Advancing Probiotic Delivery in Functional Yogurt: Encapsulation in Prebiotic-Based Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Development of Encapsulated Structures
2.2.1. Preparation of Polymer Solution
2.2.2. Electrohydrodynamic Processing (Electrospraying)
2.2.3. Freeze Drying Method
2.3. Characterization of Encapsulated Structures
2.3.1. Encapsulation Efficiency (EE%)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Survival in Simulated Gastrointestinal Conditions
2.3.4. Stability of Encapsulated L. rhamnosus LGG® During Storage at 4 °C
2.3.5. Stability of Encapsulated L. rhamnosus LGG® After Incorporation in Yogurt Products and Storage at 4 °C
2.3.6. Sensory Evaluation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Encapsulated L. rhamnosus LGG®
3.1.1. Encapsulation Efficiency (EE%)
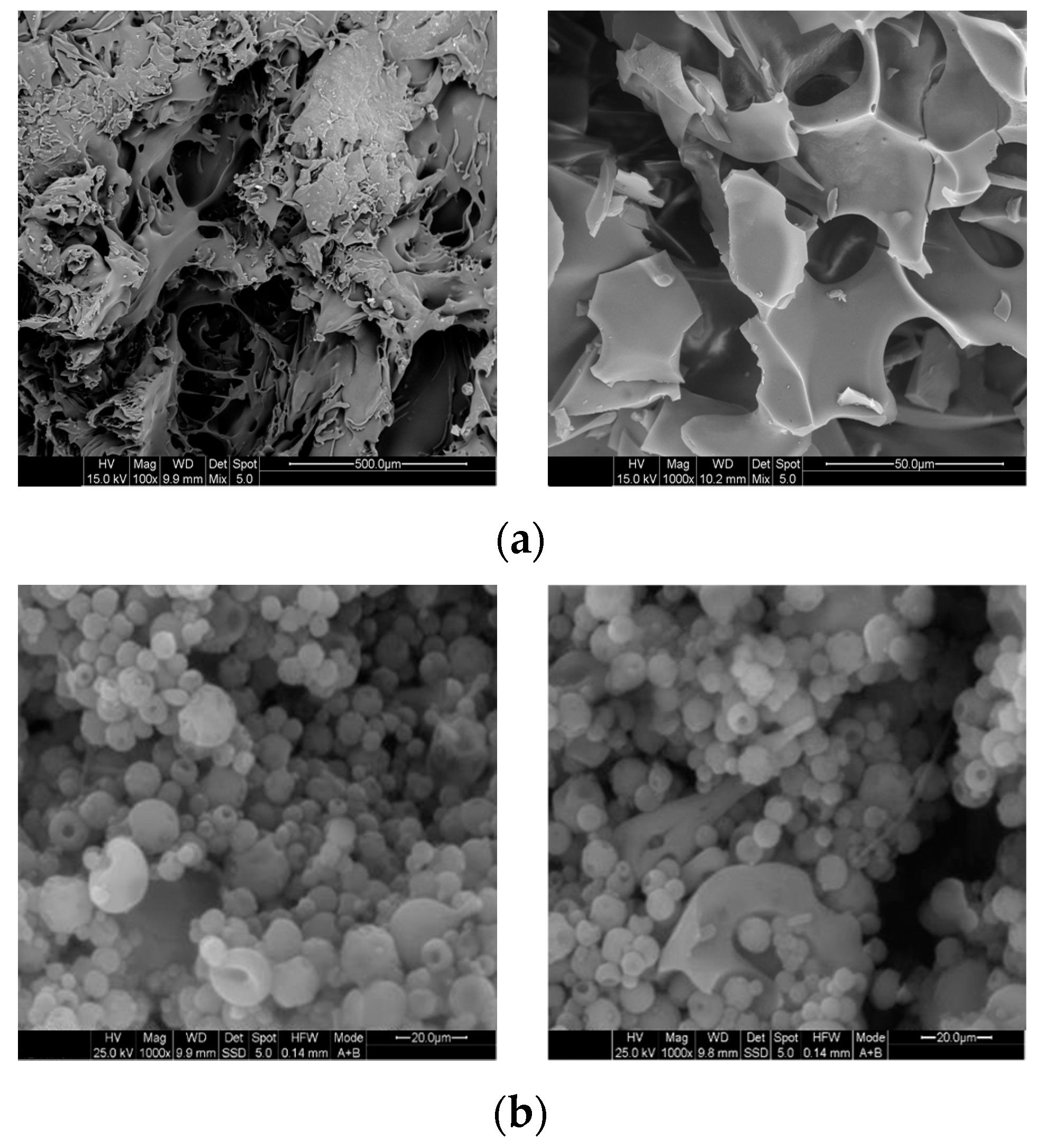
3.1.2. Morphological Characterization of FD and ES Encapsulants
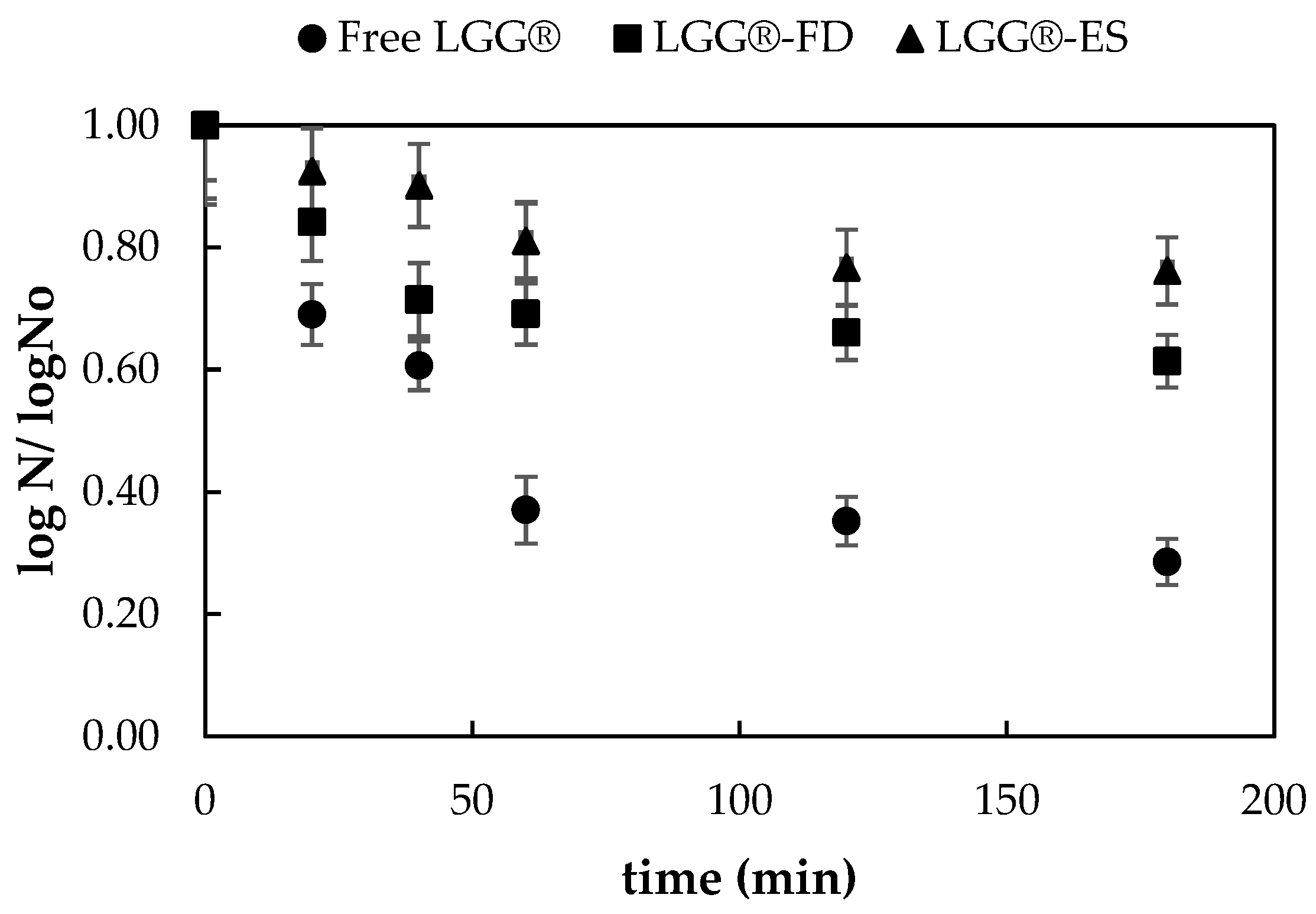
3.1.3. Survival of Encapsulated Probiotic Cells in Simulated Gastric and Intestinal Conditions
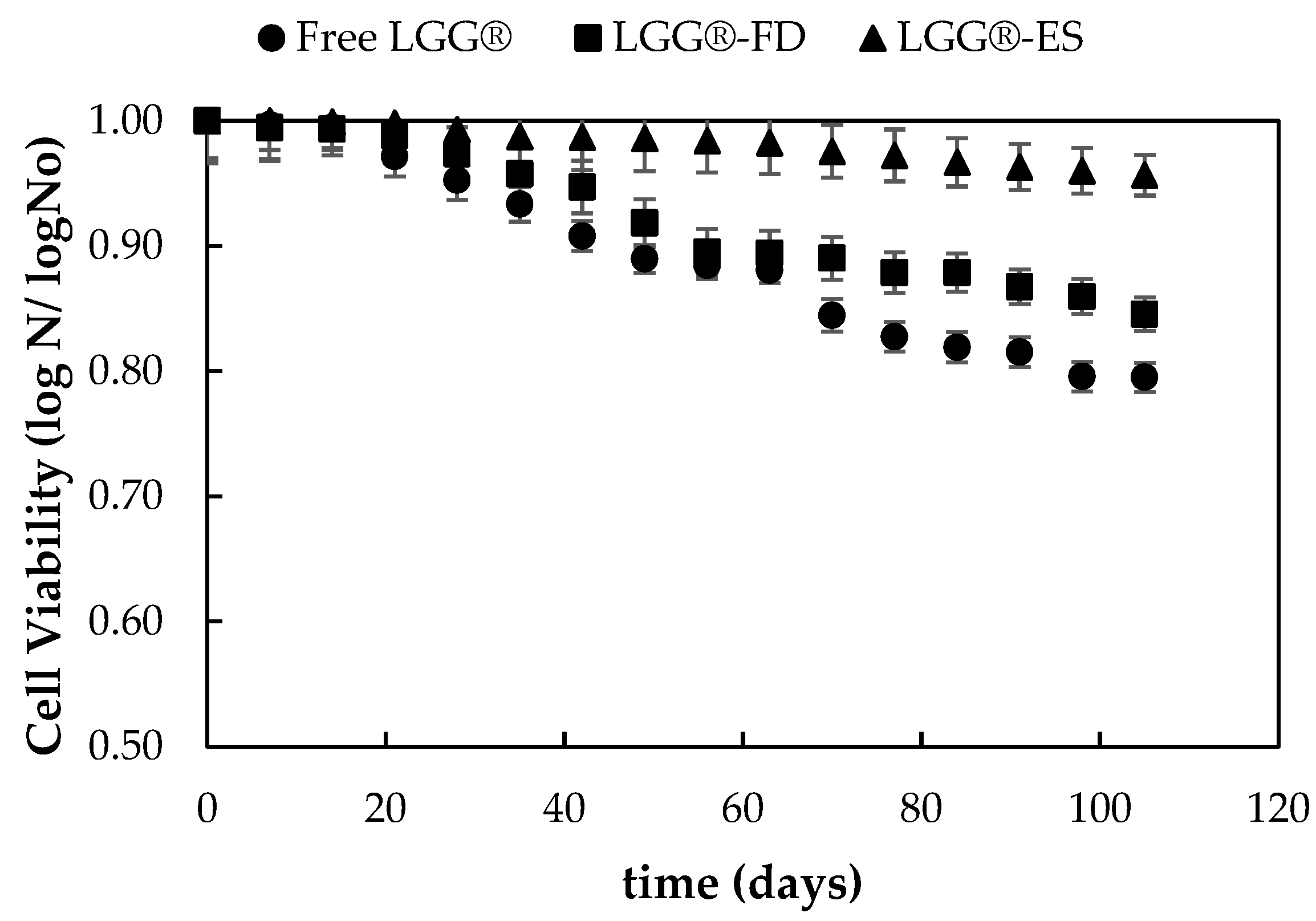
3.1.4. Evaluation of Viability of Encapsulated Probiotic Strains During Storage at 4 °C
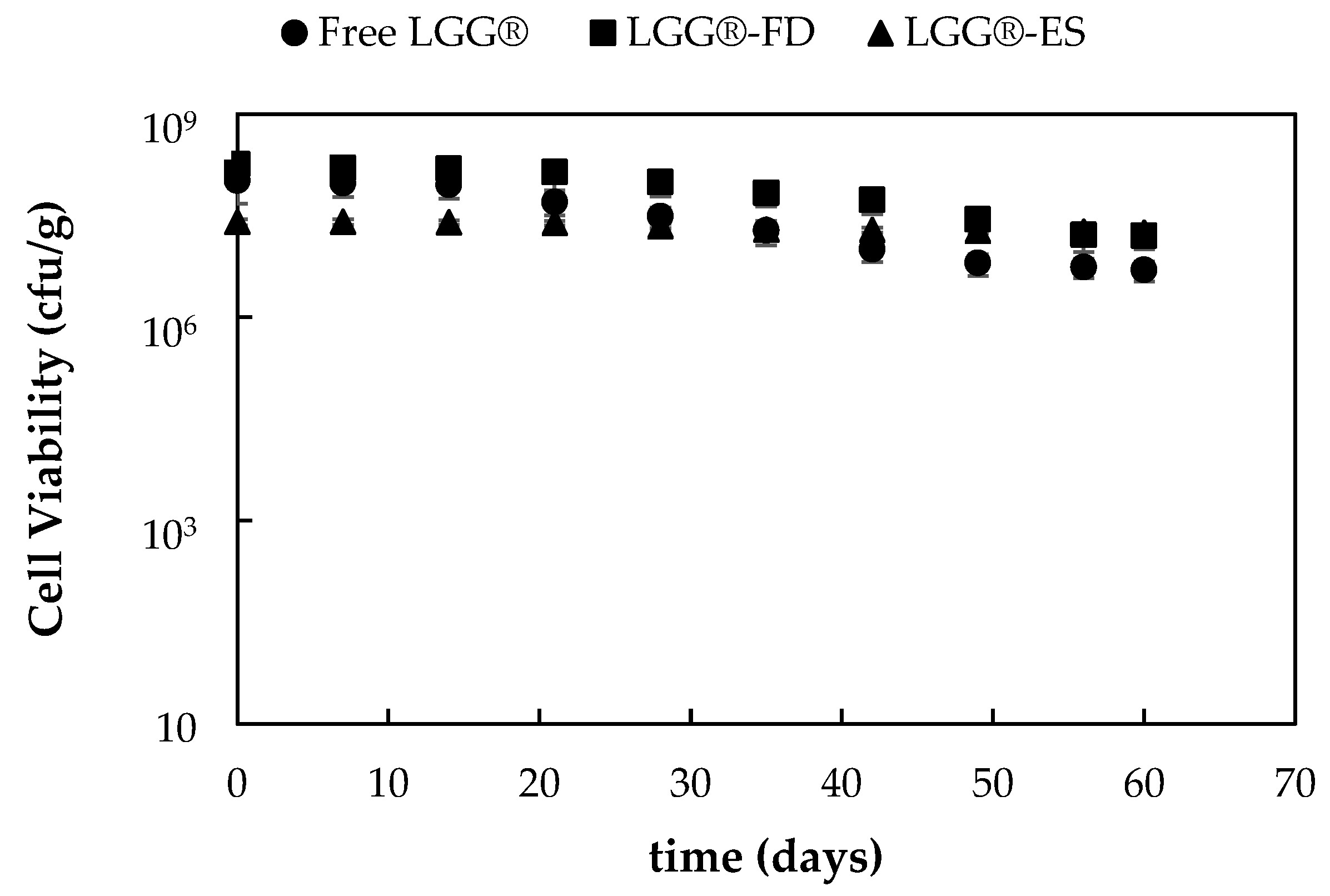
3.1.5. Evaluation of Stability and Viability of Encapsulated Probiotic Strains’ Incorporation in Yogurt Products and Storage at 4 °C
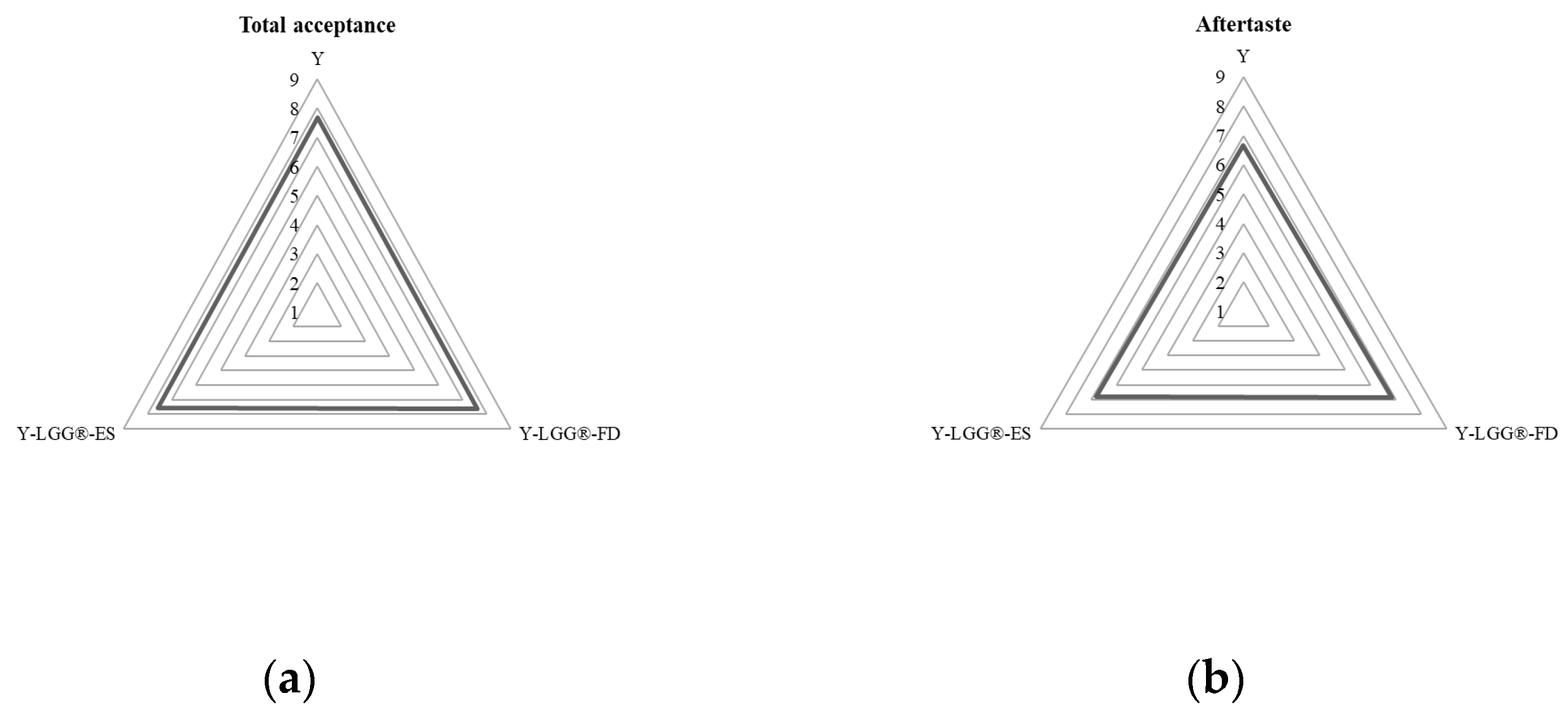
3.1.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajam, R.; Subramanian, P. Encapsulation of probiotics: Past, present and future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, W.; Liang, J.; Dou, J.; Guo, F.; Zhang, D.; Xu, Z.; Wang, T. Probiotics: Functional food ingredients with the potential to reduce hypertension. Front. Cell. Infect. Microbiol. 2023, 13, 1220877. [Google Scholar] [CrossRef]
- Peruzzolo, M.; Ceni, G.C.; Junges, A.; Zeni, J.; Cansian, R.L.; Backes, G.T. Probiotics: Health benefits, microencapsulation, and viability, combination with natural compounds, and applications in foods. Food Biosci. 2025, 66, 106253. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Suo, H.; Taip, F.S.; Cheng, K.-W. Lactoferrin-chia seed mucilage complex coacervates for intestinal delivery of quercetin and fortification of set yogurt. Food Chem. 2024, 456, 139818. [Google Scholar] [CrossRef] [PubMed]
- Frakolaki, G.; Katsouli, M.; Giannou, V.; Tzia, C. Novel encapsulation approach for bifidobacterium subsp. Lactis (bb-12) viability enhancement through its incorporation into a double emulsion prior to the extrusion process. LWT 2020, 130, 109671. [Google Scholar] [CrossRef]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Dry. Technol. 2017, 35, 139–162. [Google Scholar] [CrossRef]
- Laina, K.T.; Drosou, C.; Krokida, M. Comparative assessment of encapsulated essential oils through the innovative electrohydrodynamic processing and the conventional spray drying, and freeze-drying techniques. Innov. Food Sci. Emerg. Technol. 2024, 95, 103720. [Google Scholar] [CrossRef]
- Tsakiri-Mantzorou, Z.; Drosou, C.; Mari, A.; Stramarkou, M.; Laina, K.T.; Krokida, M. Edible coating with encapsulated antimicrobial and antibrowning agents via the emerging electrospinning process and the conventional spray drying: Effect on quality and shelf life of fresh-cut potatoes. Potato Res. 2024, 68, 587–619. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocoll. 2018, 77, 726–735. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Encapsulation of β-carotene into food-grade nanofibers via coaxial electrospinning of hydrocolloids: Enhancement of oxidative stability and photoprotection. Food Hydrocoll. 2022, 133, 107949. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M. Enrichment of white chocolate with microencapsulated β-carotene: Impact on quality characteristics and β-carotene stability during storage. Foods 2024, 13, 2699. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Krokida, M. A comparative study of encapsulation of β-carotene via spray-drying and freeze-drying techniques using pullulan and whey protein isolate as wall material. Foods 2024, 13, 1933. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Huangfu, L.; Liu, C.; Bonfili, L.; Xiang, Q.; Wu, H.; Bai, Y. Electrospinning and electrospraying: Emerging techniques for probiotic stabilization and application. Polymers 2023, 15, 2402. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tan, Z.; Wei, X.; Tian, Z.; Wang, V.Y.; Wang, E.; Xu, D. Electrospray whey protein-polysaccharide microcapsules co-encapsulating lactiplantibacillus plantarum and egcg: Enhanced probiotic viability and antioxidant activity. Int. J. Biol. Macromol. 2025, 290, 138734. [Google Scholar] [CrossRef]
- Dima, P.; Stubbe, P.R.; Mendes, A.C.; Chronakis, I.S.J.C.R.i.F.S. Enhanced electric field and charge polarity modulate the microencapsulation and stability of electrosprayed probiotic cells (Streptococcus thermophilus, st44). Curr. Res. Food Sci. 2023, 7, 100620. [Google Scholar] [CrossRef]
- Farahmand, A.; Ghorani, B.; Emadzadeh, B.; Sarabi-Jamab, M.; Emadzadeh, M.; Modiri, A.; Mendes, A.C.J.L. Double protection of probiotics in alginate hydrogel through emulsification incorporated with freeze drying and coaxial wet-electrospraying: Survivability and targeted delivery. LWT 2024, 204, 116459. [Google Scholar] [CrossRef]
- Amiri, S.; Teymorlouei, M.J.; Bari, M.R.; Khaledabad, M.A.J.F.b. Development of lactobacillus acidophilus la5-loaded whey protein isolate/lactose bionanocomposite powder by electrospraying: A strategy for entrapment. Food Biosci. 2021, 43, 101222. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic viability in yoghurt: A review of influential factors. Int. Dairy J. 2020, 109, 104793. [Google Scholar] [CrossRef]
- Mazlum, H.; Atasever, M. Probiotic cheese as a functional food. Asian Australas. J. Food Saf. Secur. 2023, 7, 20–32. [Google Scholar] [CrossRef]
- Anwer, M.; Wei, M.Q. Harnessing the power of probiotic strains in functional foods: Nutritive, therapeutic, and next-generation challenges. Food Sci. Biotechnol. 2024, 33, 2081–2095. [Google Scholar] [CrossRef]
- Ranadheera, R.D.C.S.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Al Zahrani, A.J.; Shori, A.B. Viability of probiotics and antioxidant activity of soy and almond milk fermented with selected strains of probiotic Lactobacillus spp. LWT 2023, 176, 114531. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef]
- Nole-Jaramillo, J.M.; Muñoz-More, H.D.; Ruiz-Flores, L.A.; Gutiérrez-Valverde, K.S.; Nolazco-Cama, D.M.; Espinoza-Silva, C.R.; Espinoza-Espinoza, L.A. Evaluation of the physicochemical, bioactive, and sensory properties of yogurt fortified with microencapsulated iron. Appl. Food Res. 2024, 4, 100525. [Google Scholar] [CrossRef]
- Wichchukit, S.; O’Mahony, M. The 9-point hedonic and unstructured line hedonic scales: An alternative analysis with more relevant effect sizes for preference. Food Qual. Prefer. 2022, 99, 104575. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.-T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated lactobacillus rhamnosus atcc 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Gaiani, C.; Edorh, J.-M.; Borges, F.; Beaupeux, E.; Maudhuit, A.; Desobry, S. Comparison of electrostatic spray drying, spray drying, and freeze drying for lacticaseibacillus rhamnosus gg dehydration. Foods 2023, 12, 3117. [Google Scholar] [CrossRef]
- Hossain, M.N.; Ranadheera, C.S.; Fang, Z.; Hutchinson, G.; Ajlouni, S. Protecting the viability of encapsulated lactobacillus rhamnosus lgg using chocolate as a carrier. Emir. J. Food Agric. 2021, 33, 647–656. [Google Scholar] [CrossRef]
- Azizi, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Microencapsulation of lactobacillus rhamnosus using sesame protein isolate: Effect of encapsulation method and transglutaminase. Food Biosci. 2021, 41, 101012. [Google Scholar] [CrossRef]
- Ta, P.L.; Bujna, E.; Kun, S.; Charalampopoulos, D.; Khutoryanskiy, V. Electrosprayed mucoadhesive alginate-chitosan microcapsules for gastrointestinal delivery of probiotics. Int. J. Pharm. 2021, 597, 120342. [Google Scholar]
- Ying, D.Y.; Phoon, M.C.; Sanguansri, L.; Weerakkody, R.; Burgar, I.; Augustin, M.A. Microencapsulated lactobacillus rhamnosus gg powders: Relationship of powder physical properties to probiotic survival during storage. J. Food Sci. 2010, 75, E588–E595. [Google Scholar] [CrossRef] [PubMed]
- Romero-Chapol, O.O.; Varela-Pérez, A.; Castillo-Olmos, A.G.; García, H.S.; Singh, J.; García-Ramírez, P.J.; Viveros-Contreras, R.; Figueroa-Hernández, C.Y.; Cano-Sarmiento, C. Encapsulation of lacticaseibacillus rhamnosus gg: Probiotic survival, in vitro digestion and viability in apple juice and yogurt. Appl. Sci. 2022, 12, 2141. [Google Scholar] [CrossRef]
- Qi, X.; Simsek, S.; Ohm, J.-B.; Chen, B.; Rao, J. Viability of lactobacillus rhamnosus gg microencapsulated in alginate/chitosan hydrogel particles during storage and simulated gastrointestinal digestion: Role of chitosan molecular weight. Soft Matter 2020, 16, 1877–1887. [Google Scholar] [CrossRef]
- Adhikari, P.; Kim, W.K. Roles of prebiotics and probiotics in poultry—A review. Ann. Anim. Sci. 2017, 17, 949–966. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Microencapsulating polymers for probiotics delivery systems: Preparation, characterization, and applications. Food Hydrocoll. 2021, 120, 106882. [Google Scholar] [CrossRef]
- Norouzbeigi, S.; Vahid-Dastjerdi, L.; Yekta, R.; Farhoodi, M.; Mortazavian, A.M. Effects of using different o2 scavengers on the qualitative attributes of bifidus yogurt during refrigerated storage. Food Res. Int. 2021, 140, 109953. [Google Scholar] [CrossRef] [PubMed]
- Marcial-Coba, M.S.; Knøchel, S.; Nielsen, D.S. Low-moisture food matrices as probiotic carriers. FEMS Microbiol. Lett. 2019, 366, fnz006. [Google Scholar] [CrossRef]
- Santhosh, A.V.; Sweety; Asif Ali, T.S.; Ramachandra, B. Storage stability and probiotic viability of foxtail millet probiotic powder enriched with high protein and fiber. J. Adv. Microbiol. 2025, 25, 27–36. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Oguntoye, M.A.; Ezekiel, O.O.; Oridupa, O.A. Viability of lactobacillus rhamnosus gg in provitamin a cassava hydrolysate during fermentation, storage, in vitro and in vivo gastrointestinal conditions. Food Biosci. 2021, 40, 100845. [Google Scholar] [CrossRef]
- Abbasi Saadi, M.; Sekhavatizadeh, S.S.; Barzegar, H.; Alizadeh Behbahani, B.; Mehrnia, M.A. Date yogurt supplemented with Lactobacillus rhamnosus (atcc 53103) encapsulated in wild sage (Salvia macrosiphon) mucilage and sodium alginate by extrusion: The survival and viability against the gastrointestinal condition, cold storage, heat, and salt with low ph. Food Sci. Nutr. 2024, 12, 7630–7643. [Google Scholar] [PubMed]
- Tański, T.A.; Jarka, P.; Smok, W. Electrospraying and electrospinning in food industry. In New Topics in Electrospraying; Smok, W., Tański, T.A., Jarka, P., Eds.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Adinepour, F.; Pouramin, S.; Rashidinejad, A.; Jafari, S.M. Fortification/enrichment of milk and dairy products by encapsulated bioactive ingredients. Food Res. Int. 2022, 157, 111212. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the application of microcapsules as carriers of functional compounds for food products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef]
- Niu, B.; Shao, P.; Luo, Y.; Sun, P. Recent advances of electrosprayed particles as encapsulation systems of bioactives for food application. Food Hydrocoll. 2020, 99, 105376. [Google Scholar] [CrossRef]
| Sample | EE% |
|---|---|
| LGG®-FD | 90.41 ± 2.48 a |
| LGG®-ES | 82.67 ± 1.31 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laina, K.T.; Drosou, C.; Frakolaki, G.; Krokida, M. Advancing Probiotic Delivery in Functional Yogurt: Encapsulation in Prebiotic-Based Matrices. Foods 2025, 14, 1423. https://doi.org/10.3390/foods14081423
Laina KT, Drosou C, Frakolaki G, Krokida M. Advancing Probiotic Delivery in Functional Yogurt: Encapsulation in Prebiotic-Based Matrices. Foods. 2025; 14(8):1423. https://doi.org/10.3390/foods14081423
Chicago/Turabian StyleLaina, Konstantina Theodora, Christina Drosou, Georgia Frakolaki, and Magdalini Krokida. 2025. "Advancing Probiotic Delivery in Functional Yogurt: Encapsulation in Prebiotic-Based Matrices" Foods 14, no. 8: 1423. https://doi.org/10.3390/foods14081423
APA StyleLaina, K. T., Drosou, C., Frakolaki, G., & Krokida, M. (2025). Advancing Probiotic Delivery in Functional Yogurt: Encapsulation in Prebiotic-Based Matrices. Foods, 14(8), 1423. https://doi.org/10.3390/foods14081423








