High Pressure Processing of Bivalve Shellfish and HPP’s Use as a Virus Intervention †
Abstract
:1. Introduction
1.1. Toxins
1.2. Vibrio
2. Fecal Bacteria and Sanitary Standards for Shellfish
3. HPP as a Microbiologic Intervention
3.1. Bacteria and Spoilage
3.2. Spores and Fungi
3.3. Vibrio
3.4. Fecal Bacteria
3.5. Viruses
3.6. Parameters
3.7. Inactivation Mechanisms
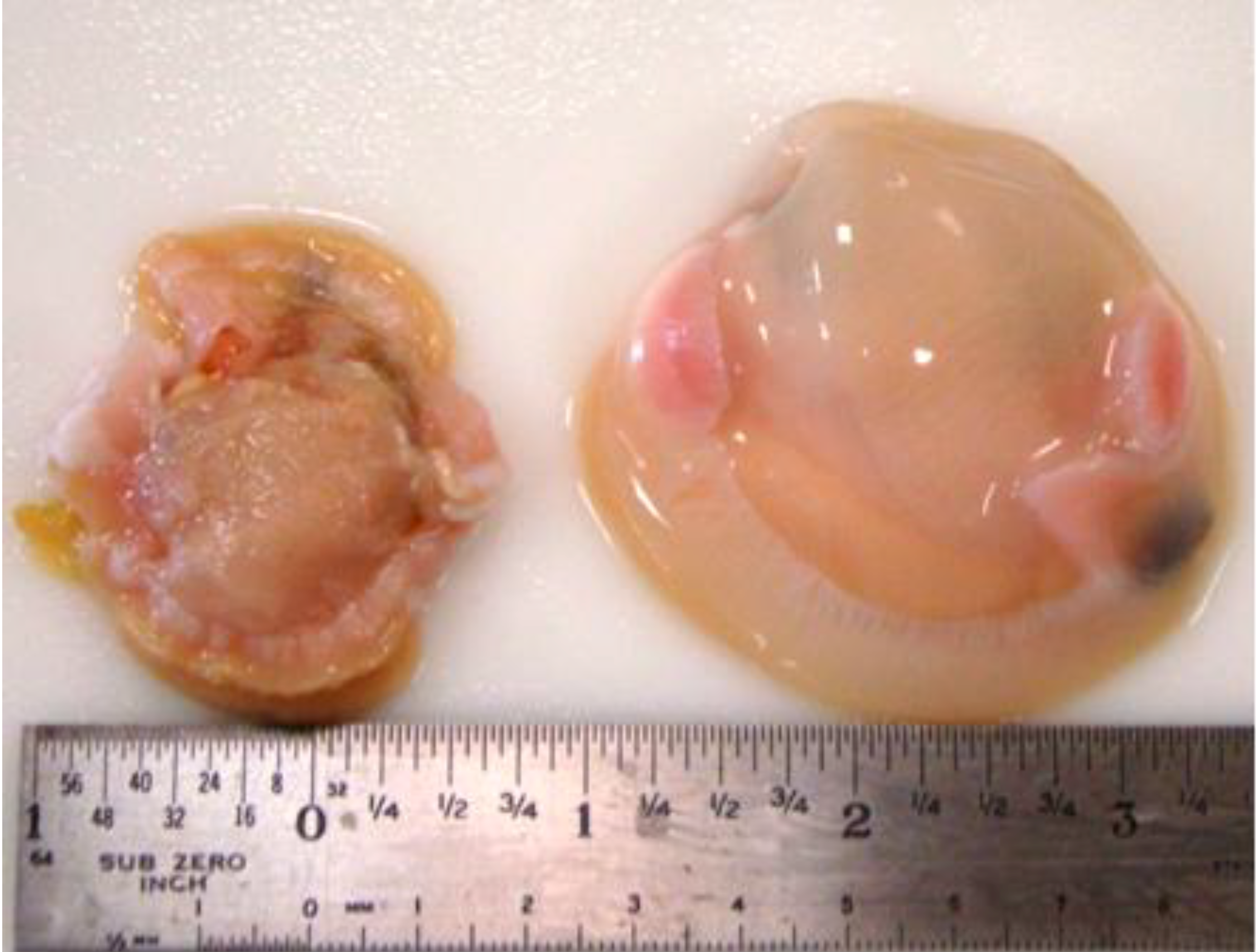
3.8. Organoleptic Considerations
| Control | 300 MPa 22 °C | 400 MPa 22 °C | 500 MPa 22 ° C | 400 MPa 6 °C | 500 MPa 6 °C | 600 MPa 6 °C | F value (Sig p value) | |
|---|---|---|---|---|---|---|---|---|
| Appearance | 4.11 ± 1.6 | 5.46 ± 1.5 | 5.39 ± 1.4 | 5.20 ± 1.6 | 5.39 ± 1.5 | 5.45 ± 1.4 | 5.22 ± 1.7 | 5.78 (0.000) |
| Texture | 4.54 ± 1.9 | 5.23 ± 1.7 | 5.36 ± 1.8 | 5.55 ± 1.6 | 5.20 ± 1.6 | 5.47 ± 1.5 | 5.43 ± 1.7 | 2.46 (0.024) |
| Flavor | 4.64 ± 1.7 | 5.04 ± 1.8 | 5.05 ± 1.7 | 5.13 ± 1.7 | 4.86 ± 1.6 | 5.35 ± 1.6 | 5.27 ± 1.6 | 1.24 (0.287) |
| Aroma | 4.90 ± 1.4 | 5.27 ± 1.3 | 5.04 ± 1.2 | 5.30 ± 1.3 | 5.27 ± 1.4 | 5.33 ± 1.3 | 5.33 ± 1.4 | 0.94 (0.469) |
| Acceptability | 4.64 ± 1.6 | 5.14 ± 1.6 | 5.13 ± 1.6 | 5.28 ± 1.6 | 5.02 ± 1.5 | 5.53 ± 1.4 | 5.38 ± 1.6 | 2.05 (0.058) |
3.9. Challenges and Future Directions
4. Conclusions
Conflicts of Interest
References
- Martin, D.E.; Hall, S.G. Oyster shucking technologies: Past and present. Int. J. Food Sci. Technol. 2006, 41, 223–232. [Google Scholar] [CrossRef]
- Hicks, D.T.; Pivarnik, L.F.; McDermott, R.; Richard, N.; Hoover, D.G.; Kniel, K.E. Consumer awareness and willingness to pay for high pressure processing of ready-to eat food. J. Food Sci. Ed. Res. 2009, 8, 32–38. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Vandevijvere, S.; van Klaveren, J.; van Oyen, H.; van Loco, J. Exposure to domoic acid through shellfish consumption in Belgium. Environ. Int. 2012, 49, 115–119. [Google Scholar] [CrossRef]
- Ciminello, P.; Fattorusso, E. Bivalve molluscs as vectors of marine biotoxins involved in seafood poisoning. Prog. Mol. Subcell. Biol. 2006, 43, 53–82. [Google Scholar] [CrossRef]
- Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.; van den Top, H.J.; de Boer, J. Marine toxins: Chemistry, toxicity, occurrence and detection, with special reference to the Dutch situation. Toxins 2010, 2, 878–904. [Google Scholar] [CrossRef]
- Hinder, S.L.; Hays, G.C.; Brooks, C.J.; Davies, A.P.; Edwards, M.; Walne, A.W.; Gravenor, M.B. Toxic marine microalgae and shellfish poisoning in the British Isles: History, review of epidemiology and future implications. Environ. Health 2011, 10. [Google Scholar] [CrossRef]
- Jeffery, B.; Barlow, T.; Moizer, K.; Paul, S.; Boyle, C. Amnesic shellfish poison. Food Chem. Toxicol. 2004, 42, 545–557. [Google Scholar] [CrossRef]
- Lee, K.J.; Mok, J.S.; Song, K.C.; Yu, H.; Jung, J.H.; Kim, J.H. Geographic and annual variation in lipophilic shellfish toxins from oysters and mussels along the south coast of Korea. J. Food Prot. 2011, 74, 2127–2133. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef]
- Suzuki, H. Susceptibility of different mice strains to okadaic acid, a diarrhetic shellfish poisoning toxin. Food Addit. Contam. A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1307–1310. [Google Scholar] [CrossRef]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef]
- McNabb, P.S.; Selwood, A.I.; van Ginkel, R.; Boundy, M.; Holland, P.T. Determination of brevetoxins in shellfish by LC/MS/MS: Single-laboratory validation. J. AOAC Int. 2012, 95, 1097–1105. [Google Scholar] [CrossRef]
- Murchie, L.W.; Cruz-Promero, M.; Kerry, J.P.; Linton, M.; Patterson, M.F.; Smiddy, M.; Kelly, A.L. High pressure processing of shellfish: A review of microbiological and other quality aspects. Innov. Food Sci. Emerg. Technol. 2005, 6, 257–270. [Google Scholar] [CrossRef]
- Turner, J.W.; Malayil, L.; Guadagnoli, D.; Cole, D.; Lipp, E.K. Detection of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae with respect to seasonal fluctuations in temperature and plankton abundance. Environ. Microbiol. 2014, 4, 1019–1028. [Google Scholar]
- Motes, M.L.; DePaola, A.; Cook, D.W.; Veazey, J.E.; Hunsucker, J.C.; Garthright, W.E.; Blodgett, R.J.; Chirtel, S.J. Influence of water temperature and salinity on Vibrio vulnificus in northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 1998, 64, 1459–1465. [Google Scholar]
- Desenclos, J.A.; Klontz, K.C.; Wolfe, L.E.; Hoecheri, S. The risk of Vibrio illness in the Florida raw oyster eating population, 1981–1988. Am. J. Epidemiol. 1991, 134, 290–297. [Google Scholar]
- Drake, S.L.; DePaola, A.; Jaykus, L. An overview of Vibrio vulnificus and Vibrio parahaemolyticus. Comp. Rev. Food Sci. Saf. 2007, 6, 120–144. [Google Scholar] [CrossRef]
- Thacket, C.O.; Brenner, F.; Blake, P.A. Clinical features and epidemiological study of Vibrio vulnificus infections. J. Infect. Dis. 1984, 149, 558–561. [Google Scholar] [CrossRef]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bressee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Bishop, R.D.; Baldy, L.M.; Thompson, S.G.; Wilson, S.A.; Ray, B.J.; Griffin, P.M. Vibrio gastroenteritis in the US Gulf of Mexico region: The role of raw oysters. Epidemiol. Infect. 2000, 124, 489–495. [Google Scholar] [CrossRef]
- Cook, D.W. Effect of time and temperature on multiplication of Vibrio vulnificus in postharvest Gulf coast shellstock oysters. Appl. Environ. Microbiol. 1994, 60, 3483–3484. [Google Scholar]
- Cook, D.W.; Ruple, A.D. Indicator bacteria and Vibrionaceae multiplication in post-harvest shellstock oysters. J. Food Prot. 1989, 52, 343–349. [Google Scholar]
- Gooch, J.A.; DePaola, A.; Bowers, J.; Marshall, D.L. Growth and survival of Vibrio parahaemolyticus in postharvest American oysters. J. Food Prot. 2002, 65, 970–974. [Google Scholar]
- Johnson, W.G.; Salinger, A.C.; King, W.C. Survival of Vibrio parahaemolyticus in oyster shellstock at two different storage temperatures. Appl. Microbiol. 1973, 26, 122–123. [Google Scholar]
- Lorca, T.A.; Pierson, M.D.; Flick, G.J.; Hackney, C.R. Levels of Vibrio vulnificus and organoleptic quality of raw shellstock oysters (Crassostrea virginica) maintained at different storage temperatures. J. Food Prot. 2001, 64, 1716–1721. [Google Scholar]
- Burnham, V.E.; Janes, M.E.; Jaykus, L.A.; Supan, J.; DePaola, A.; Bell, J. Growth and survival differences of Vibrio vulnificus and Vibrioparahaemolyticus strains during cold storage. J. Food Sci. 2009, 74, 314–318. [Google Scholar] [CrossRef]
- Cook, D.W. Refrigeration of oyster shellstock: Conditions which minimize the outgrowth of Vibrio vulnificus. J. Food Prot. 1997, 60, 349–352. [Google Scholar]
- Muth, M.K.; Karns, S.A.; Anderson, D.W.; Murray, B.C. Effects of post-harvest treatment requirements for the markets for oysters. Agric. Resour. Econ. Rev. 2002, 31, 171–186. [Google Scholar]
- Jakabi, M.; Gelli, D.S.; Torre, J.C.; Rodas, M.A.; Franco, B.D.; Destro, M.T.; Landgrafi, M. Inactivation by ionizing radiation of Salmonella enteritidis, Salmonella infantis, and Vibrio parahaemolyticus in oysters (Crassostrea brasiliana). J. Food Prot. 2003, 66, 1025–1029. [Google Scholar]
- Liu, C.; Lu, J.; Su, Y. Effects of flash freezing, followed by frozen storage, on reducing Vibrio parahaemolyticus in Pacific raw oysters (Crassostrea gigas). J. Food Prot. 2009, 72, 174–177. [Google Scholar]
- Parker, R.W.; Maurer, E.M.; Childers, A.B.; Lewis, D.H. Effect of frozen storage and vacuum packaging on survival of Vibrio vulnificus in Gulf Coast oysters (Crassostrea virginica). J. Food Prot. 1994, 57, 604–606. [Google Scholar]
- Andrews, L.S.; Park, D.L.; Chen, Y.P. Low temperature pasteurization to reduce the risk of vibrio infections from raw shell-stock oysters. Food. Add. Cont. 2000, 17, 787–791. [Google Scholar]
- Berlin, D.L.; Herson, D.S.; Hicks, D.T.; Hoover, D.G. Response of pathogenic Vibrio species to high hydrostatic pressure. Appl. Environ. Microbiol. 1999, 65, 2776–2780. [Google Scholar]
- Ye, M.; Huang, Y.; Gurtler, J.B.; Niemera, B.A.; Sites, J.E.; Chen, H. Effects of pre- and post-processing storage conditions on high hydrostatic pressure inactivation of Vibrio parahaemolyticus and V. vulnificus in oysters. Int. J. Food Microbiol. 2013, 163, 146–152. [Google Scholar] [CrossRef]
- Rippey, S.R. Infectious diseases associated with molluscan shellfish consumption. Clin. Microbiol. Rev. 1994, 7, 419–425. [Google Scholar]
- Calci, K.R.; DePaola, A.; Burkhardt, W., III. Chapter 49: Molluscan shellfish; oysters, mussels, clams. In Compendium of Methods for the Microbiological Examination of Foods; Doores, S., Salfinger, Y., Tortorello, M.L., Wilcke, B.W., Eds.; American Public Health Association: Washington, DC, USA, 2013. [Google Scholar]
- Olivera, J.; Cunha, A.; Catilho, F.; Romalde, J.L.; Pereira, M.J. Microbial contamination and purification of bivalve shellfish: Crucial aspects in monitoring and future perspectives—A mini-review. Food Cont. 2011, 22, 805–816. [Google Scholar] [CrossRef]
- Hood, M.A.; Ness, G.E.; Blake, N.J. Relationship among fecal coliforms, Escherichia coli and Salmonella spp. in shellfish. Appl. Environ. Microbiol. 1983, 45, 122–126. [Google Scholar]
- Brands, D.A.; Inman, A.E.; Gerba, C.P.; Maré, C.J.; Billington, S.J.; Saif, L.A.; Levine, J.F.; Joens, L.A. Prevalence of Salmonella ssp. in oysters in the United States. Appl. Environ. Microbiol. 2005, 71, 893–897. [Google Scholar] [CrossRef]
- DePaola, A.; Jones, J.L.; Woods, J.; Burkhardt, W., III; Calci, K.R.; Krantz, J.A.; Bowers, J.C.; Kasturi, K.; Byars, R.H.; Jacobs, E.; et al. Bacterial and viral pathogens in live oysters: 2007 United States market survey. Appl. Environ. Microbiol. 2010, 76, 2754–2768. [Google Scholar] [CrossRef]
- Morrison, C.M.; Armstrong, A.E.; Evans, S.; Mild, R.M.; Langdon, C.J.; Joens, L.A. Survival of Salmonella newport in oysters. Int. J. Food Microbiol. 2011, 148, 93–98. [Google Scholar] [CrossRef]
- Linton, M.; McClements, J.M.J.; Patterson, M.F. Changes in the microbial quality of shellfish brought about by treatment with high hydrostatic pressure. Int. J. Food Sci. Technol. 2003, 38, 713–727. [Google Scholar] [CrossRef]
- Smelt, J.P.P.M. Recent advances in the microbiology of high pressure processing. Trends Food Sci. Technol. 1998, 9, 152–158. [Google Scholar] [CrossRef]
- He, H.; Adams, R.M.; Farkas, D.F.; Morrissey, M.T. Use of high pressure processing for oyster shucking and shelf-life extension. J. Food Prot. 2002, 67, 640–645. [Google Scholar]
- Shearer, A.E.; Dunne, C.P.; Sikes, A.; Hoover, D.G. Bacterial spore inhibition and inactivation in foods by pressure, chemical preservatives, and mild heat. J. Food Prot. 2000, 63, 1503–1510. [Google Scholar]
- Kural, A.G.; Shearer, A.E.H.; Kingsley, D.H.; Chen, H. Conditions for high pressure inactivation of Vibrio parahaemolyticus in oysters. Int. J. Food Microbiol. 2008, 127, 1–5. [Google Scholar] [CrossRef]
- Ma, L.; Su, Y.C. Validation of high pressure processing for inactivating Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas). Int. J. Food Microbiol. 2011, 144, 469–474. [Google Scholar] [CrossRef]
- Sherry, A.E.; Patterson, M.F.; Madden, R.H. Comparison of 40 Salmonella enterica servars injured by thermal, high pressure and irradiation stress. J. Appl. Microbiol. 2004, 96, 887–893. [Google Scholar] [CrossRef]
- Griffin, M.R.; Dalley, E.; Fitzpatrick, M.; Austin, S.H. Campylobacter gastroenteritis associated with raw clams. J. Med. Soc. N. J. 1983, 80, 607–609. [Google Scholar]
- Reeve., G.; Martin, D.L.; Pappas, J.; Thompson, R.E.; Greene, K.D. An outbreak of shigellosis associated with the consumption of raw oysters. N. Engl. J. Med. 1989, 321, 224–227. [Google Scholar] [CrossRef]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High pressure processing—Effects on microbial food safety and food quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef]
- Kingsley, D.H. High pressure processing and its application to the challenge of virus-contaminated Foods. Food Environ. Virol. 2013, 5, 1–12. [Google Scholar] [CrossRef]
- Kingsley, D.H. Food-borne noroviruses. In Genomes of Food- and Water-borne Pathogens; Fratamico, P., Kathariou, S., Liu, Y., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 237–245. [Google Scholar]
- Teunis, P.F.; Moe, C.L.; Liu, P.; Miller, S.E.; Lindesmith, L.; Baric, R.S.; le Pendu, J.; Calderon, R.L. Norwalk virus: How infectious is it? J. Med. Virol. 2008, 80, 1468–1476. [Google Scholar]
- Kingsley, D.H.; Richards, G.P. Persistence of hepatitis A virus within oysters. J. Food Prot. 2003, 66, 331–334. [Google Scholar]
- Provost, K.; Dancho, B.A.; Ozbay, G.; Anderson, R.; Richards, G.; Kingsley, D.H. Hemocytes are sites of persistence for enteric viruses within oysters. Appl. Environ. Microbiol. 2011, 77, 8360–8369. [Google Scholar] [CrossRef]
- Love, D.C.; Lovelace, G.L.; Sobsey, M.D. Removal of Escherichia coli, Enterococcus fecalis, coliphage MS2, poliovirus, and hepatitis A virus from oysters (Crassostrea virginica) and hard shell clams (Mercinaria mercinaria) by depuration. Int. J. Food Microbiol. 2010, 143, 211–217. [Google Scholar]
- Grohmann, G.S.; Murphy, A.M.; Christopher, P.J.; Auty, E.; Greenberg, H.B. Norwalk gastroenteritis in volunteers consuming depurated oysters. Aust. J. Exp. Biol. Med. Sci. 1981, 59, 219–228. [Google Scholar] [CrossRef]
- Chalmers, J.W.; McMillan, J.H. An outbreak of viral gastroenteritis associated with adequately prepared oysters. Epidemiol. Infect. 1995, 115, 163–167. [Google Scholar] [CrossRef]
- Kirkland, K.B.; Meriwether, R.A.; Leiss, J.K.; MacKenzie, W.R. Steaming oysters does not prevent Norwalk-like gastroenteritis. Public Health Rep. 1996, 111, 527–530. [Google Scholar]
- McDonnell, S.; Kirkland, K.B.; Hlady, W.G.; Aristeguieta, C.; Hopkins, R.S.; Monroe, S.S.; Glass, R.I. Failure of cooking to prevent shellfish associated gastroenteritis. Arch. Int. Med. 1997, 157, 111–116. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Butt, A.A.; Aldridge, K.E.; Sanders, C.V. Infections related to the ingestion of seafood part I: Viral and bacterial infections. Lancet Infect. Dis. 2004, 4, 201–212. [Google Scholar] [CrossRef]
- Hall, A.J.; Eisenbart, V.G.; Etingüe, A.L.; Gould, L.H.; Lopman, B.A.; Parashar, U.D. Epidemiology of foodborne norovirus outbreaks, United States, 2001–2008. Emerg. Infect. Dis. 2012, 18, 1566–1573. [Google Scholar] [CrossRef]
- Buckow, R.; Isbarn, S.; Knorr, D.; Hienz, V.; Lehmacher, A. Predictive model for inactivation of feline calcivirus, a norovirus surrogate, by heat and high hydrostatic pressure. Appl. Environ. Microbiol. 2008, 74, 1030–1038. [Google Scholar] [CrossRef]
- Chen, H.; Hoover, D.G.; Kingsley, D.H. Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. J. Food Prot. 2005, 68, 2389–2394. [Google Scholar]
- Kingsley, D.H.; Chen, H. Aqueous matrix composition influences feline calicivirus inactivation by high pressure processing. J. Food Prot. 2008, 71, 1598–1603. [Google Scholar]
- Kingsley, D.H.; Hoover, D.; Papafragkou, E.; Richards, G.P. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J. Food Prot. 2002, 65, 1605–1609. [Google Scholar]
- Kingsley, D.H.; Holliman, D.R.; Calci, K.R.; Chen, H.; Flick, G.J. Inactivation of a norovirus by high pressure processing. Appl. Environ. Microbiol. 2007, 73, 581–585. [Google Scholar] [CrossRef]
- Duizer, E.; Schwab, K.J.; Neill, F.H.; Atmar, R.L.; Koopmans, M.P.G.; Estes, M.K. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 2004, 85, 79–87. [Google Scholar] [CrossRef]
- Herbst-Kralovetz, M.M.; Radtke, A.L.; Lay, M.K.; Hjelm, B.E.; Bolick, A.N.; Sarker, S.S.; Atmar, R.L.; Kingsley, D.H.; Arntzen, C.J.; Estes, M.K.; et al. Lack of norovirus replication and histo-blood group antigen expression in 3-D intestinal epithelial cell cultures. Emerg. Infect. Dis. 2013, 19, 431–438. [Google Scholar] [CrossRef]
- Grove, S.F.; Forsyth, S.; Wan, J.; Coventry, J.; Cole, M.; Stewart, C.M.; Lewis, T.; Ross, T.; Lee, A. Inactivation of hepatitis A virus, poliovirus, and a norovirus surrogate by high pressure processing. Innov. Food Sci. Emerg. Technol. 2008, 9, 206–210. [Google Scholar] [CrossRef]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W., IV. Murine norovirus: A model system to study norovirus biology and pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef]
- Arcangeli, G.; Terregino, C.; de Benedictis, P.; Zecchin, B.; Manfrin, A.; Rossetti, E.; Magnabosco, C.; Mancin, M.; Brutti, A. Effect of high hydrostatic pressure on murine norovirus in Manila clams. Lett. Appl. Microbiol. 2012, 54, 325–329. [Google Scholar] [CrossRef]
- Gogal, R.M., Jr.; Kerr, R.; Kingsley, D.H.; Granata, L.A.; LeRoith, T.; Holliman, S.D.; Dancho, B.A.; Flick, G.J., Jr. High hydrostatic pressure processing of murine norovirus-1 contaminated oysters inhibits oral infection in STAT-1−/− deficient female mice. J. Food Prot. 2011, 74, 209–214. [Google Scholar] [CrossRef]
- Leon, J.S.; Kingsley, D.H.; Montes, J.S.; Richards, G.P.; Lyon, G.M.; Abdulhafid, G.M.; Seitz, S.R.; Fernandez, M.L.; Teunis, P.F.; Flick, G.J.; et al. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl. Environ. Microbiol. 2011, 77, 5476–5482. [Google Scholar] [CrossRef]
- Dancho, B.A.; Chen, H.; Kingsley, D.H. A method to discriminate between infectious and inactive human noroviruses. Int. J. Food Microbiol. 2012, 155, 222–226. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Kingsley, D.H. The influence of temperature, pH, and water immersion on the high hydrostatic pressure inactivation of G.1 and GII.4 human noroviruses. Int. J. Food Microbiol. 2013, 167, 138–143. [Google Scholar] [CrossRef]
- Ye, M.; Li, X.; Kingsley, D.H.; Jiang, X.; Chen, H. Inactivation of human norovirus in contaminated oysters and clams by high-hydrostatic pressure. Appl. Environ. Microbiol. 2014, 80, 2248–2253. [Google Scholar] [CrossRef]
- Jacobsen, K.H.; Koopman, J.S. Declining hepatitis A virus seroprevalence: A global review and analysis. Epidemiol. Infect. 2004, 132, 1005–1022. [Google Scholar] [CrossRef]
- Franco, E.; Meleleo, C.; Serino, L.; Sorbara, D.; Zaratti, L. Hepatitis A: Epidemiology and prevention in developing countries. World J. Hepatol. 2012, 4, 68–73. [Google Scholar] [CrossRef]
- Fiore, A.E. Hepatitis A transmitted by food. Clin. Infect. Dis. 2004, 38, 705–715. [Google Scholar] [CrossRef]
- FitzSimons, D.; Hendrickx, G.; Vorsters, A.; van Damme, P. Hepatitis A and E: Update on prevention and epidemiology. Vaccine 2010, 28, 583–588. [Google Scholar] [CrossRef]
- Hepatitis A outbreak associated with green onions at a restaurant—Monaca, Pennsylvania, 2003. Morb. Mortal. Wkly. Rep. 2003, 52, 1155–1157.
- Sánchez, G.; Pintó, R.M.; Vanaclocha, H.; Bosch, A. Molecular characterization of hepatitis A virus isolates from a transcontinental shellfish-borne outbreak. J. Clin. Microbiol. 2002, 40, 4148–4155. [Google Scholar] [CrossRef]
- Calci, K.R.; Meade, G.K.; Tetzloff, R.C.; Kingsley, D.H. High pressure inactivation of hepatitis A virus within oysters. Appl. Environ. Microbiol. 2005, 71, 339–343. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; le Saux, J.C.; Ambert-Balay, K.; Krol, J.; Serais, O.; Parnaudeau, S.; Giraudon, H.; Delmas, G.; Pommepuy, M.; Pothier, P.; et al. Aichi virus, norovirus, astrovirus enterovirus and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J. Clin. Microbiol. 2008, 46, 4011–4017. [Google Scholar] [CrossRef]
- Crossan, C.; Baker, P.J.; Craft, J.; Takeuchi, Y.; Dalton, H.R.; Scobie, L. Hepatitis E virus genotype 3 in shellfish United Kingdom. Emerg. Infect. Dis. 2012, 18, 2085–2087. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Chen, H.; Hoover, D. Inactivation of selected picornaviruses by high hydrostatic pressure. Virus Res. 2004, 102, 221–224. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Li, X.; Chen, H. Temperature effects for high pressure processing of picornaviruses. Food Environ. Virol. 2014, 6, 58–61. [Google Scholar] [CrossRef]
- Chen, H.; Hoover, D.G. Use of Weibull model to describe and predict pressure inactivation of Listeria monocytogenes Scott A in whole milk. Innov. Food Sci. Emerg. Technol. 2004, 5, 269–276. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Chen, H. Influence of pH, salt, and temperature on pressure inactivation of hepatitis A virus. Int. J. Food Microbiol. 2009, 130, 61–64. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Guan, D.; Hoover, D.G.; Chen, H. Inactivation of hepatitis A virus by high pressure processing: The role of temperature and pressure oscillation. J. Food Prot. 2006, 69, 2454–2459. [Google Scholar]
- Kingsley, D.H.; Guan, D.; Hoover, D.G. Pressure inactivation of hepatitis A virus in strawberry puree and sliced green onions. J. Food Prot. 2005, 68, 1748–1751. [Google Scholar]
- Balasubramanian, S.; Balasubramaniam, V.M. Compression heating influence of pressure transmitting fluids on bacteria inactivation during high pressure processing. Food Res. Int. 2003, 36, 661–668. [Google Scholar] [CrossRef]
- Mootian, G.K.; Flimlin, G.E.; Karwe, M.; Schaffner, D.W. Inactivation of Vibrio parahaemolyticus in hard clams (Mercanaria mercanaria) by high hydrostatic pressure (HHP) and the effect of HHP on the physical characteristics of hard clam meat. J. Food Sci. 2013, 78, E251–E257. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2007, 8, 30–38. [Google Scholar] [CrossRef]
- Narwanker, S.P.; Flimlin, G.E.; Schaffner, D.W.; Tepper, B.J.; Karwe, M.V. Microbial safety and consumer acceptability of high-pressure processed hard clams (Mercenaria mercenaria). J. Food Sci. 2011, 76, M375–M380. [Google Scholar] [CrossRef]
- Lopez-Caballero, M.E.; Perez-Mateos, M.; Montero, P.; Borderias, A.J. Oyster preservation by high-pressure treatment. J. Food Prot. 2000, 63, 196–201. [Google Scholar]
- Kingsley, D.H.; Kuhn, D.D.; Flick, G.J.; Oh, J.; Lawson, L.S.; Meade, G.K.; Giesecke, C.C. Desirability of oysters treated by high pressure processing at different temperatures and elevated pressures. Am. J. Food Technol. 2014, in press. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kingsley, D.H. High Pressure Processing of Bivalve Shellfish and HPP’s Use as a Virus Intervention. Foods 2014, 3, 336-350. https://doi.org/10.3390/foods3020336
Kingsley DH. High Pressure Processing of Bivalve Shellfish and HPP’s Use as a Virus Intervention. Foods. 2014; 3(2):336-350. https://doi.org/10.3390/foods3020336
Chicago/Turabian StyleKingsley, David H. 2014. "High Pressure Processing of Bivalve Shellfish and HPP’s Use as a Virus Intervention" Foods 3, no. 2: 336-350. https://doi.org/10.3390/foods3020336
APA StyleKingsley, D. H. (2014). High Pressure Processing of Bivalve Shellfish and HPP’s Use as a Virus Intervention. Foods, 3(2), 336-350. https://doi.org/10.3390/foods3020336




