Use of Donkey Milk in Children with Cow’s Milk Protein Allergy
Abstract
:1. Introduction
| Donkey | Human | |
|---|---|---|
| pH | 7.0–7.2 | 7.0–7.5 |
| Protein (g/100g) | 1.5–1.8 | 0.9–1.7 |
| Fat (g/100 g) | 0.3–1.8 | 3.5–4.0 |
| Lactose (g/100 g) | 5.8–7.4 | 6.3–7.0 |
| Ash (g/100 g) | 0.3–0.5 | 0.2–0.3 |
| Total solids (g/100 g) | 8.8–11.7 | 11.7–12.9 |
| Caseins (g/100 g) | 0.64–1.03 | 0.32–0.42 |
| Whey proteins (g/100 g) | 0.49–0.80 | 0.68–0.83 |
2. Experimental Section
3. Results and Discussion
| Protein | kDa | N-Terminal sequence |
|---|---|---|
| Lysozyme | 14.60 | KVFSKXELA |
| α-lactalbumin | 14.12 | KQFTKXELSQVLXSM |
| β-lactoglobulin | 22.40 | TNIPQTMQ |
| αs1-casein | 33.30 | RPKLPHQPE |
| β-casein | 37.50 | REKEELNVS |
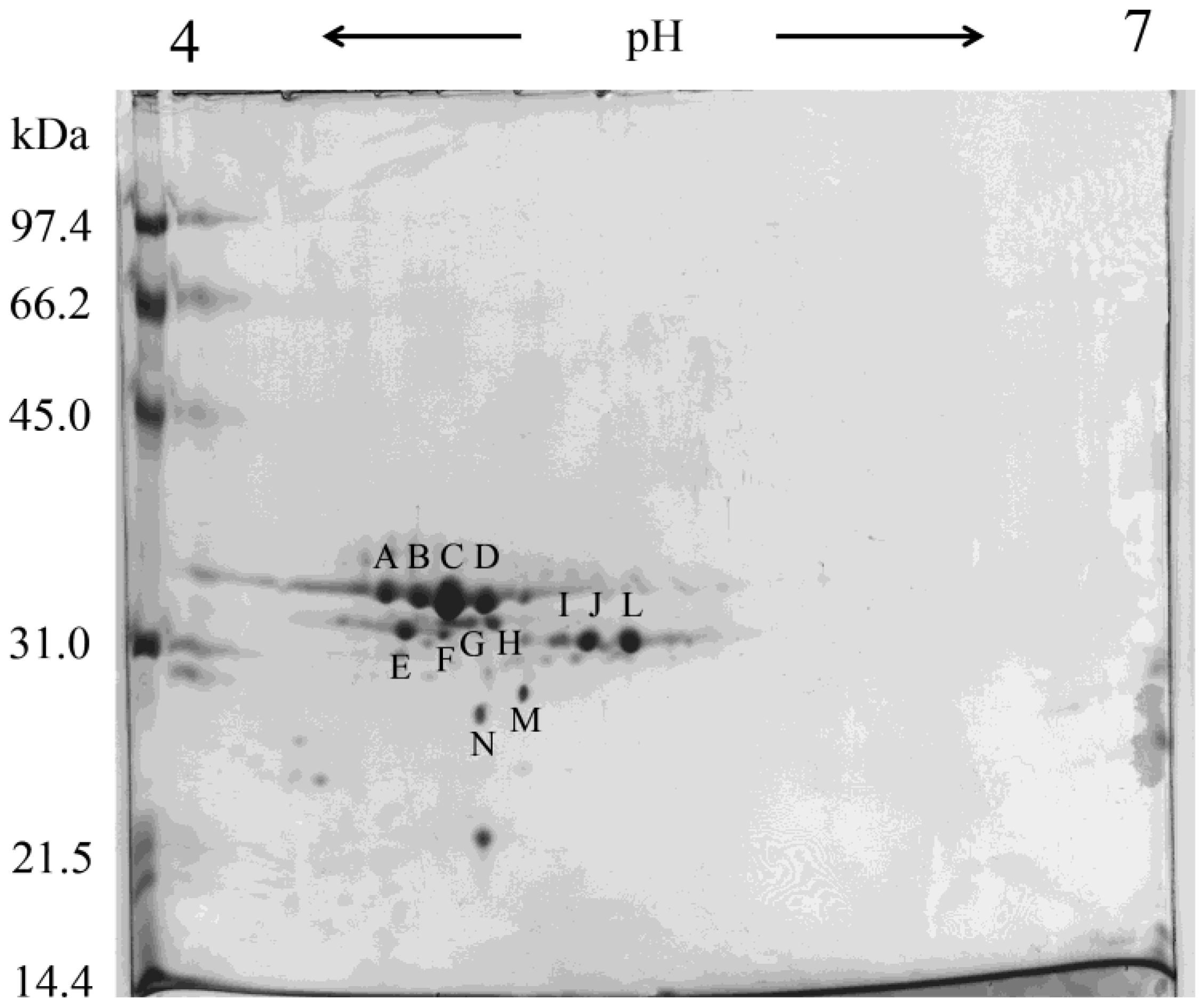
| Days after parturition | Lysozyme (mg/mL) | β-Lactoglobulin | α-Lactalbumin |
|---|---|---|---|
| 60 | 1.34 | Not determined | 0.81 |
| 90 | 0.94 | 4.13 | 1.97 |
| 120 | 1.03 | 3.60 | 1.87 |
| 160 | 0.82 | 3.69 | 1.74 |
| 190 | 0.76 | 3.60 | 1.63 |
| Milk | Lactoperoxidase (mg/L) | Lysozyme (g/L) | Lactoferrin (g/L) |
|---|---|---|---|
| Human | 0.77 | 0.12 | 0.3–4.2 |
| Donkey | 0.11 | 1.0 | 0.080 |
| Bovine | 30–100 | Trace | 0.10 |
4. Conclusions
Conflicts of Interest
References
- Hill, D.J.; Firer, M.A.; Shelton, M.J.; Hosking, C.S. Manifestations of milk allergy in infancy: Clinical and immunologic findings. J. Pediatr. 1986, 109, 270–276. [Google Scholar] [CrossRef]
- Host, A. Frequency of cow’s milk allergy in childhood. Ann. Allergy Asthma Immunol. 2002, 89, 33–37. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Koletzko, S.; Isolauri, E.; Hill, D.; Oranje, A.P.; Brueton, M.; Staiano, A.; Dupont, C. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch. Dis. Child. 2007, 92, 902–908. [Google Scholar] [CrossRef]
- Viera, M.C.; Spolidoro, J.V.N.; Toporovski, M.S.; Cardoso, A.L.; Traujo, G.T.B.; Nudelman, V.; Fonseca, M.C.M. A survey on clinical presentation and nutritional status of infants with suspected cow’ milk allergy. BMC Pediatr. 2010, 10, 25. [Google Scholar] [CrossRef]
- Crittenden, R.G.; Bennett, L.E. Cow’s milk allergy: A complex disorder. J. Am. Coll. Nutr. 2005, 24, 582S–591S. [Google Scholar]
- Hill, D.J.; Hosking, C.S.; Zhie, C.Y.; Leung, R.; Baratwidjaja, K.; Iikura, Y.; Iyngkaran, N.; Gonzalez-Andaya, A.; Wah, L.B.; Hsieh, K.H. The frequency of food allergy in Australia and Asia. Environ. Toxicol. Pharmacol. 1997, 4, 101–110. [Google Scholar] [CrossRef]
- Garcia-Ara, M.C.; Boyano-Martinez, M.T.; Diaz-Pena, J.M.; Martin-Munoz, M.F.; Martin-Esteban, M. Cow’s milk-specific immuno-globulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin. Exp. Allergy 2004, 34, 866–870. [Google Scholar] [CrossRef]
- Woods, R.K.; Thien, F.; Raven, J.; Walters, E.H.; Abramson, M.A. Prevalence of food allergies in young adults and their relationship to asthma, nasal allergies, and eczema. Ann. Allergy Asthma Immunol. 2002, 88, 183–189. [Google Scholar] [CrossRef]
- Bindels, J.G.; Hoijer, M. Allergens: Latest developments, newest techniques. Bull. Int. Dairy Fed. 2000, 351, 31–32. [Google Scholar]
- Høst, A. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on HypoallergenicFormulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch. Dis. Child. 1999, 81, 80–84. [Google Scholar] [CrossRef]
- Distler, J.W. Food allergy children. An update on diagnosis and treatment. Adv. NPs PAs 2010, 1, 26–30. [Google Scholar]
- Jansen, J.J.; Kardinaal, A.F.; Huijbers, G.; Vlieg-Boerstra, B.J.; Martens, B.P.; Ockhuizen, T. Prevalence of food allergy and intolerance in the adult Dutch population. J. Allergy Clin. Immunol. 1994, 93, 446–456. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Noone, S.A.; Koerner, C.B.; Christie, L.; Burks, A.W.; Sampson, H.A. Hypoallergenicity and efficacy of an amino acid-based formula in children with cow’s milk and multiple food hypersensitivities. J. Pediatr. 2001, 138, 688–693. [Google Scholar] [CrossRef]
- Hill, D.J.; Hosking, C.S. Cow milk allergy in infancy and early childhood. Clin. Exp. Allergy 1996, 26, 254–261. [Google Scholar]
- Stintzing, G.; Zetterstrom, R. Cow’s milk allergy, incidence and pathogenetic role of early exposure to cow’s milk formula. Acta Paediatr. Scand. 1979, 68, 383–387. [Google Scholar]
- Bock, S.A. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Paediatrics 1987, 79, 683–688. [Google Scholar]
- Host, A.; Husby, S.; Osterballe, O. A prospective study of cow’s milk allergy in exclusively breast-fed infants. Incidence, pathogenic role of early exposure to cow’s milk formula, and characterization of bovine milk protein in human milk. Acta Paediatr. Scand. 1988, 77, 663–670. [Google Scholar] [CrossRef]
- Host, A.; Halken, S. A prospective study of cow’s milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy 1990, 45, 587–596. [Google Scholar] [CrossRef]
- Restani, P.; Beretta, B.; Fiocchi, A.; Ballabio, C.; Galli, C.L. Cross-reactivity between mammalian proteins. Ann. Allergy Asthma Immunol. 2002, 89, 11–15. [Google Scholar] [CrossRef]
- De Greef, E.; Veereman-Wauters, G.; Devreker, T.; Hauser, B.; Vandenplas, Y. Diagnosis and Management of Cows’ Milk Protein Allergy in Infants. In Allergic Diseases—Highlights in the Clinic, Mechanisms and Treatment; Pereira, C., Ed.; Intech Publisher: Rijeka, Croatia, 2012; pp. 279–288. [Google Scholar]
- Malacarne, M.; Martuzzi, F.; Summer, A.; Mariani, P. Protein and fat composition of mare’s milk: Some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Herrouin, M.; Mollé, D.; Fauquant, J.; Ballestra, F.; Maubois, J.L.; Léonil, J. New genetic variants identified in donkey’s milk whey proteins. J. Protein Chem. 2000, 19, 105–115. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T.; Huppertz, T.; Fox, P.F. Equine milk proteins: Chemistry, structure and nutritional significance. Int. Dairy J. 2010, 20, 609–629. [Google Scholar] [CrossRef]
- Guo, H.Y.; Pang, K.; Zhang, X.Y.; Zhao, L.; Chen, S.W.; Dong, M.L.; Ren, F.Z. Composition, physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. J. Dairy Sci. 2007, 90, 1635–1643. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Polidori, P.; Mariani, P.; Cammertoni, N.; Fantuz, F.; Vita, A. Donkey’s milk protein fractions characterization. Food Chem. 2008, 106, 640–649. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Amici, A.; Pucciarelli, S.; Vita, A.; Micozzi, D.; Carpi, F.M.; Polzonetti, V.; Natalini, P.; Polidori, P. A proteomic study on donkey milk. Biochem. Anal. Biochem. 2012, 1, 109. [Google Scholar]
- Egito, A.S.; Miclo, L.; Lopez, C.; Adam, A.; Girardet, J.M.; Gaillard, J.L. Separation and characterization of mare’s milk αs1-, β-, κ-caseins, γ-casein-like and proteose peptone component 5-like peptides. J. Dairy Sci. 2002, 85, 697–706. [Google Scholar]
- Miranda, G.; Mahé, M.F.; Leroux, C.; Martin, P. Proteomic tools to characterize the protein fractions of Equidae milk. Proteomics 2004, 4, 2496–2509. [Google Scholar] [CrossRef]
- Stelwagen, K. Milk Protein. In Encyclopedia of Dairy Sciences; Roginski, H., Fuquay, J.W., Fox, P.F., Eds.; Academic Press: London, UK, 2003; pp. 1835–1842. [Google Scholar]
- Hennart, P.F.; Brasseu, D.J.; Delogne-Desnoeck, J.B.; Dramaix, M.M.; Robyn, C.E. Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: Influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am. J. Clin. Nutr. 1991, 53, 32–39. [Google Scholar]
- Pruitt, K.M.; Kamau, D.N. Indigenous Antimicrobial Agents of Milk. In International Dairy. Federation Editions; International Dairy Federation: Bruxelles, Belgium, 1993; pp. 73–87. [Google Scholar]
- Shin, K.; Hayasawa, H.; Lönnerdal, B. Purification and quantification of lactoperoxidase in human milk with use of immunoadsorbent with antibodies against recombinant human lactoperoxidase. Am. J. Clin. Nutr. 2001, 73, 984–989. [Google Scholar]
- Polidori, P.; Vincenzetti, S. Protein Profile Characterization of Donkey Milk. In Milk Protein; Hurley, W.L., Ed.; InTech Publisher: Rijeka, Croatia, 2012; pp. 215–232. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Polidori, P.; Vincenzetti, S. Use of Donkey Milk in Children with Cow’s Milk Protein Allergy. Foods 2013, 2, 151-159. https://doi.org/10.3390/foods2020151
Polidori P, Vincenzetti S. Use of Donkey Milk in Children with Cow’s Milk Protein Allergy. Foods. 2013; 2(2):151-159. https://doi.org/10.3390/foods2020151
Chicago/Turabian StylePolidori, Paolo, and Silvia Vincenzetti. 2013. "Use of Donkey Milk in Children with Cow’s Milk Protein Allergy" Foods 2, no. 2: 151-159. https://doi.org/10.3390/foods2020151
APA StylePolidori, P., & Vincenzetti, S. (2013). Use of Donkey Milk in Children with Cow’s Milk Protein Allergy. Foods, 2(2), 151-159. https://doi.org/10.3390/foods2020151






