Advances in Reducing Salt Content in Processed Meats with Basic Amino Acids
Abstract
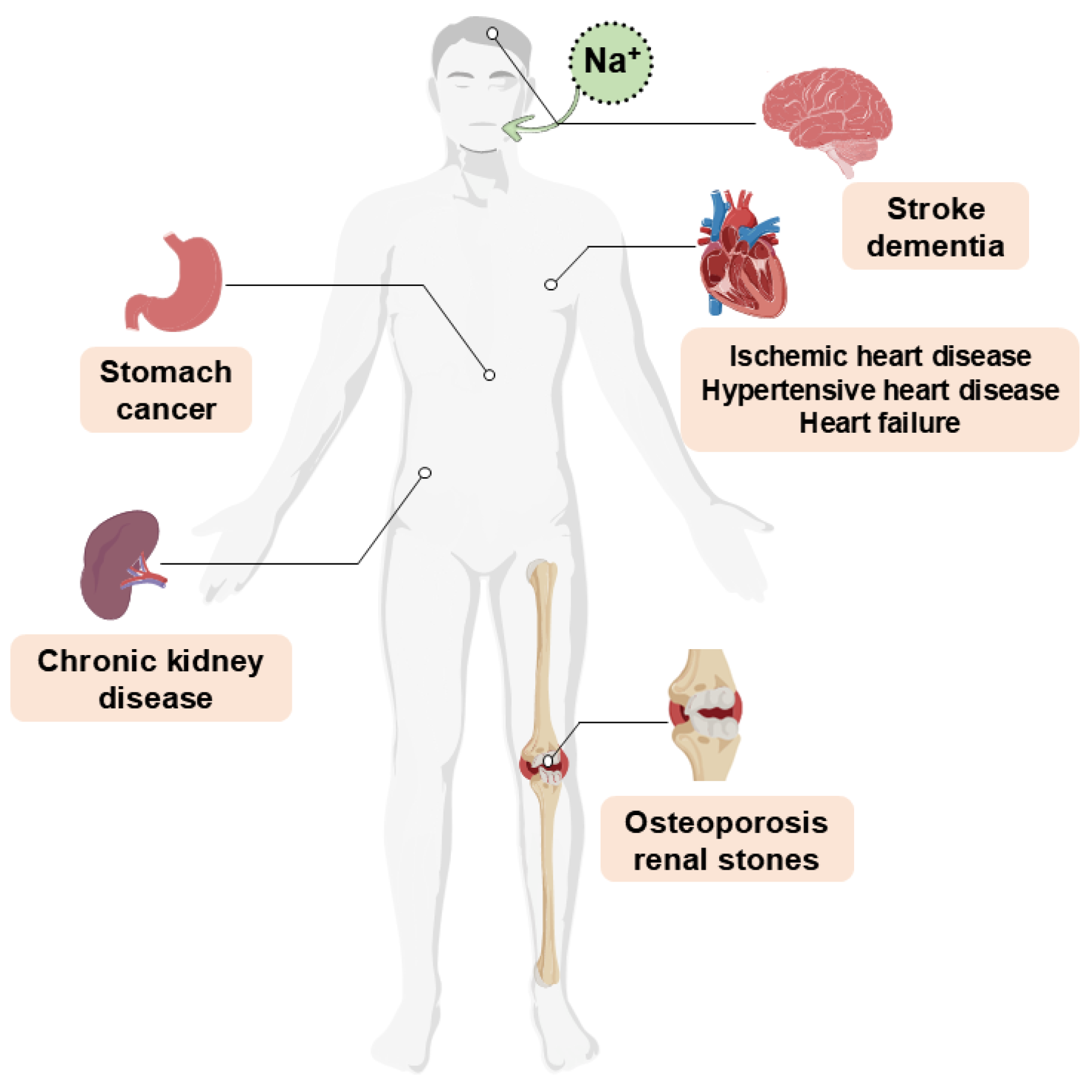
1. Introduction
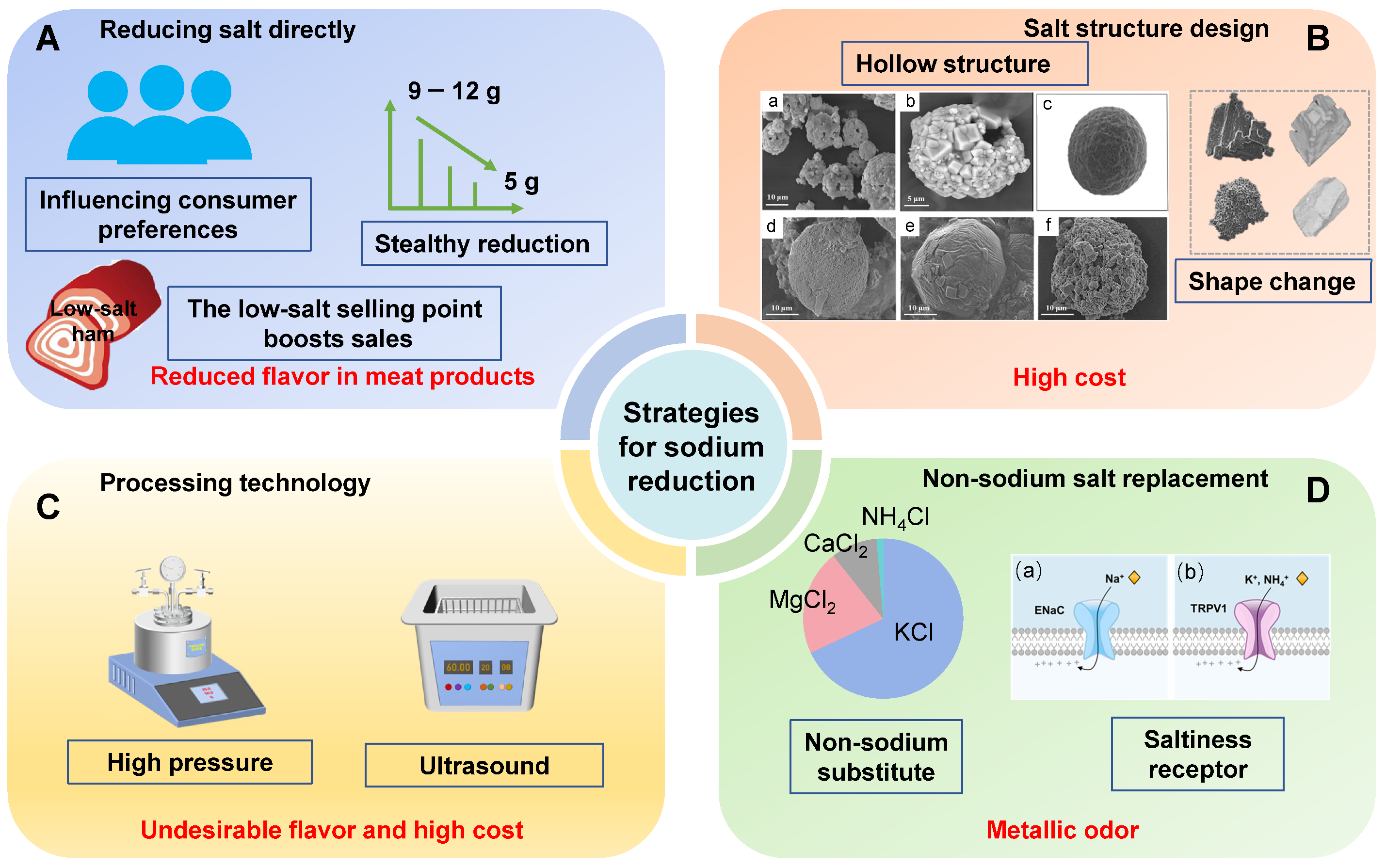
2. Strategies for Reducing Sodium in Meat Products
2.1. Gradual Reduction in Salt Content
2.2. Optimization of Salt Crystal Shape
2.3. Advancements in Processing Technology
2.4. Sodium Salt Substitutes
3. Salt Reduction Potential of Basic Amino Acids
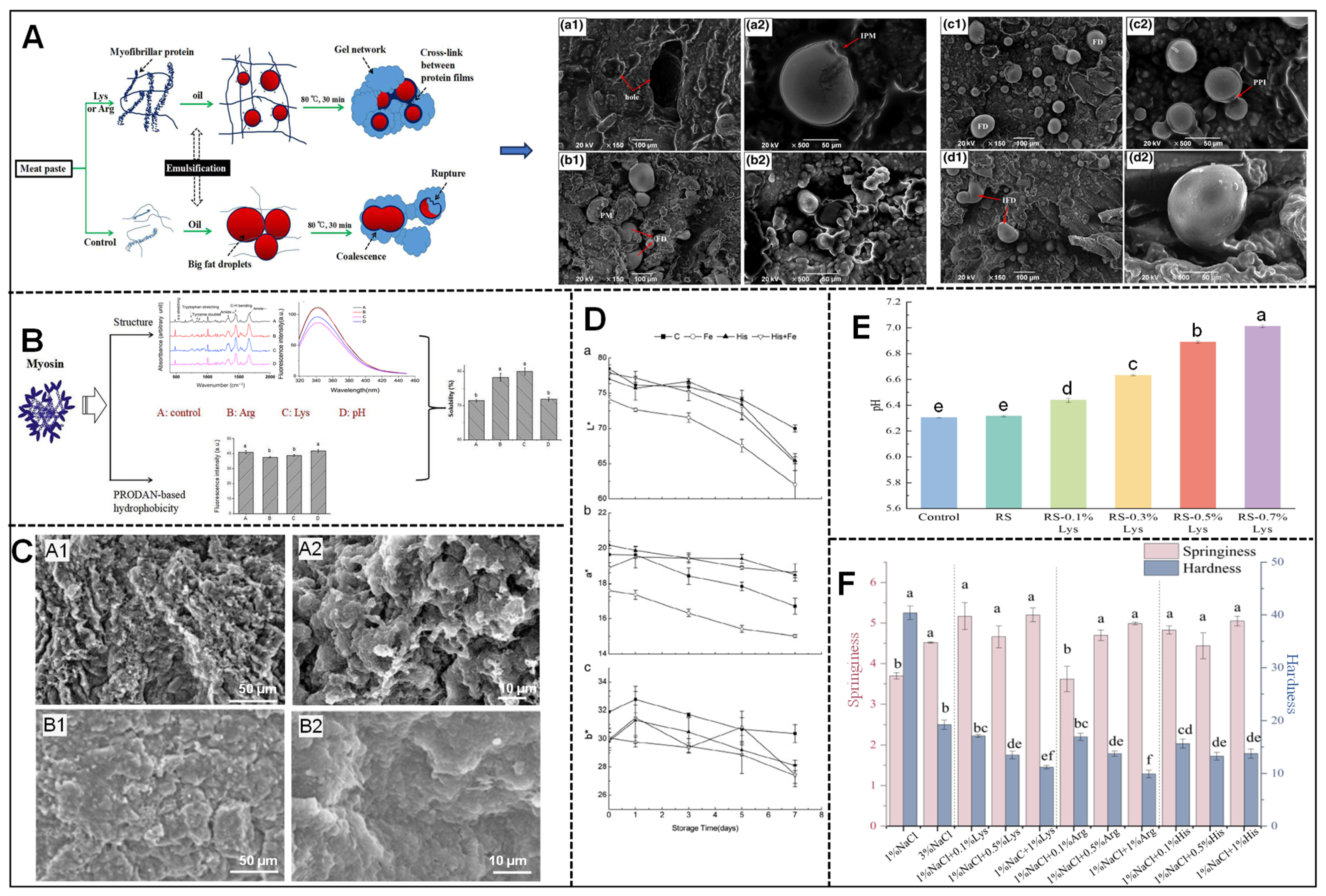
4. Effect of Basic Amino Acids on the Quality of Meat Products
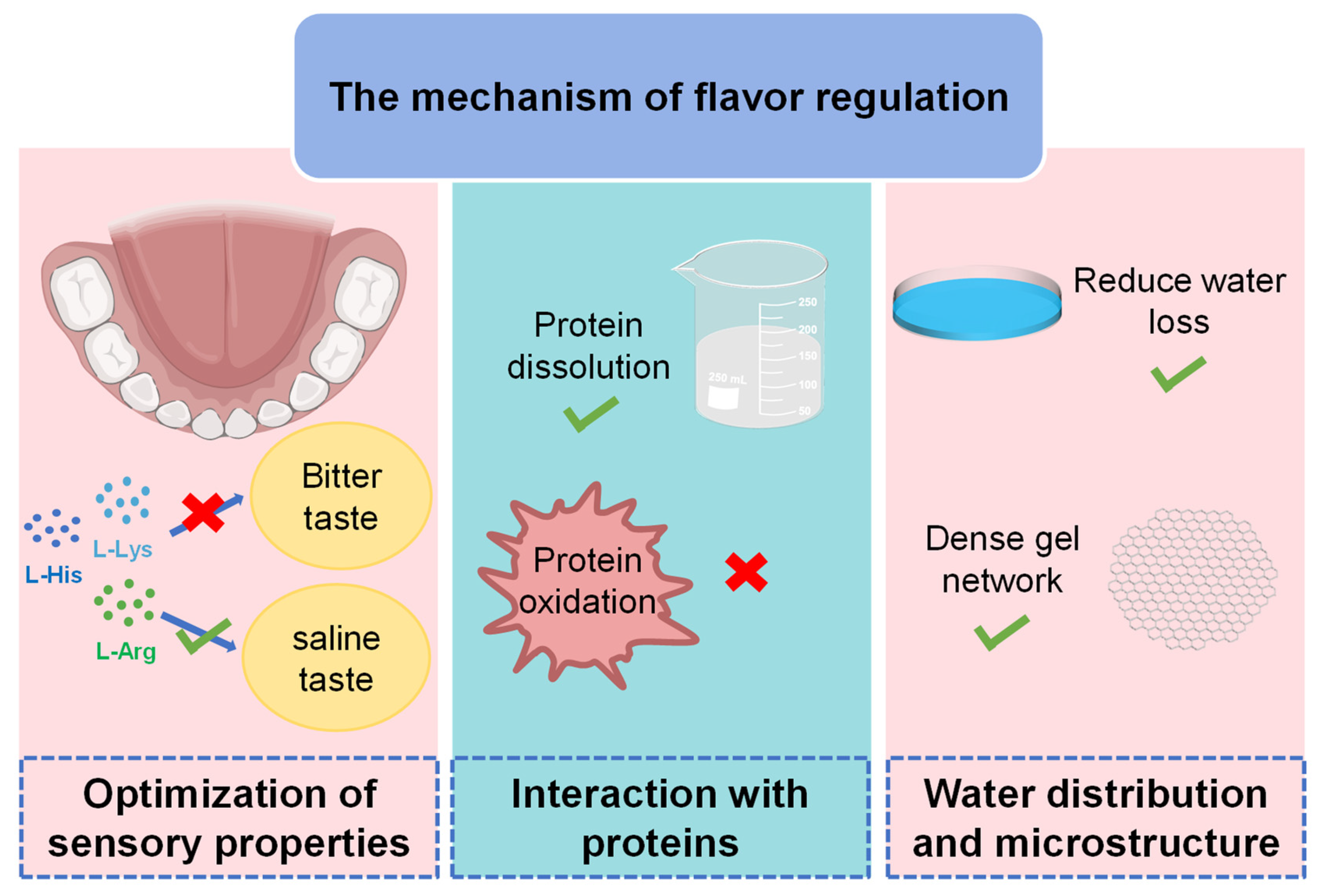
5. Molecular Mechanisms of Basic Amino Acids in Flavor Regulation
5.1. Optimization of Sensory Properties
5.2. Protein Interactions
5.3. Moisture Distribution and Microstructure
6. Challenges and Development Trends of Basic Amino Acid-Based Salt Reduction Technology in Industrial Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Zheng, B.; Huang, L.; Zhang, Y.; Zeng, H. Saltiness perception mechanism and salt reduction strategies in food. Trends Food Sci. Technol. 2024, 148, 104521. [Google Scholar] [CrossRef]
- Le, B.; Yu, B.; Amin, M.S.; Liu, R.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. Salt taste receptors and associated salty/salt taste-enhancing peptides: A comprehensive review of structure and function. Trends Food Sci. Technol. 2022, 129, 657–666. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Venkatakrishnan, K.; Wang, C.-K. Chapter 20—Nutraceuticals and functional foods in the prevention of hypertension induced by excessive intake of dietary salt. In Dietary Sugar, Salt and Fat in Human Health; Preuss, H.G., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 423–450. [Google Scholar] [CrossRef]
- Mancilha-Carvalho Jde, J.; Souza e Silva, N.A. The Yanomami Indians in the INTERSALT Study. Arq. Bras. Cardiol. 2003, 80, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, M.A.; van Raaij, J.M.; Geleijnse, J.M.; Breda, J.; Boshuizen, H.C. Health gain by salt reduction in europe: A modelling study. PLoS ONE 2015, 10, e0118873. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.C.; Marklund, M.; Anderson, C.A.M.; Cobb, L.K.; Dalcin, A.T.; Henry, M.; Appel, L.J. Potassium-Enriched Salt Substitutes as a Means to Lower Blood Pressure: Benefits and Risks. Hypertension 2020, 75, 266–274. [Google Scholar] [CrossRef]
- Ikeda, N.; Yamaguchi, M.; Kashino, I.; Sugiyama, T.; Miura, K.; Nishi, N. Evaluation of public health and economic impacts of dietary salt reduction initiatives on social security expenditures for cardiovascular disease control in Japan. Hypertens. Res. 2025, 48, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Moran, A.E.; Liu, J.; Coxson, P.G.; Penko, J.; Goldman, L.; Bibbins-Domingo, K.; Zhao, D. Projected Impact of Salt Restriction on Prevention of Cardiovascular Disease in China: A Modeling Study. PLoS ONE 2016, 11, e0146820. [Google Scholar] [CrossRef]
- Hashem, K.M.; Pombo-Rodrigues, S.; Capewell, S. Reducing Sodium in the Global Food Supply to Reduce Population Burden of Cardiovascular Disease. Curr. Cardiovasc. Risk Rep. 2015, 9, 7. [Google Scholar] [CrossRef]
- Smith-Spangler, C.M.; Juusola, J.L.; Enns, E.A.; Owens, D.K.; Garber, A.M. Population strategies to decrease sodium intake and the burden of cardiovascular disease: A cost-effectiveness analysis. Ann. Intern. Med. 2010, 152, 481–487. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Chen, R.; Liu, X.-C.; Xiang, J.; Sun, W.; Tomasevic, I. Prospects and challenges for the application of salty and saltiness-enhancing peptides in low-sodium meat products. Meat Sci. 2023, 204, 109261. [Google Scholar] [CrossRef] [PubMed]
- Rios-Mera, J.D.; Selani, M.M.; Patinho, I.; Saldaña, E.; Contreras-Castillo, C.J. Modification of NaCl structure as a sodium reduction strategy in meat products: An overview. Meat Sci. 2021, 174, 108417. [Google Scholar] [CrossRef] [PubMed]
- Desmond, E. Reducing salt: A challenge for the meat industry. Meat Sci. 2006, 74, 188–196. [Google Scholar] [CrossRef]
- Bahuaud, D.; Gaarder, M.; Veiseth-Kent, E.; Thomassen, M. Fillet texture and protease activities in different families of farmed Atlantic salmon (Salmo salar L.). Aquaculture 2010, 310, 213–220. [Google Scholar] [CrossRef]
- Estévez, A.; Camacho, C.; Correia, T.; Barbosa, V.; Marques, A.; Lourenço, H.; Serrano, C.; Sapata, M.; Duarte, M.P.; Pires, C.; et al. Strategies to reduce sodium levels in European seabass sausages. Food Chem. Toxicol. 2021, 153, 112262. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.-H.; Zhang, Y.-Y.; Li, S.; Dong, X.; Qin, L. Effect of sodium salt on meat products and reduction sodium strategies—A review. Meat Sci. 2023, 205, 109296. [Google Scholar] [CrossRef]
- Żochowska-Kujawska, J. Effects of fibre type and structure of longissimus lumborum (Ll), biceps femoris (Bf) and semimembranosus (Sm) deer muscles salting with different Nacl addition on proteolysis index and texture of dry-cured meats. Meat Sci. 2016, 121, 390–396. [Google Scholar] [CrossRef]
- França, F.; Harada-Padermo, S.d.S.; Frasceto, R.A.; Saldaña, E.; Lorenzo, J.M.; Vieira, T.M.F.d.S.; Selani, M.M. Umami ingredient from shiitake (Lentinula edodes) by-products as a flavor enhancer in low-salt beef burgers: Effects on physicochemical and technological properties. LWT Food Sci. Technol. 2022, 154, 112724. [Google Scholar] [CrossRef]
- Vaclavik, V.A.; Christian, E.W.; Campbell, T. Proteins in Food: An Introduction. In Essentials of Food Science; Vaclavik, V.A., Christian, E.W., Campbell, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 123–136. [Google Scholar] [CrossRef]
- Khetra, Y.; Kanawjia, S.K.; Puri, R.; Kumar, R.; Meena, G.S. Using taste-induced saltiness enhancement for reducing sodium in Cheddar cheese: Effect on physico-chemical and sensorial attributes. Int. Dairy J. 2019, 91, 165–171. [Google Scholar] [CrossRef]
- Bae, I.; Park, J.H.; Choi, H.Y.; Jung, H.K. Emerging Innovations to Reduce the Salt Content in Cheese; Effects of Salt on Flavor, Texture, and Shelf Life of Cheese; and Current Salt Usage: A Review. Korean J. Food Sci. Anim. Resour. 2017, 37, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Yadong, Z.; Peng, X.; Shiyi, L.; Xiaoxu, Z.; Conggui, C.; Cunliu, Z. Effects of L-lysine/L-arginine on the Physicochemical Properties and Quality of Sodium-Reduced and Phosphate-Free Pork Sausage. Int. J. Nutr. Food Sci. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Wachirasiri, K.; Wanlapa, S.; Uttapap, D.; Rungsardthong, V. Use of amino acids as a phosphate alternative and their effects on quality of frozen white shrimps (Penaeus vanamei). LWT-Food Sci. Technol. 2016, 69, 303–311. [Google Scholar] [CrossRef]
- Turk, R. Metal Free and Low Metal Salt Substitutes Containing Lysine. US Patent 5,229,161, 5 March 1993. [Google Scholar]
- dos Santos, B.A.; Campagnol, P.C.; Morgano, M.A.; Pollonio, M.A. Monosodium glutamate, disodium inosinate, disodium guanylate, lysine and taurine improve the sensory quality of fermented cooked sausages with 50% and 75% replacement of NaCl with KCl. Meat Sci. 2014, 96, 509–513. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Zhang, L.; Hui, T.; Guo, X.Y.; Peng, Z.Q. Influence of partial replacement of NaCl by KCl, l-histidine and l-lysine on the lipase activity and lipid oxidation in dry-cured loin process. LWT-Food Sci. Technol. 2015, 64, 966–973. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Tan, S.; Sun, G. Effects of L-Arginine on Physicochemical and Sensory Characteristics of Pork Sausage. Adv. J. Food Sci. Technol. 2014, 6, 660–667. [Google Scholar] [CrossRef]
- Germond, A.; Fardet, A.; Álvarez García, C.; Boland, M.; Ming Hoang, H.; Mullen, A.-M.; Kaur, L. Analyzing the complexity of animal products’ processing and its impact on sustainability. Front. Sustain. Food Syst. 2024, 8, 1424282. [Google Scholar] [CrossRef]
- Nilson, E.A.F.; Metlzer, A.B.; Labonté, M.-E.; Jaime, P.C. Modelling the effect of compliance with WHO salt recommendations on cardiovascular disease mortality and costs in Brazil. PLoS ONE 2020, 15, e0235514. [Google Scholar] [CrossRef]
- Riis, N.L.; Bjoernsbo, K.S.; Toft, U.; Trolle, E.; Hyldig, G.; Hartley, I.E.; Keast, R.; Lassen, A.D. Impact of salt reduction interventions on salt taste sensitivity and liking, a cluster randomized controlled trial. Food Qual. Prefer. 2021, 87, 104059. [Google Scholar] [CrossRef]
- He, F.J.; Brinsden, H.C.; MacGregor, G.A. Salt reduction in the United Kingdom: A successful experiment in public health. J. Hum. Hypertens. 2014, 28, 345–352. [Google Scholar] [CrossRef]
- Zandstra, E.H.; Lion, R.; Newson, R.S. Salt reduction: Moving from consumer awareness to action. Food Qual. Prefer. 2016, 48, 376–381. [Google Scholar] [CrossRef]
- Hurst, K.E.; Hewson, L.; Fisk, I.D. Sensory perception and consumer acceptance of commercial and salt-reduced potato crisps formulated using salt reduction design rules. Food Res. Int. 2022, 155, 111022. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ullah, N.; Shen, Y.; Sun, Z.; Wang, X.; Feng, T.; Zhang, X.; Huang, Q.; Xia, S. Emulsion delivery of sodium chloride: A promising approach for modulating saltiness perception and sodium reduction. Trends Food Sci. Technol. 2021, 110, 525–538. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Duizer, L.; Aguilera, J.M. The morphology of salt crystals affects the perception of saltiness. Food Res. Int. 2015, 76, 675–681. [Google Scholar] [CrossRef]
- Rodrigues, D.M.; de Souza, V.R.; Mendes, J.F.; Nunes, C.A.; Pinheiro, A.C.M. Microparticulated salts mix: An alternative to reducing sodium in shoestring potatoes. LWT-Food Sci. Technol. 2016, 69, 390–399. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.; Mosqueda-Melgar, J.; Rosales-Oballos, Y.; Citti de Petricone, R.; Frágenas, N.N.; Zambrano-Durán, A.; Sayago, K.; Lara, M.; Urbina, G. New alternative to reduce sodium chloride in meat products: Sensory and microbiological evaluation. LWT Food Sci. Technol. 2019, 108, 253–260. [Google Scholar] [CrossRef]
- He, M.; Tan, M. Hollow salt for sodium reduction in foods: Mechanisms, influence factors, applications and challenges. Trends Food Sci. Technol. 2024, 147, 104451. [Google Scholar] [CrossRef]
- Guillou, S.; Lerasle, M.; Simonin, H.; Federighi, M. High-pressure processing of meat and meat products. In Emerging Technologies in Meat Processing: Production, Processing and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 37–101. [Google Scholar] [CrossRef]
- Ferreira, N.B.M.; Rodrigues, M.I.; Cristianini, M. Modeling the protective effect of water activity of a meat emulsion matrix model on Listeria monocytogenes inactivation by high pressure processing. Food Control 2025, 167, 110751. [Google Scholar] [CrossRef]
- Nuygen, M.; Arvaj, L.; Balamurugan, S. The use of high pressure processing to compensate for the effects of salt reduction in ready-to-eat meat products. Crit. Rev. Food Sci. Nutr. 2024, 64, 2533–2547. [Google Scholar] [CrossRef]
- Khan, A.W.; Roobab, U.; Wang, Z.; Raza, M.M.; Nawazish, H.; Islam, F.; Aadil, R.M. Salt reduction in food products: A systematic review of clean-label ingredients and non-thermal technologies. Trends Food Sci. Technol. 2024, 153, 104695. [Google Scholar] [CrossRef]
- Khaliq, A.; Chughtai, M.F.J.; Mehmood, T.; Ahsan, S.; Liaqat, A.; Nadeem, M.; Sameed, N.; Saeed, K.; Rehman, J.U.; Ali, A. Chapter 3—High-Pressure Processing; Principle, Applications, Impact, and Future Prospective. In Sustainable Food Processing and Engineering Challenges; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 75–108. [Google Scholar] [CrossRef]
- Yang, H.; Qu, Y.; Su, Y.; Liu, Y.; Chen, T.; Wang, H.; Shen, Q. Uncovering the chemical bonding basis for ultrasound treatment-induced improvement in the molecular flexibility of myofibrillar proteins from low-salt meat batters with added methylcellulose. LWT Food Sci. Technol. 2024, 203, 116408. [Google Scholar] [CrossRef]
- Liu, L.; Niu, F.; Xiong, Y.; Wang, P.; Lyu, X.; Yang, Z. Ultrasound-assisted low-sodium salt curing to modify the quality characteristics of beef for aging. Ultrason. Sonochemistry 2024, 111, 107134. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Huang, X.; Chen, X.; Cai, X.; Huang, J.; Vincent, G.; Wang, S. Advances in flavor peptides with sodium-reducing ability: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 9568–9584. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.d.S.A.; Kato, L.S.; Carvalho, A.P.A.d.; Almeida, A.E.C.C.d.; Conte-Junior, C.A. Sodium replacement on fish meat products—A systematic review of microbiological, physicochemical and sensory effects. Trends Food Sci. Technol. 2021, 118, 639–657. [Google Scholar] [CrossRef]
- Azevedo, C.; Saiardi, A. Why always lysine? The ongoing tale of one of the most modified amino acids. Adv. Biol. Regul. 2016, 60, 144–150. [Google Scholar] [CrossRef]
- Armstrong, C.T.; Mason, P.E.; Anderson, J.L.R.; Dempsey, C.E. Arginine side chain interactions and the role of arginine as a gating charge carrier in voltage sensitive ion channels. Sci. Rep. 2016, 6, 21759. [Google Scholar] [CrossRef]
- Raum, H.N.; Modig, K.; Akke, M.; Weininger, U. Proton Transfer Kinetics in Histidine Side Chains Determined by pH-Dependent Multi-Nuclear NMR Relaxation. J. Am. Chem. Soc. 2024, 146, 22284–22294. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Li, J.H.; Teng, S.; Peng, Z.Q.; Jamali, M.A. Quality improvement of prerigor salted ground chicken breast with basic amino acids at low NaCl level. Poult. Sci. 2023, 102, 102871. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Kong, B.; Cao, C.; Sun, F.; Zhang, H.; Liu, Q. Application of lysine as a potential alternative to sodium salt in frankfurters: With emphasis on quality profile promotion and saltiness compensation. Meat Sci. 2024, 217, 109609. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.-D.; Wang, Y.-R.; Zhou, T.-Q.; Wang, Y.-Q.; Yan, J.-N.; Lai, B.; Wang, C.; Wu, H.-T. Gelation improvement of low-salt Chinese shrimp (Fenneropenaeus chinensis) surimi gel by L-arginine. Food Chem. 2025, 465, 142020. [Google Scholar] [CrossRef]
- Guo, X.; Tao, S.; Pan, J.; Lin, X.; Ji, C.; Liang, H.; Dong, X.; Li, S. Effects of l-Lysine on the physiochemical properties and sensory characteristics of salt-reduced reconstructed ham. Meat Sci. 2020, 166, 108133. [Google Scholar] [CrossRef] [PubMed]
- Man, H.; Sun, P.; Lin, J.; Ren, X.; Li, D. Based on hydrogen and disulfide-mediated bonds, l-lysine and l-arginine enhanced the gel properties of low-salt mixed shrimp surimi (Antarctic krill and Pacific white shrimp). Food Chem. 2024, 445, 138735. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Teng, S.; Zhou, Y.; Liu, Y.; Liao, B.; Ren, X.; Zhang, Y. Effects of basic amino acids on heterocyclic amines and quality characteristics of fried beef patties at low NaCl level. Meat Sci. 2024, 215, 109541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, N.; Gao, P.; Yu, D.; Yang, F.; Xu, Y.; Xia, W. Influence of L-arginine addition on the gel properties of reduced-salt white leg shrimp (Litopenaeus vannamei) surimi gel treated with microbial transglutaminase. LWT Food Sci. Technol. 2023, 173, 114310. [Google Scholar] [CrossRef]
- da Silva, S.L.; Lorenzo, J.M.; Machado, J.M.; Manfio, M.; Cichoski, A.J.; Fries, L.L.M.; Morgano, M.A.; Campagnol, P.C.B. Application of arginine and histidine to improve the technological and sensory properties of low-fat and low-sodium bologna-type sausages produced with high levels of KCl. Meat Sci. 2020, 159, 107939. [Google Scholar] [CrossRef]
- Arakawa, T.; Ejima, D.; Tsumoto, K.; Obeyama, N.; Tanaka, Y.; Kita, Y.; Timasheff, S.N. Suppression of protein interactions by arginine: A proposed mechanism of the arginine effects. Biophys. Chem. 2007, 127, 1–8. [Google Scholar] [CrossRef]
- Arakawa, T.; Tsumoto, K. The effects of arginine on refolding of aggregated proteins: Not facilitate refolding, but suppress aggregation. Biochem. Biophys. Res. Commun. 2003, 304, 148–152. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Zhu, X.; Ning, C.; Cai, K.; Zhou, C. Conformational and charge changes induced by L-arginine and L-lysine increase the solubility of chicken myosin. Food Hydrocoll. 2019, 89, 330–336. [Google Scholar] [CrossRef]
- Fu, Y.; Zheng, Y.; Lei, Z.; Xu, P.; Zhou, C. Gelling properties of myosin as affected by L-lysine and L-arginine by changing the main molecular forces and microstructure. Int. J. Food Prop. 2017, 20, S884–S898. [Google Scholar] [CrossRef]
- Lei, Z.; Fu, Y.; Xu, P.; Zheng, Y.; Zhou, C. Effects of l-arginine on the physicochemical and gel properties of chicken actomyosin. Int. J. Biol. Macromol. 2016, 92, 1258–1265. [Google Scholar] [CrossRef]
- Zhu, X.; Ning, C.; Li, S.; Xu, P.; Zheng, Y.; Zhou, C. Effects of l-lysine/l-arginine on the emulsion stability, textural, rheological and microstructural characteristics of chicken sausages. Int. J. Food Sci. Technol. 2018, 53, 88–96. [Google Scholar] [CrossRef]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ye, H.; Nishiumi, T.; Qin, H.; Chen, C. L-Histidine enhances stability of hemoglobin concentrates by coordinating with free iron. Food Res. Int. 2014, 62, 637–643. [Google Scholar] [CrossRef]
- Zhou, C.; Ye, H.; Wang, H.; Qin, H.; Li, J. Coordination of L-arginine and iron cation improves stability of hemoglobin concentrates. Eur. Food Res. Technol. 2015, 240, 743–751. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Q.; Yao, Y.; Guo, X.; Wang, R.; Peng, Z. A preliminary study: Saltiness and sodium content of aqueous extracts from plants and marine animal shells. Eur. Food Res. Technol. 2014, 238, 565–571. [Google Scholar] [CrossRef]
- Breslin, P.A.S. Interactions among salty, sour and bitter compounds. Trends Food Sci. Technol. 1996, 7, 390–399. [Google Scholar] [CrossRef]
- Harmon, C.P.; Ahmed, O.M.; Breslin, P.A.S. Amino Acid Bitterness: Characterization and Suppression. J. Agric. Food Chem. 2024, 72, 22753–22765. [Google Scholar] [CrossRef]
- Lee, C.L.; Lee, S.M.; Kim, K.-O. Use of Consumer Acceptability as a Tool to Determine the Level of Sodium Reduction: A Case Study on Beef Soup Substituted With Potassium Chloride and Soy-Sauce Odor. J. Food Sci. 2015, 80, S2570–S2577. [Google Scholar] [CrossRef]
- He, N.; Chen, X.; Li, L.; Wang, S.; Lan, M.; Yuan, Y.; Zhang, Z.; Li, T.; Zhang, X.; He, X.; et al. κ-Carrageenan masking bitterness perception in surimi gels containing potassium chloride-based salt substitutes: Gel properties, oral processing, and sensory evaluation. Food Chem. 2024, 456, 139859. [Google Scholar] [CrossRef]
- Bansal, V.; Mishra, S.K. Reduced-sodium cheeses: Implications of reducing sodium chloride on cheese quality and safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 733–758. [Google Scholar] [CrossRef]
- Felicio, T.L.; Esmerino, E.A.; Vidal, V.A.S.; Cappato, L.P.; Garcia, R.K.A.; Cavalcanti, R.N.; Freitas, M.Q.; Conte Junior, C.A.; Padilha, M.C.; Silva, M.C.; et al. Physico-chemical changes during storage and sensory acceptance of low sodium probiotic Minas cheese added with arginine. Food Chem. 2016, 196, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Sinesio, F.; Raffo, A.; Peparaio, M.; Moneta, E.; Saggia Civitelli, E.; Narducci, V.; Turfani, V.; Ferrari Nicoli, S.; Carcea, M. Impact of sodium reduction strategies on volatile compounds, sensory properties and consumer perception in commercial wheat bread. Food Chem. 2019, 301, 125252. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, N.; Guo, C.; Luo, J.; Wei, J.; Zhang, N.; Yin, X.; Feng, X.; Wang, X.; Cao, J. Current trends and perspectives on salty and salt taste–enhancing peptides: A focus on preparation, evaluation and perception mechanisms of salt taste. Food Res. Int. 2024, 190, 114593. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.-Y.; Pan, F.; Yang, Z.-C.; Song, H.-L.; Zou, T.-t.; Xiong, J.; Li, K.; Li, P.; Hu, N.; Xue, D.-d. Identification of novel saltiness-enhancing peptides from yeast extract and their mechanism of action for transmembrane channel-like 4 (TMC4) protein through experimental and integrated computational modeling. Food Chem. 2022, 388, 132993. [Google Scholar] [CrossRef]
- Li, K.; Wang, L.-M.; Gao, H.-J.; Du, M.-T.; Bai, Y.-H. Use of basic amino acids to improve gel properties of PSE-like chicken meat proteins isolated via ultrasound-assisted alkaline extraction. J. Food Sci. 2023, 88, 5136–5148. [Google Scholar] [CrossRef]
- Takai, E.; Yoshizawa, S.; Ejima, D.; Arakawa, T.; Shiraki, K. Synergistic solubilization of porcine myosin in physiological salt solution by arginine. Int. J. Biol. Macromol. 2013, 62, 647–651. [Google Scholar] [CrossRef]
- Guo, X.Y.; Peng, Z.Q.; Zhang, Y.W.; Liu, B.; Cui, Y.Q. The solubility and conformational characteristics of porcine myosin as affected by the presence of l-lysine and l-histidine. Food Chem. 2015, 170, 212–217. [Google Scholar] [CrossRef]
- Chen, X.; Zou, Y.; Han, M.; Pan, L.; Xing, T.; Xu, X.; Zhou, G. Solubilisation of myosin in a solution of low ionic strength L-histidine: Significance of the imidazole ring. Food Chem. 2016, 196, 42–49. [Google Scholar] [CrossRef]
- Wu, L.; Wu, T.; Wu, J.; Chang, R.; Lan, X.; Wei, K.; Jia, X. Effects of cations on the “salt in” of myofibrillar proteins. Food Hydrocoll. 2016, 58, 179–183. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.; Xu, P.; Zhu, X.; Zhou, C. l-Lysine and l-arginine inhibit myosin aggregation and interact with acidic amino acid residues of myosin: The role in increasing myosin solubility. Food Chem. 2018, 242, 22–28. [Google Scholar] [CrossRef]
- Qin, H.; Xu, P.; Zhou, C.; Wang, Y. Effects of l-Arginine on water holding capacity and texture of heat-induced gel of salt-soluble proteins from breast muscle. LWT-Food Sci. Technol. 2015, 63, 912–918. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Mu, J.; Shi, T.; Yuan, L. Effect of l-histidine on the heat-induced aggregation of bighead carp (Aristichthys nobilis) myosin in low/high ionic strength solution. Food Hydrocoll. 2018, 75, 174–181. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, Q.; Gao, P.; Yu, D.; Yu, P.; Xia, W. L-histidine-assisted ultrasound improved physicochemical properties of myofibrillar proteins under reduced-salt condition—Investigation of underlying mechanisms. Int. J. Biol. Macromol. 2023, 253, 126820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, C.; Zhang, Y.; Pan, J.; Huang, S.; Lichao, H.; Jin, G. Impact of salt content and hydrogen peroxide-induced oxidative stress on protein oxidation, conformational/morphological changes, and micro-rheological properties of porcine myofibrillar proteins. Food Chem. 2022, 370, 131074. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, Y.; Zhu, X.; Li, S.; Zhou, C. L-lysine and L-arginine inhibit the oxidation of lipids and proteins of emulsion sausage by chelating iron ion and scavenging radical. Asian-Australas. J. Anim. Sci. 2018, 31, 905–913. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Lass, A.; Suessenbacher, A.; Wölkart, G.; Mayer, B.; Brunner, F. Functional and analytical evidence for scavenging of oxygen radicals by L-arginine. Mol. Pharmacol. 2002, 61, 1081–1088. [Google Scholar] [CrossRef]
- Mao, R.; Tang, J.; Swanson, B. Water holding capacity and microstructure of gellan gels. Carbohydr. Polym. 2001, 46, 365–371. [Google Scholar] [CrossRef]
- Lyutova, E.M.; Kasakov, A.S.; Gurvits, B.Y. Effects of arginine on kinetics of protein aggregation studied by dynamic laser light scattering and tubidimetry techniques. Biotechnol. Prog. 2007, 23, 1411–1416. [Google Scholar] [CrossRef]
- Reddy, K.R.C.; Lilie, H.; Rudolph, R.; Lange, C. L-Arginine increases the solubility of unfolded species of hen egg white lysozyme. Protein Sci. 2005, 14, 929–935. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Huang, Y.; Chen, L.; Bao, P.; Fang, H.; Xu, B.; Zhou, C. Effects of basic amino acid on the tenderness, water binding capacity and texture of cooked marinated chicken breast. LWT Food Sci. Technol. 2020, 129, 109524. [Google Scholar] [CrossRef]
- Xu, K.; Lv, A.-J.; Dong, R.-L.; Li, Y.-C.; Zeng, L.-T.; Wang, Y.; Li, H.-S.; Qi, J.; Wang, H.-H.; Zhang, C.-H.; et al. The research on the synergistic improvement of water retention capacity and eating quality of marinated pork meat by the combination of basic arginine and acidic aspartic acid. Food Chem. 2025, 470, 142649. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. The Nature of Human Hazards Associated with Excessive Intake of Amino Acids1. J. Nutr. 2004, 134, 1633S–1639S. [Google Scholar] [CrossRef] [PubMed]
- Tonouchi, N.; Ito, H. Present Global Situation of Amino Acids in Industry. In Amino Acid Fermentation; Yokota, A., Ikeda, M., Eds.; Springer: Tokyo, Japan, 2017; pp. 3–14. [Google Scholar] [CrossRef]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino acids production focusing on fermentation technologies—A review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef]
- Scheper, T.H.; Lammers, F. Fermentation monitoring and process control. Curr. Opin. Biotechnol. 1994, 5, 187–191. [Google Scholar] [CrossRef]
- Hermann, T. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 2003, 104, 155–172. [Google Scholar] [CrossRef]
- Utagawa, T. Production of arginine by fermentation. J. Nutr. 2004, 134, 2854S–2857S. [Google Scholar] [CrossRef]
- Cao, J.; Yan, H.; Liu, L. Optimized preparation and antioxidant activity of glucose-lysine Maillard reaction products. LWT Food Sci. Technol. 2022, 161, 113343. [Google Scholar] [CrossRef]
| Product | Salt Mixtures (%) | Organisational Changes | Effect of Sensory Properties | Main Findings | Reference |
|---|---|---|---|---|---|
| Salted chicken breast | C: 0% NaCl; 1% NaCl; 3% NaCl; SS1: 1% NaCl + 0.06% L-Lys SS2: 1% NaCl + + 0.06% L-Arg; SS3: 1% NaCl + 0.06% L-His | SS1 and SS2 increased protein solubility. | The addition of basic amino acids enhanced the WHC and texture. | The addition of basic amino acids improved solubility, emulsification, and gelation ability. | [53] |
| Frankfurter sausage | C: 1.75% NaCl; SS1: 0.875% NaCl + 0.1% L-Lys SS2: 0.875% NaCl + 0.3% L-Lys; SS3:0.875% NaCl + 0.5% L-Lys; SS4: 0.875% NaCl + 0.7% L-Lys | The gel network structure becomes denser and more uniform. | The addition of lysine enhanced the perception of salinity in the sodium-reduced group. | SS2 reduced cooking losses, improved emulsion stability and textural properties, and maintained high product quality with a 50% sodium reduction. | [54] |
| Fenneropenaeus chinensis | C: 2% NaCl; SS1: 0.5% NaCl SS2: 0.5% NaCl + 0.25% L-Arg; SS3: 0.5% NaCl + 0.5% L-Arg; SS4: 0.5% NaCl + 0.75% L-Arg; SS5: 0.5% NaCl + 1% L-Arg | SS4 has the lowest gel porosity and forms a denser gel network. | The L* values decreased. Hardness, elasticity, and chewiness increased. | 0.75% L-Arg is regarded as the optimal level for enhancing the gelation properties of low-salt surimi. | [55] |
| Ham | C: 2.5% NaCl; SS1: 1.25% NaCl + 0.2% L-Lys; SS2: 1.25% NaCl + 0.4% L-Lys; SS3: 1.25% NaCl + 0.6% L-Lys; SS4: 1.25% NaCl + 0.8% L-Lys | The addition of L-Lys creates a finer network structure. | The SS4 group was comparable to the C group in taste and overall acceptability. | The optimal concentration of L-Lys for enhancing the physicochemical and organoleptic properties of salt-reduced ham is 0.8%. | [56] |
| Low-salt mixed shrimp and fish mince gel | C: 2.25% NaCl; SS1: 1% NaCl SS2: 1% NaCl + 0.3% STPP; SS3: 1% NaCl + 0.3% L-Lys; SS4: 1% NaCl + 0.6% L-Lys SS5: 1% NaCl + 0.3% L-Arg; SS6: 1% NaCl + 0.6% L-Arg | The gel network structure transitioned from a loose configuration to a homogeneous and dense form. | The addition of L-Lys and L-Arg increased the hardness, elasticity, and gel strength, particularly in the 0.3% Lys group. | L-Lys and L-Arg improved the strength and texture of low-salt mixed shrimp and fish surimi gels, surpassing traditional phosphates (STPPs). The optimal concentration of L-Lys was found to be 0.3%. | [57] |
| Beef patty | C: 0% NaCl, 1% NaCl, 2% NaCl, 3% NaCl, 4% NaCl, 5% NaCl; SS1: 1% NaCl +0.1%/0.5%/1%L-Lys; SS2: 1% NaCl +0.1%/0.5%/1%L-Arg; SS3: 1% NaCl +0.1%/0.5%/1%L-His | The hardness of beef patties decreased, while their elasticity increased with the addition of basic amino acids. | The 1% L-Lys and L-Arg groups exhibited the best organoleptic quality, with improved textural homogeneity and palatability. | 1% L-Lys significantly inhibits the production of heterocyclic amines and improves the product quality. | [58] |
| Reduced-salt white leg shrimp (Litopenaeus vannamei) surimi gel | C: 1% NaCl; SS1: 0.5% NaCl and L-Arg (0.5%, 1.5%, 2.5%); SS2: 1% NaCl and 0.75% microbial transglutaminase (MTGase); SS3: 0.5% NaCl, 0.75% MTGase and L-Arg (0.5%, 1.5%, 2.5%) | The surimi developed a denser network structure, resulting in a decrease in porosity from 3.60% to 0.62%. | Synergistic treatment with L-Arg and MTGase significantly enhanced the firmness and chewiness of surimi. | The combination of L-Arg and MTGase reduced the NaCl content and significantly enhanced the gel strength, WHC, and textural properties of surimi. | [59] |
| Gna-type sausage | C: 2.5% NaCl; SS1: 1% NaCl + 1.5% KCl; SS2: 1% NaCl + 1.5% KCl + 1% Arg; SS3: 1% NaCl + 1.5% KCl + 0.2% His; SS4: 1% NaCl + 1.5% KCl + 1% Arg + 0.2% His | The addition of L-Arg and L-His enhanced emulsion stability and decreased water release. | The addition of L-Arg and L-His significantly improved the flavor and masked the metallic and astringent taste of KCl. | L-Arg and L-His improved the emulsion stability and textural properties by enhancing protein solubility and altering pH. | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, R.; Zhu, Z. Advances in Reducing Salt Content in Processed Meats with Basic Amino Acids. Foods 2025, 14, 940. https://doi.org/10.3390/foods14060940
Fang R, Zhu Z. Advances in Reducing Salt Content in Processed Meats with Basic Amino Acids. Foods. 2025; 14(6):940. https://doi.org/10.3390/foods14060940
Chicago/Turabian StyleFang, Rui, and Zongshuai Zhu. 2025. "Advances in Reducing Salt Content in Processed Meats with Basic Amino Acids" Foods 14, no. 6: 940. https://doi.org/10.3390/foods14060940
APA StyleFang, R., & Zhu, Z. (2025). Advances in Reducing Salt Content in Processed Meats with Basic Amino Acids. Foods, 14(6), 940. https://doi.org/10.3390/foods14060940





