Analysis of Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Organs of Carotenoid-Supplemented Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Carotenoid Standards and Solvents
2.2. Supplementation and Feeding
2.3. Sample Preparation
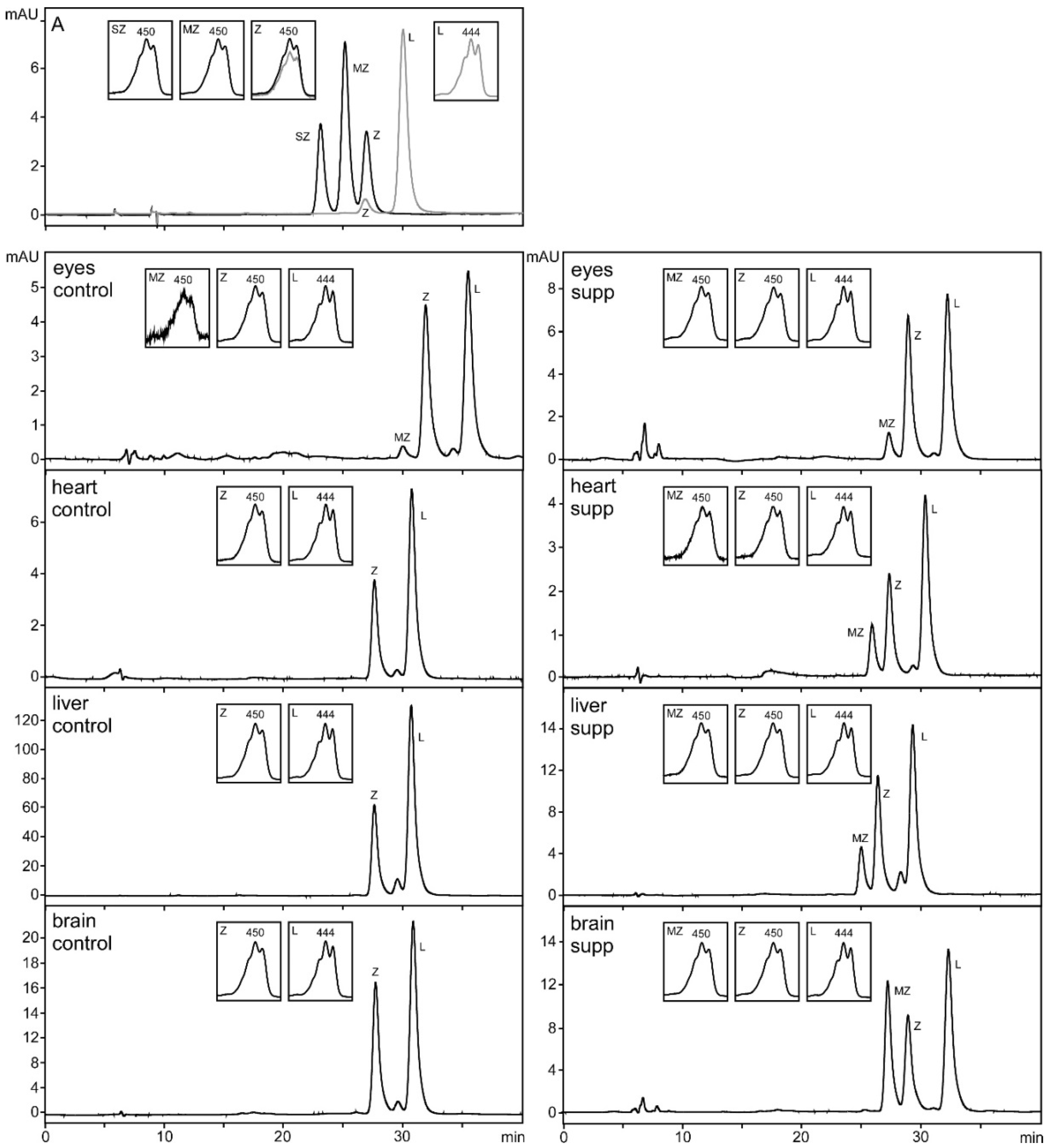
2.4. HPLC Analysis
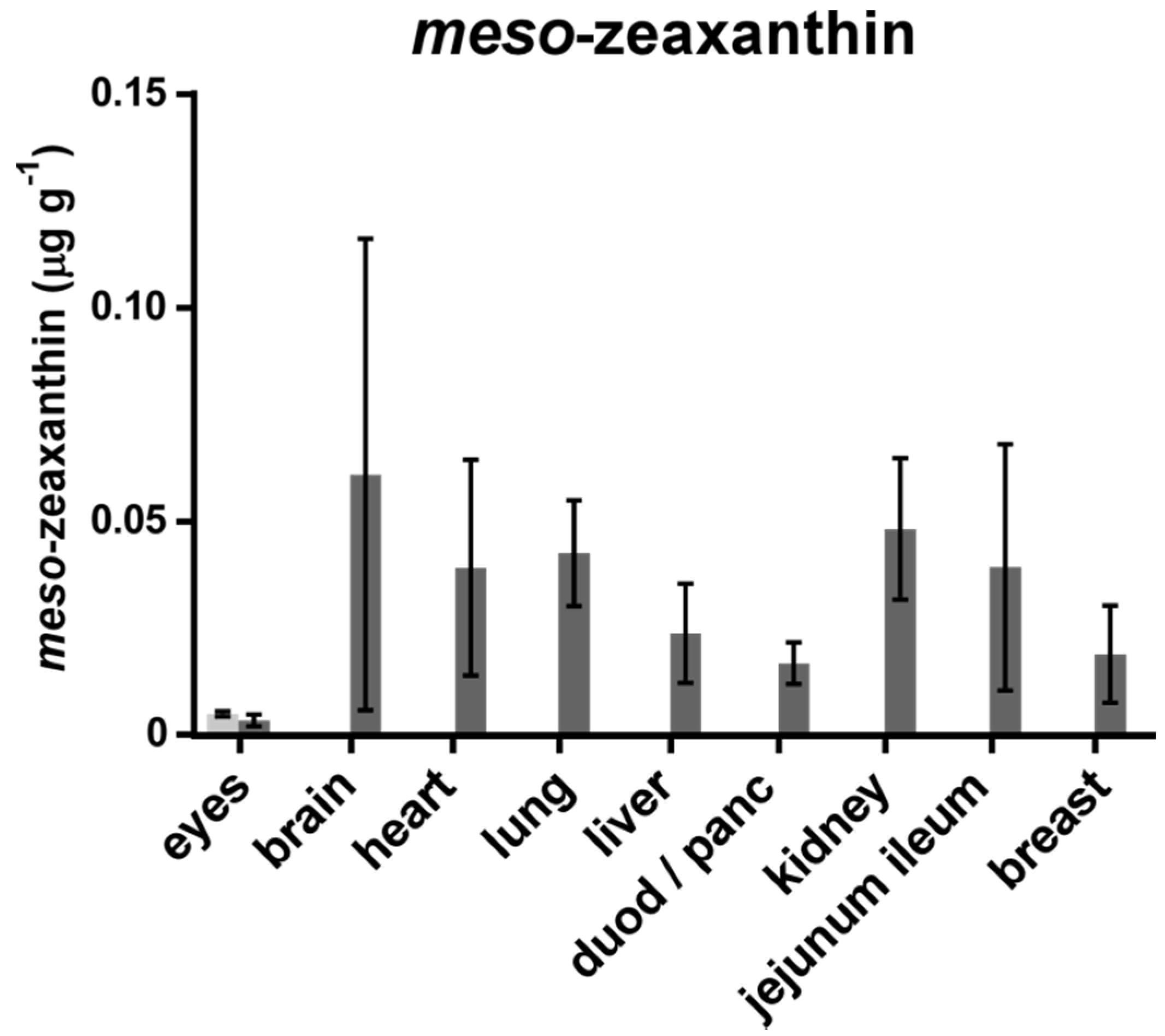
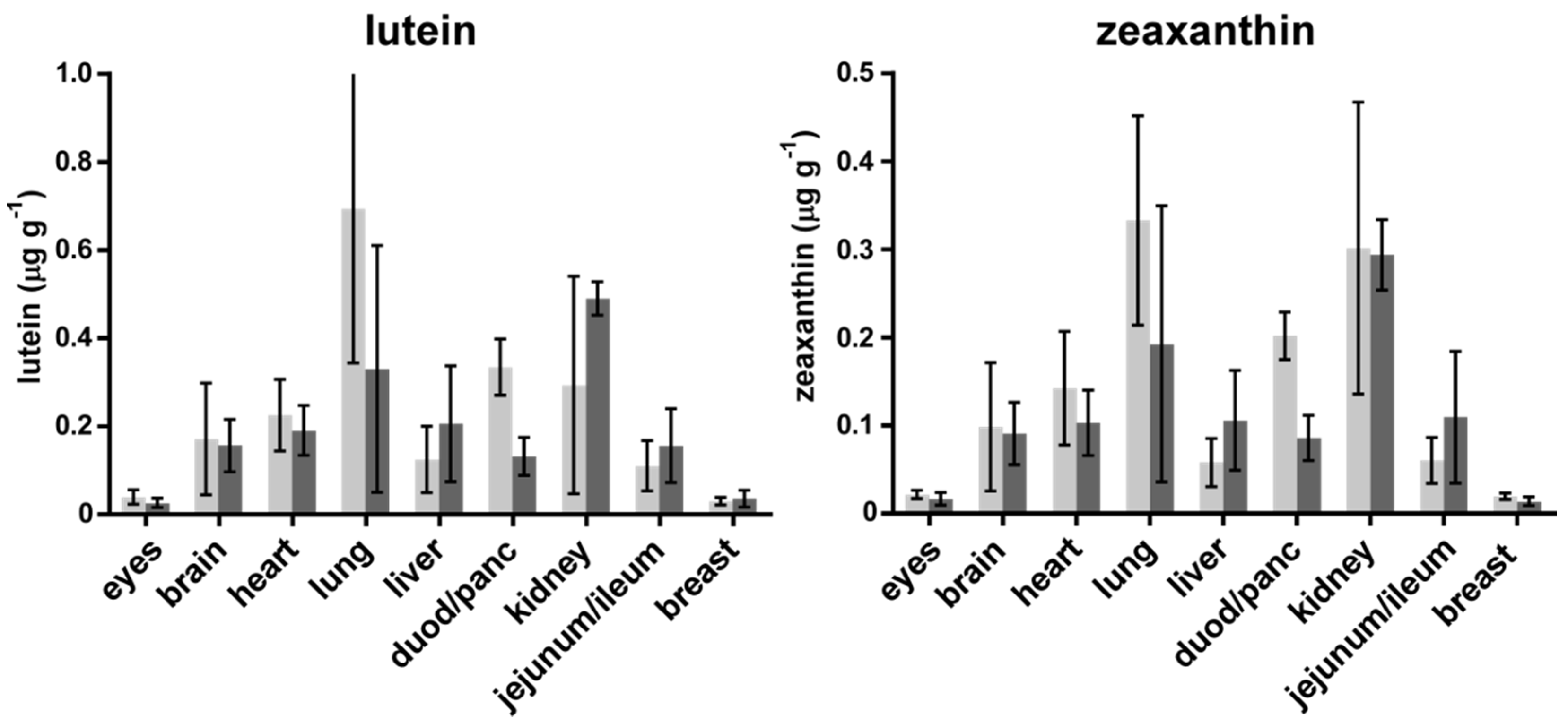
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retinal Eye Res. 2016, 50, 34–66. [Google Scholar]
- Mercadante, A.; Egeland, E.; Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Handbook; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Springer Science & Business Media: Berlin, Germany, 2004. [Google Scholar]
- Furr, H.C.; Clark, R.M. Intestinal absorption and tissue distribution of carotenoids. J. Nutr. Biochem. 1997, 8, 364–377. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Mares-Perlman, J.A.; Wooten, B.R.; Oxton, L.; Gruber, N. Carotenoids and age-related eye disease: Evaluation of a method for measurement of macular pigment in large samples of older subjects. Investig. Ophthalmol. Vis. Sci. 2002, 43, 4394. [Google Scholar]
- Crosby-Nwaobi, R.; Hykin, P.; Peto, T.; Sivaprasad, S. An exploratory study evaluating the effects of macular carotenoid supplementation in various retinal diseases. Clin. Ophthalmol. (Auckland N.Z.) 2016, 10, 835–844. [Google Scholar]
- Akuffo, K.; Nolan, J.; Howard, A.; Moran, R.; Stack, J.; Klein, R.; Klein, B.; Meuer, S.; Sabour-Pickett, S.; Thurnham, D. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye 2015, 29, 902–912. [Google Scholar] [PubMed]
- Nolan, J.M.; Power, R.; Stringham, J.; Dennison, J.; Stack, J.; Kelly, D.; Moran, R.; Akuffo, K.O.; Corcoran, L.; Beatty, S. Enrichment of Macular Pigment Enhances Contrast Sensitivity in Subjects Free of Retinal Disease: Central Retinal Enrichment Supplementation Trials—Report 1. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Trieschmann, M.; Beatty, S.; Nolan, J.M.; Hense, H.W.; Heimes, B.; Austermann, U.; Fobker, M.; Pauleikhoff, D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: The LUNA study. Exp. Eye Res. 2007, 84, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ahmed, F.; Bernstein, P.S. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch. Biochem. Biophys. 2010, 504, 56–60. [Google Scholar] [PubMed]
- Johnson, E.J.; Maras, J.E.; Rasmussen, H.M.; Tucker, K.L. Intake of lutein and zeaxanthin differ with age, sex, and ethnicity. J. Am. Diet. Assoc. 2010, 110, 1357–1362. [Google Scholar] [PubMed]
- Nebeling, L.C.; Forman, M.R.; Graubard, B.I.; Snyder, R.A. Changes in carotenoid intake in the United States: The 1987 and 1992 National Health Interview Surveys. J. Am. Diet. Assoc. 1997, 97, 991–996. [Google Scholar] [CrossRef]
- Maoka, T.; Arai, A.; Shimizu, M.; Matsuno, T. The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp. Biochem. Physiol. B Comp. Biochem. 1986, 83, 121–124. [Google Scholar] [CrossRef]
- Khachik, F.; de Moura, F.F.; Zhao, D.Y.; Aebischer, C.P.; Bernstein, P.S. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3383–3392. [Google Scholar]
- Prado-Cabrero, A.; Beatty, S.; Stack, J.; Howard, A.; Nolan, J.M. Quantification of zeaxanthin stereoisomers and lutein in trout flesh using chiral high-performance liquid chromatography-diode array detection. J. Food Compos. Anal. 2016, 50, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Beatty, S.; Meagher, K.A.; Howarth, P.A.; Kelly, D.; Thurnham, D.I. Verification of Meso-Zeaxanthin in Fish. J. Food Process. Technol. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.; Meagher, K.; Kashani, S.; Beatty, S. What is meso-zeaxanthin, and where does it come from? Eye 2013, 27, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Gorusupudi, A.; Nelson, K.; Horvath, M.P.; Bernstein, P.S. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc. Natl. Acad. Sci. USA 2017, 114, 10882–10887. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Ito, Y.; Sasaki, R.; Ohtani, M.; Hamajima, N.; Asano, A. Smoking, alcohol drinking and serum carotenoids levels. Jpn. J. Cancer Res. 1987, 78, 1049–1056. [Google Scholar] [PubMed]
- Bone, R.A.; Landrum, J.T.; Dixon, Z.; Chen, Y.; Lerena, C.M. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp. Eye Res. 2000, 71, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.D.; Curran-Celentano, J.; Wenzel, A.J. Diet and serum carotenoid concentrations affect macular pigment optical density in adults 45 years and older. J. Nutr. 2005, 135, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Min, K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer's disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T. Distribution of macular pigment components, zeaxanthin and lutein, in human retina. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1992; Volume 213, pp. 360–366. [Google Scholar]
- Handelman, G.J.; Snodderly, D.M.; Krinsky, N.I.; Russett, M.D.; Adler, A.J. Biological control of primate macular pigment. Biochemical and densitometric studies. Investig. Ophthalmol. Vis. Sci. 1991, 32, 257–267. [Google Scholar]
- Lee, C.M.; Boileau, A.C.; Boileau, T.W.; Williams, A.W.; Swanson, K.S.; Heintz, K.A.; Erdman, J.W., Jr. Review of animal models in carotenoid research. J. Nutr. 1999, 129, 2271–2277. [Google Scholar] [PubMed]
- Moreno, J.A.; Diaz-Gomez, J.; Nogareda, C.; Angulo, E.; Sandmann, G.; Portero-Otin, M.; Serrano, J.C.; Twyman, R.M.; Capell, T.; Zhu, C.; et al. The distribution of carotenoids in hens fed on biofortified maize is influenced by feed composition, absorption, resource allocation and storage. Sci. Rep. 2016, 6, 35346. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Meagher, K.A.; Howard, A.N.; Moran, R.; Thurnham, D.I.; Beatty, S. Lutein, zeaxanthin and meso-zeaxanthin content of eggs laid by hens supplemented with free and esterified xanthophylls. J. Nutr. Sci. 2016, 5, e1. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K. Hen egg carotenoids (lutein and zeaxanthin) and nutritional impacts on human health: A review. CyTA J. Food 2017, 15, 474–487. [Google Scholar] [CrossRef]
- Gorusupudi, A.; Shyam, R.; Li, B.; Vachali, P.; Subhani, Y.K.; Nelson, K.; Bernstein, P.S. Developmentally Regulated Production of meso-Zeaxanthin in Chicken Retinal Pigment Epithelium/Choroid and Retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vachali, P.; Bernstein, P.S. Human ocular carotenoid-binding proteins. Photochem. Photobiological Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2010, 9, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Whittow, G.C. Sturkie’s Avian Physiology; Elsevier Inc.: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Karadas, F.; Wood, N.A.R.; Surai, P.F.; Sparks, N.H.C. Tissue-specific distribution of carotenoids and vitamin E in tissues of newly hatched chicks from various avian species. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Fisinin, V.I.; Karadas, F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Kelly, D.; Nolan, J.M.; Howard, A.N.; Stack, J.; Akuffo, K.O.; Moran, R.; Thurnham, D.I.; Dennison, J.; Meagher, K.A.; Beatty, S. Serum and macular response to carotenoid-enriched egg supplementation in human subjects: The Egg Xanthophyll Intervention clinical Trial (EXIT). Br. J. Nutr. 2017, 117, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Connor, S.L.; Wang, W.; Johnson, E.J.; Connor, W.E. The selective retention of lutein, meso-zeaxanthin and zeaxanthin in the retina of chicks fed a xanthophyll-free diet. Exp. Eye Res. 2007, 84, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Roger Illingworth, D.; Connor, S.L.; Barton Duell, P.; Connor, W.E. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur. J. Nutr. 2010, 49, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Vachali, P.; Gorusupudi, A.; Nelson, K.; Bernstein, P.S. All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids. Arch. Biochem. Biophys. 2017, 634, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and Characterization of a Pi Isoform of Glutathione S-Transferase (GSTP1) as a Zeaxanthin-binding Protein in the Macula of the Human Eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Firdous, A.P.; Kuttan, G.; Kuttan, R. Anti-inflammatory potential of carotenoid meso-zeaxanthin and its mode of action. Pharm. Biol. 2015, 53, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Power, R.; Coen, R.F.; Beatty, S.; Mulcahy, R.; Moran, R.; Stack, J.; Howard, A.N.; Nolan, J.M. Supplemental Retinal Carotenoids Enhance Memory in Healthy Individuals with Low Levels of Macular Pigment in A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Alzheimer’s Dis. 2018, 61, 947–961. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Analysis of Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Organs of Carotenoid-Supplemented Chickens. Foods 2018, 7, 20. https://doi.org/10.3390/foods7020020
Phelan D, Prado-Cabrero A, Nolan JM. Analysis of Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Organs of Carotenoid-Supplemented Chickens. Foods. 2018; 7(2):20. https://doi.org/10.3390/foods7020020
Chicago/Turabian StylePhelan, David, Alfonso Prado-Cabrero, and John M. Nolan. 2018. "Analysis of Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Organs of Carotenoid-Supplemented Chickens" Foods 7, no. 2: 20. https://doi.org/10.3390/foods7020020
APA StylePhelan, D., Prado-Cabrero, A., & Nolan, J. M. (2018). Analysis of Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Organs of Carotenoid-Supplemented Chickens. Foods, 7(2), 20. https://doi.org/10.3390/foods7020020






