020027




