The Composition and Biological Activity of Honey: A Focus on Manuka Honey
Abstract
:1. Introduction
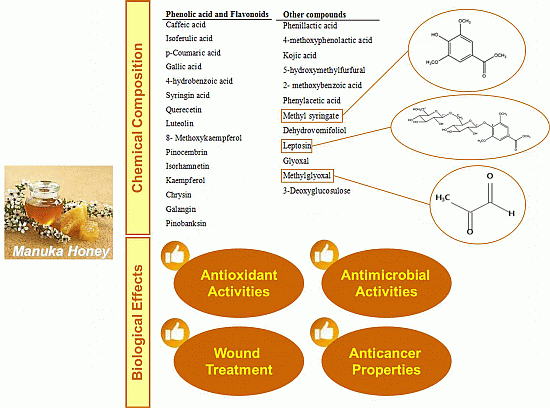
2. Chemical Composition
| Phenolic Acid and Flavonoids | Ref. | Other Compounds | Ref. |
|---|---|---|---|
| Caffeic acid | [12,13] | Phenyllactic acid | [13] |
| Isoferulic acid | [12] | 4-Methoxyphenolactic acid | [13] |
| p-Coumaric acid | [12] | Kojic acid | [13] |
| Gallic acid | [13,17] | 5-Hydroxymethylfurfural | [13] |
| 4-Hydrobenzoic acid | [13] | 2-Methoxybenzoic acid | [13] |
| Syringin acid | [13] | Phenylacetic acid | [13] |
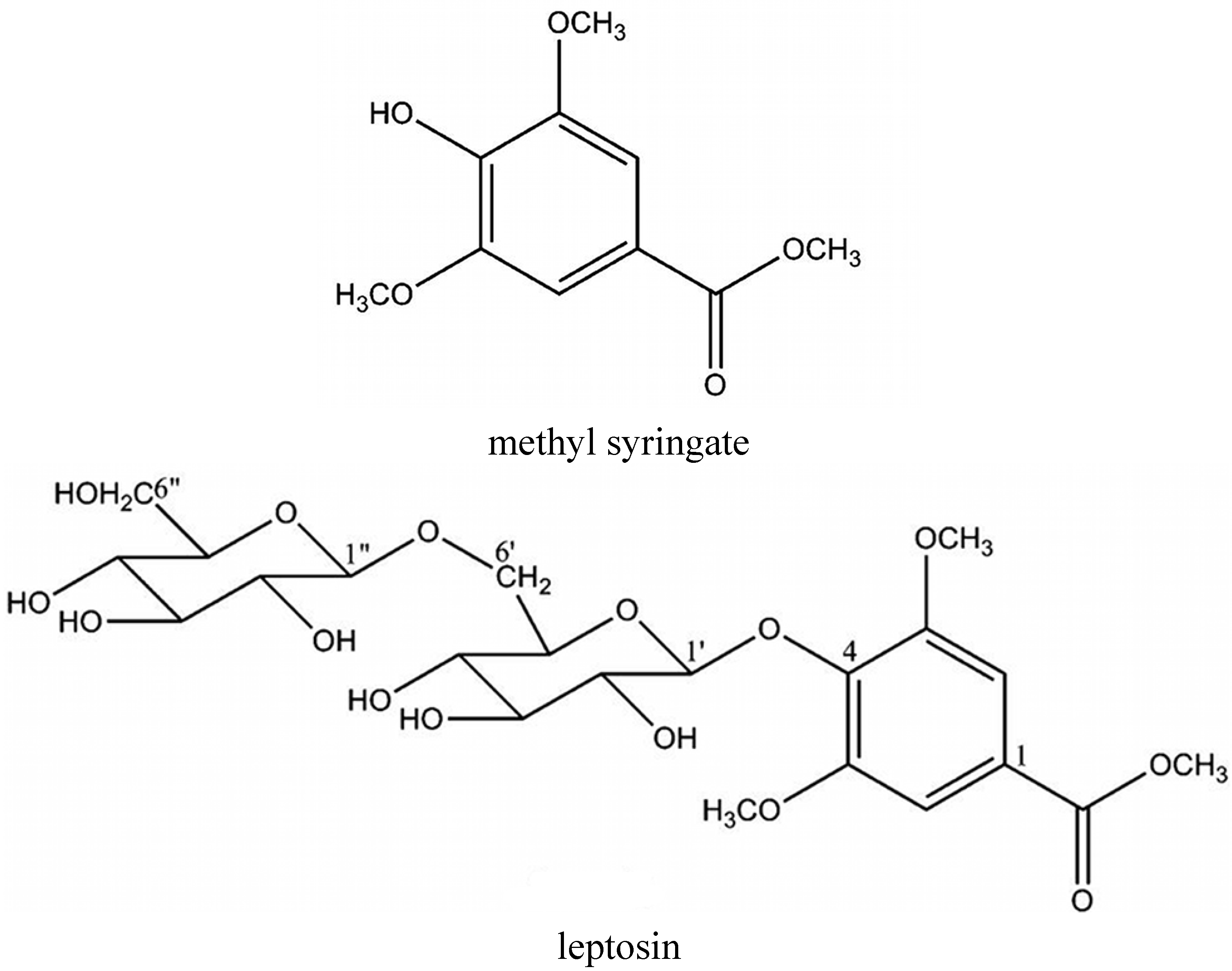
| Quercetin | [12,17] | Methyl syringate | [13] |
| Luteolin | [12,13] | Dehydrovomifoliol | [13] |
| 8-Methoxykaempferol | [12] | Leptosin | [13] |
| Pinocembrin | [12] | Glyoxal | [13,16] |
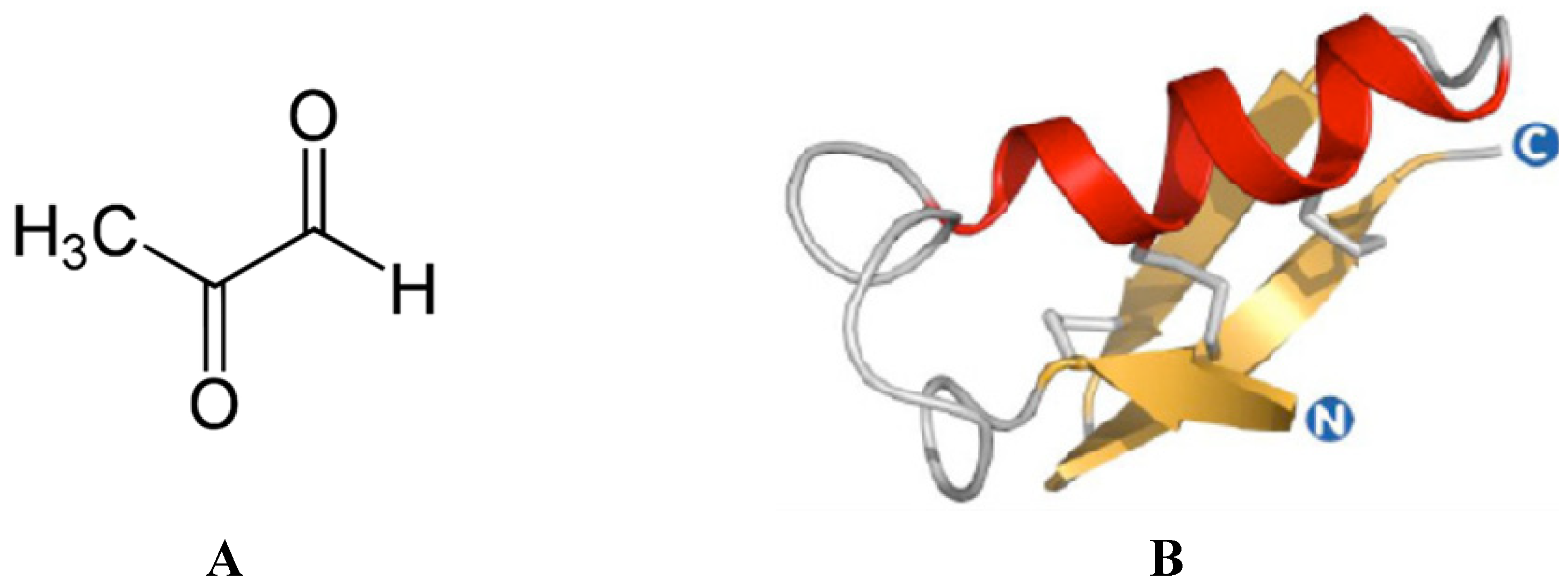
| Isorhamnetin | [12,17] | Methylglyoxal | [13,16] |
| Kaempferol | [12] | 3-Deoxyglucosulose | [13,16] |
| Chrysin | [12] | - | - |
| Galangin | [12] | - | - |
| Pinobanksin | [12] | - | - |
3. Use of Manuka Honey in Wound Treatments
| Gram Positive Strains | Gram Negative Strains |
|---|---|
| Streptococcus pyogenes | Stenotrophomonas maltophilia |
| Coagulase negative staphylococci | Acinetobacter baumannii |
| Methicillin-resistant Staphylococcus aureus (MRSA) | Salmonella enterica serovar typhi |
| Streptococcus agalactiae | Pseudomonas aeruginosa |
| Staphylococcus aureus | Proteus mirabilis |
| Coagulase-negative Staphylococcus aureus (CONS) | Shigella flexneri |
| Hemolytic streptococci | Escherichia coli |
| Enterococcus | Enterobacter cloacae |
| Streptococcus mutans | Shigella sonnei |
| Streptococcus sobrinus | Salmonella typhi |
| Actinomyces viscosus | Klebsiella pneumonia |
| - | Stenotrophomonas maltophilia |
| - | Burkholderia cepacia |
| - | Helicobacter pylori |
| - | Campylobacter spp. |
| - | Porphyromonas gingivalis |
4. Antioxidant Activity
5. Other Effects
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. Am. J. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Mediterr. J. Nutr. Metab. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; González-Paramás, A.M.; Santos-Buelga, C.; Battino, M. Antioxidant characterization of native monofloral Cuban honeys. J. Agric. Food Chem. 2010, 58, 9817–9824. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; González-Paramás, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Umeda, N.; Maeda, A.; Matsumoto, D.; Kitamoto, N.; Kikuzaki, H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J. Agric. Food Chem. 2012, 60, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Cichello, S. Manuka honey: An emerging natural food with medicinal use. Nat. Prod. Bioprospect. 2013, 3, 121–128. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Ferreres, F.; Garcia-Viguera, C.; Tomas-Lorente, F. Flavonoids in honey of different geographical origin. Z. Lebensm. Unters. Forsch. 1993, 196, 38–44. [Google Scholar] [CrossRef]
- Yaoa, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids in Australian Melaleuca, Guioa, Laphostemon, Banksia and Helianthus honeys and their potential for floral authentication. Food Res. Internat. 2005, 38, 651–658. [Google Scholar] [CrossRef]
- Chan, C.W.; Deadman, B.J.; Manley-Harris, M.; Wilkins, A.L.; Alber, D.G.; Harry, E. Analysis of the flavonoid component of bioactive New Zealand manuka (Leptospermum scoparium) honey and the isolation, characterisation and synthesis of an unusual pyrrole. Food Chem. 2013, 141, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaegel, S.; Gruner, M.; Wang, P.; Boettcher, A.; Koelling-Speer, I.; Speer, K. Classification and characterization of Manuka honeys based on phenolic compounds and methylglyoxal. J. Agric. Food Chem. 2012, 60, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.; Bifulco, E.; Jerkovic, I.; Caboni, P.; Cabras, P.; Floris, I. Methyl syringate: A chemical marker of asphodel (Asphodelus microcarpus Salzm. et Viv.) monofloral honey. J. Agric. Food Chem. 2009, 57, 3895–3900. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic composition and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 2010, 120, 78–86. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Tonks, J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Kovacova, E.; Bılikova, K.; Simuth, J. The immunostimulatory effect of the recombinant apalbumin 1-major honeybee royal jelly protein-on TNFα release. Int. Immunopharmacol. 2006, 6, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, A.J.; van den Worm, E.; van Ufford, H.C.; Halkes, S.B.; Hoekstra, M.J.; Beukelman, C.J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J. Wound Care 2008, 17, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, R.A.; Mesaik, M.A. Anti-inflammatory effect of natural honey on bovine thrombin-induced oxidative burst in phagocytes. Phytother. Res. 2009, 23, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp. Dermatol. 2010, 19, 73–79. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Spence, A.J.; Rodriguez, I.A.; McCool, J.M.; Petrella, R.L.; Garg, K.; Ericksen, J.J.; Bowlin, G.L. A Preliminary study on the potential of manuka honey and platelet-rich plasma in wound healing. Int. J. Biomater. 2012, 2012, 313781. [Google Scholar] [CrossRef] [PubMed]
- Engemann, J.J.; Carmeli, Y.; Cosgrove, S.E.; Fowler, V.G.; Bronstein, M.Z.; Trivette, S.L.; Briggs, J.P.; Sexton, D.J.; Kaye, K.S. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 2003, 36, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Woods, E.; Nutekpor, M.; Bowler, P.; Radford, A.; Cochrane, C. Feature: Prevalence of silver resistance in bacteria isolated from diabetic foot ulcers and efficacy of silver-containing wound dressings. Ostomy Wound Manag. 2008, 54, 30–40. [Google Scholar]
- Loh, J.V.; Percival, S.L.; Woods, E.J.; Williams, N.J.; Cochrane, C. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int. Wound J. 2009, 6, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.C.; Perez, R. Cosmeceuticals and natural products: Wound healing. Clin. Dermatol. 2009, 27, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Cooper, R. Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS One 2012, 7, e45600. [Google Scholar] [PubMed]
- Tomblin, V.; Ferguson, L.R.; Han, D.Y.; Murray, P.; Schlothauer, R. Potential pathway of anti-inflammatory effect by New Zealand honeys. Int. J. Gen. Med. 2014, 7, 149–158. [Google Scholar]
- Visavadia, B.G.; Honeysett, J.; Danford, M.H. Manuka honey dressing: An effective treatment for chronic wound infections. Br. J. Oral Maxillofac. Surg. 2008, 46, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Tonks, A.; Cooper, R.A.; Price, A.J.; Molan, P.C.; Jones, K.P. Stimulation of TNF-alpha release in monocytes by honey. Cytokine 2001, 14, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Riches, D.W. Macrophage involvement in wound repair, remodeling and fibrosis. In The Molecular and Cellular Biology of Wound Repair; Clarke, R., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 95–141. [Google Scholar]
- Tonks, J.; Dudley, E.; Porter, N.G.; Parton, J.; Brazier, J.; Smith, E.L.; Tonks, A. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J. Leukoc. Biol. 2007, 82, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Hern, T.T.; Rosliza, A.R.; Siew, H.G.; Ahmad, S.H.; Siti, A.H.; Siti, A.S.; Kirnpal-Kaur, B.S. The antibacterial properties of Malaysian tualang honey. BMC Complement. Altern. Med. 2009, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.K.; Hoekstra, M.J.; Hage, J.; Karim, R.B. Honey-medicated dressing: Transformation of an ancient remedy into modern therapy. Ann. Plast. Surg. 2003, 50, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Shupp, J.W.; Nasabzadeh, T.J.; Rosenthal, D.S.; Jordan, M.H.; Fidler, P.; Jeng, J.C. A review of the local pathophysiologic bases of burn wound progression. J. Burn Care Res. 2010, 31, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, H.O.; Nisbet, C.; Yarim, M.; Guler, A.; Ozak, A. Effects of three types of honey on cutaneous wound healing. Wounds 2010, 22, 275–283. [Google Scholar]
- Molan, P.C. Potential of honey in the treatment of wounds and burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Swiss Model Server. Available online: http://swissmodel.expasy.org (accessed on 8 May 2014).
- Blair, S.E.; Cokcetin, N.N.; Harry, E.J.; Carter, D.A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Alandejani, T.; Marsan, J.G.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Head Neck Surg. 2008, 139, 114–118. [Google Scholar] [CrossRef]
- Maddocks, S.E.; Jenkins, R.E.; Rowlands, R.S.; Purdy, K.J.; Cooper, R.A. Manuka honey inhibits adhesion and invasion of medically important wound bacteria in vitro. Future Microbiol. 2013, 8, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Lopez, M.S.; Rowlands, R.S.; Cooper, R.A. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology 2012, 158, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Bohova, J.; Horniackova, M.; Klaudiny, J.; Majtan, V. Anti-biofilm effects of honey against wound pathogens proteus mirabilis and enterobacter cloacae. Phytother. Res. 2013, 28, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Turnbull, L.; Burke, C.M.; Liu, M.; Carter, D.A.; Schlothauer, R.C.; Whitchurch, C.B.; Harry, E.J. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. Peer J. 2014, 25, e326. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, H.N. Review of the medicinal effects of Tualang honey and a comparison with manuka honey. Malays. J. Med. Sci. 2013, 20, 6–13. [Google Scholar] [PubMed]
- Muller, P.; Alber, D.G.; Turnbull, L.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Synergism between medihoney and rifampicin against methicillin-resistant Staphylococcus aureus (MRSA). PLoS One 2013, 8, e57679. [Google Scholar] [CrossRef] [PubMed]
- Lusby, P.E.; Coombes, A.; Wilkinson, J.M. Honey: A potent agent for wound healing? J. Wound Ostomy Cont. Nurs. 2002, 29, 295–300. [Google Scholar]
- Al-Waili, N.S.; Salom, K.; Al-Ghamdi, A.A. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. ScientificWorldJournal 2011, 11, 766–787. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.; Jackson, S.; Cooper, R.; Burton, N. Free radical production and quenching in honeys with wound healing potential. J. Antimicrob. Chemother. 2006, 58, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.A.; Boukraa, L.; Bellik, Y.; Abdellah, F.; Bakhotmah, B.A.; Kolayli, S.; Sahin, H. Evaluation of the antioxidant activity of three varieties of honey from different botanical and geographical origins. Glob. J. Health Sci. 2012, 4, 191–196. [Google Scholar] [PubMed]
- Moniruzzaman, M.; Sulaiman, S.A.; Khalil, M.I.; Gan, S.H. Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with manuka honey. Chem. Cent. J. 2013, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Jubri, Z.; Rahim, N.B.; Aan, G.J. Manuka honey protects middle-aged rats from oxidative damage. Clinics 2013, 68, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abdellah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Coyle, S.; Warnock, M.; Gow, I.; Fyfe, L. Anti-microbial activity and composition of manuka and portobello honey. Phytother. Res. 2013, 27, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic acid composition and antioxidant properties of Malaysian honeys. J. Food Sci. 2011, 76, 921–928. [Google Scholar] [CrossRef]
- Inoue, K.; Murayama, S.; Seshimo, F.; Takeba, K.; Yoshimura, Y.; Nakazawa, H. Identification of phenolic compound in manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. J. Sci. Food Agric. 2005, 85, 872–878. [Google Scholar] [CrossRef]
- Fukuda, M.; Kobayashi, K.; Hirono, Y.; Miyagawa, M.; Ishida, T.; Ejiogu, E.C.; Sawai, M.; Pinkerton, K.E.; Takeuchi, M. Jungle honey enhances immune function and antitumor activity. Evid. Based Complement. Alternat. Med. 2011, 2011, 908743. [Google Scholar] [CrossRef] [PubMed]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Swellam, T.; Miyanaga, N.; Onozawa, M.; Hattori, K.; Kawai, K.; Shimazui, T.; Akaza, H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int. J. Urol. 2003, 10, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.; Eady, S.; Miles, M.; Martin, H.; McLachlan, A.; Rodier, M.; Willis, J.; Scott, R.; Sutherland, J. Demonstrating the safety of manuka honey UMF 20 in a human clinical trial with healthy individuals. Br. J. Nutr. 2010, 103, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420-432. https://doi.org/10.3390/foods3030420
Alvarez-Suarez JM, Gasparrini M, Forbes-Hernández TY, Mazzoni L, Giampieri F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods. 2014; 3(3):420-432. https://doi.org/10.3390/foods3030420
Chicago/Turabian StyleAlvarez-Suarez, José M., Massimiliano Gasparrini, Tamara Y. Forbes-Hernández, Luca Mazzoni, and Francesca Giampieri. 2014. "The Composition and Biological Activity of Honey: A Focus on Manuka Honey" Foods 3, no. 3: 420-432. https://doi.org/10.3390/foods3030420
APA StyleAlvarez-Suarez, J. M., Gasparrini, M., Forbes-Hernández, T. Y., Mazzoni, L., & Giampieri, F. (2014). The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods, 3(3), 420-432. https://doi.org/10.3390/foods3030420