Honey Differentiation Using Infrared and Raman Spectroscopy Analysis and the Employment of Machine-Learning-Based Authentication Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Acquisition
2.2. Vibrational Spectra Collection
2.3. Machine Learning Investigation
3. Results and Discussion
3.1. ATR-IR Spectra Analysis
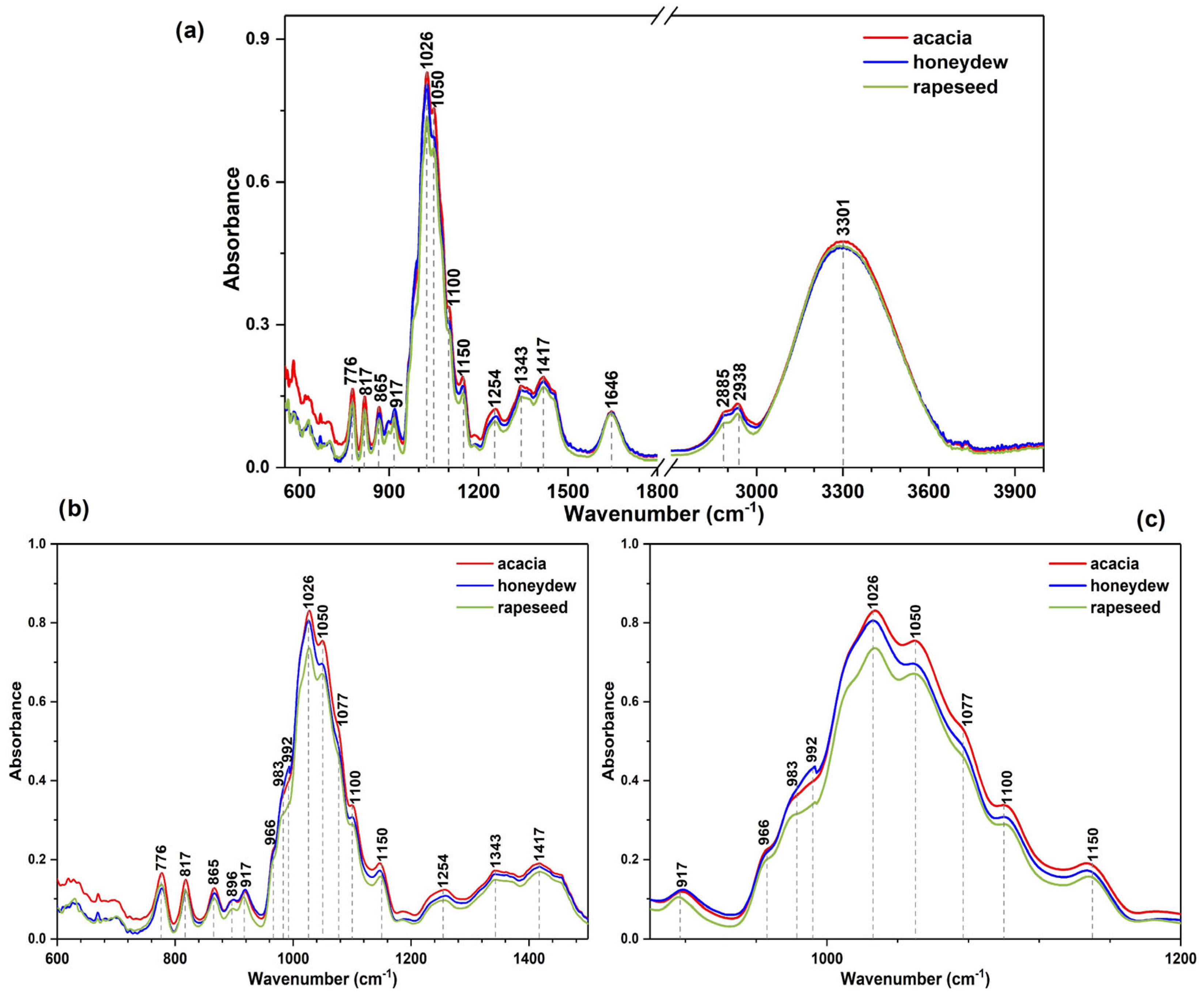
| ATR-IR Band Position (Intensity) in cm−1 | Assignment of The Vibrational Mode | Refs. |
|---|---|---|
| Fingerprint Region | ||
| 600–700 (vw) | “crystalline region” containing the exocyclic deformations of (CCO) and endocyclic deformations of (CCO, CCC) | [30,52,59] |
| 776 (w) | Anomeric region of carbohydrates; C–H bending vibrations; ring vibrations; and COH, CCH, OCH bond bending | [52,60] |
| 817 (w) | ||
| 856 (w) | ||
| 917 (w) | ||
| 966 (m) | C–O (from C–OH) and C–C stretching in carbohydrates | [30,52] |
| 983 (m) | ||
| 992 (m) | ||
| 1026 (s) | C–O stretching in carbohydrates | [52,54,61] |
| 1050 (s) | ||
| 1077 (m) | C–N stretching in proteins | [30,52,56,57] |
| 1100 (m) | The stretching of the C–O bond of the C–O–C linkage | [30,52] |
| 1150 (m) | C-CO-C bending and stretching in carbonyl group, presence of maltose | [54,61] |
| 1254 (w) | Amide III vibration mode | [56,57,59] |
| 1343 (w) | O–C–H, C–C–H, and C–O–H bending modes | [30,52] |
| 1417 (w) | Carbohydrate C–H stretching band, C–H bending of alkenes, and a CH=C Vibration mode of organic acids such as fumaric acids | [30,52] |
| Stretching vibrations area | ||
| Broad band (m) with a maximum peak at 1646 | O–H stretching/bending of water, C=O stretching mainly from carbohydrates, and N–H bending of amide I from proteins | [52,61] |
| Broad band (m) with a maximum peak at 2885 and 2938 | C–OH mode in carbohydrates, O–H stretching from carboxylic acids, and NH3 stretching from amino acids | [30,52,61] |
| Broad band (s) with a maximum peak at 3301 | O–H stretching | [30,61] |
3.2. FT-Raman Spectra Analysis

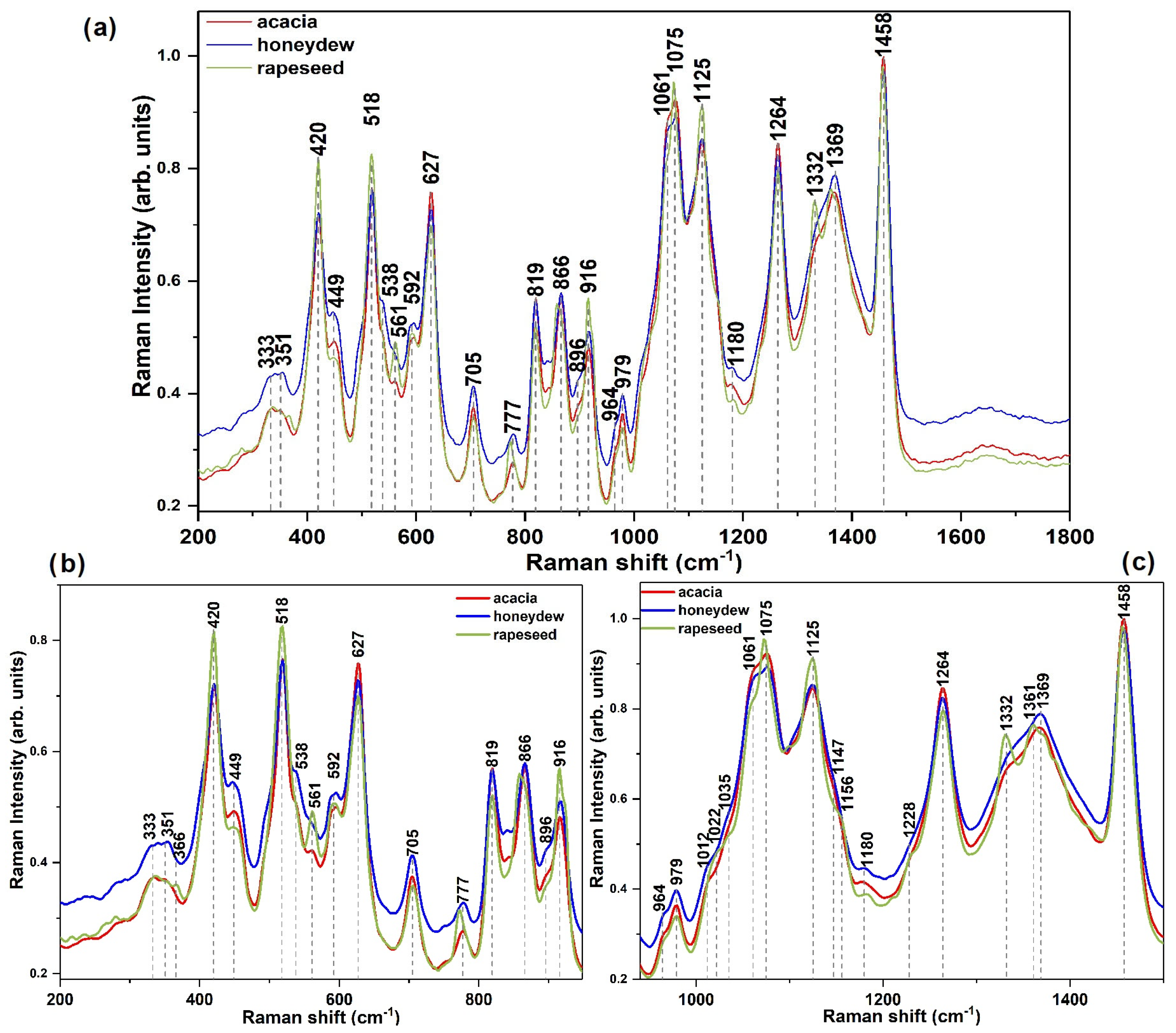
| FT-Raman Band Position (Intensity) in cm−1 | Assignment of the Vibrational Mode | Refs. |
|---|---|---|
| Region 1—Anti-Stokes Region | ||
| −523 (w) | Glucose | [62,63] |
| −627 (w) | Fructose | |
| Region 2—Fingerprint Area | ||
| 333 (w) | Sucrose’s glycosidic linkage C–O–C bending mode | [63,65] |
| 351 (w) | Presence of glucose | [62,65] |
| 420 (m) | Presence of glucose | [62,65] |
| 449 (w) | Skeletal vibration | [65] |
| 518 (m) | Presence of sucrose and fructose | [66] |
| 538 (m) | Bending of C–C–O | [65] |
| 592 (m) | Skeletal vibration | [65] |
| 627 (m) | Ring deformation vibration, fructose bending of C–C–O (exocyclic) | [66] |
| 705 (w) | Stretching vibration of CO, CCO, OCO, presence of fructose | [62,69] |
| 777(vw) | Deformation of C–H in fructose | [62] |
| 819 (m) | Presence of fructose | [62] |
| 866 (m) | Presence of fructose | [62] |
| 896 | Bending of CH | [62] |
| 916 (w) | Bending mode of CH and COH in glucose | [65] |
| 979 (w) | Presence of anomeric fructose | [62,65,69] |
| 1022 (shoulder) | Stretching vibrations of C–C and C–O from glucose | [65] |
| 1061 (s) | Stretching vibration of C–OH, C–C, bending of C–O–H | [66] |
| 1075 (s) | ||
| 1125 (s) | Bending C-OH, presence of glucose | [65] |
| 1264 (s) | Bending vibration of CH, CH2, COH in crystallized fructose | [66] |
| 1332 | Presence of fructose | [65] |
| 1361/1369 (m) | Bending in-plane asymmetric vibration of CH2, presence of disaccharides (sucrose) | [66] |
| 1458 (s) | Bending vibration of CH, symmetric in-plane CH2, C–O–H from fructose | [66] |
3.3. Use of Machine Learning Algorithms for Honey Samples Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121. [Google Scholar]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731. [Google Scholar] [CrossRef]
- Sachdev, S.; Kumar, A.; Ansari, M.I. Health Benefit, Traditional, and Modern Uses of Natural Honey. Non-Timber For. Prod. Food Healthc. Ind. Appl. 2021, 281–299. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Magdas, T.M.; David, M.; Hategan, A.R.; Filip, G.A.; Magdas, D.A. Geographical Origin Authentication—A Mandatory Step in the Efficient Involvement of Honey in Medical Treatment. Foods 2024, 13, 532. [Google Scholar] [CrossRef]
- Brar, D.S.; Pant, K.; Krishnan, R.; Kaur, S.; Rasane, P.; Nanda, V.; Saxena, S.; Gautam, S. A comprehensive review on unethical honey: Validation by emerging techniques. Food Control 2023, 145, 109482. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Berghian-Grosan, C.; Hategan, A.R.; David, M.; Magdas, D.A. Untargeted metabolomic analysis of honey mixtures: Discrimination opportunities based on ATR-FTIR data and machine learning algorithms. Microchem. J. 2023, 188, 108458. [Google Scholar] [CrossRef]
- Fakhlaei, R.; Selamat, J.; Khatib, A.; Razis, A.F.A.; Sukor, R.; Ahmad, S.; Babadi, A.A. The toxic impact of honey adulteration: A review. Foods 2020, 9, 1538. [Google Scholar] [CrossRef]
- European Parliament Resolution of 14 January 2014 on the Food Crisis, Fraud in the Food Chain and the Control Thereof (2013/2091(INI)). Available online: https://www.europarl.europa.eu/doceo/document/TA-7-2014-0011_EN.html (accessed on 2 February 2025).
- European Commission. Commission Welcomes Political Agreement on Clearer Labelling and Improved Composition of Honey, Fruit Juices, Jams and Milk [Press Release]. (31 January 2024). Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_24_563) (accessed on 2 February 2025).
- Zhang, G.; Abdulla, W. On honey authentication and adulterant detection techniques. Food Control 2022, 138, 108992. [Google Scholar] [CrossRef]
- Balkanska, R.; Stefanova, K.; Stoikova–Grigorova, R. Main honey botanical components and techniques for identification: A review. J. Apic. Res. 2022, 59, 852–861. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Halatsi, E.Z.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Volatile fraction of commercial thyme honeys produced in Mediterranean regions and key volatile compounds for geographical discrimination: A chemometric approach. Int. J. Food Prop. 2017, 20, 2699–2710. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Xiaowei, H.; Jiyong, S.; Mariod, A.A. Discrimination of honeys using colorimetric sensor arrays, sensory analysis and gas chromatography techniques. Food Chem. 2017, 206, 37–43. [Google Scholar] [CrossRef]
- Schuhfried, E.; del Pulgar, J.S.; Bobba, M.; Piro, R.; Cappellin, L.; Märk, T.D.; Biasioli, F. Classification of 7 monofloral honey varieties by PTR-ToF-MS direct headspace analysis and chemometrics. Talanta 2017, 147, 213–219. [Google Scholar] [CrossRef]
- Chudzinska, M.; Baralkiewicz, D. Application of ICP-MS method of determination of 15 elements in honey with chemometric approach for the verification of their authenticity. Food Chem. Toxicol. 2011, 49, 2741–2749. [Google Scholar] [CrossRef]
- Schellenberg, A.; Chmielus, S.; Schlicht, C.; Camin, F.; Perini, M.; Bontempo, L.; Horacek, M. Multielement stable isotope ratios (H, C, N, S) of honey from different European regions. Food Chem. 2010, 121, 770–777. [Google Scholar] [CrossRef]
- Zhou, X.; Taylor, M.P.; Salouros, H.; Prasad, S. Authenticity and geographic origin of global honeys determined using carbon isotope ratios and trace elements. Sci. Rep. 2018, 8, 14639. [Google Scholar] [CrossRef]
- Voerkelius, S.; Lorenz, G.D.; Rummel, S.; Quétel, C.R.; Heiss, G.; Baxter, M.; Brach-Papa, C.; Deters-Itzelsberger, P.; Hoelzl, S.; Hoogewerff, J.; et al. Strontium isotopic signatures of natural mineral waters, the reference to a simple geological map and its potential for authentication of food. Food Chem. 2010, 118, 933–940. [Google Scholar] [CrossRef]
- Liu, H.; Nie, J.; Liu, Y.; Wadood, S.A.; Rogers, K.M.; Yuan, Y.; Gan, R.Y. A review of recent compound-specific isotope analysis studies applied to food authentication. Food Chem. 2023, 415, 135791. [Google Scholar] [CrossRef]
- Baroni, M.V.; Podio, N.S.; Badini, R.G.; Inga, M.; Ostera, H.A.; Cagnoni, M.; Gautier, E.A.; García, P.P.; Hoogewerff, J.; Wunderlin, D.A. Linking soil, water, and honey composition to assess the geographical origin of Argentinean honey by multielemental and isotopic analyses. J. Agric. Food Chem. 2015, 63, 4638–4645. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, L.; Camin, F.; Ziller, L.; Perini, M.; Nicolini, G.; Larcher, R. Isotopic and elemental composition of selected types of Italian honey. Measurement 2017, 98, 283–289. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Quirantes, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. Rsc Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef] [PubMed]
- Lohumi, S.; Lee, S.; Lee, H.; Cho, B.K. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci. Technol. 2015, 46, 85–98. [Google Scholar] [CrossRef]
- Ciulu, M.; Hategan, A.R.; David, M.; Magdas, D.A.; Quirantes-Piné, R.; Borrás-Linares, I. Non-destructive Analytical Technologies for the Analysis of Honey and Related Bee-Based Products. In Non-Invasive and Non-Destructive Methods for Food Integrity; Jiménez-Carvelo, A.M., Arroyo-Cerezo, A., Cuadros-Rodríguez, L., Eds.; Springer: Cham, Switzerland, 2024; pp. 281–302. [Google Scholar]
- Aykas, D.P.; Shotts, M.L.; Rodriguez-Saona, L.E. Authentication of commercial honeys based on Raman fingerprinting and pattern recognition analysis. Food Control 2020, 117, 107346. [Google Scholar] [CrossRef]
- Corvucci, F.; Nobili, L.; Melucci, D.; Grillenzoni, F.V. The discrimination of honey origin using melissopalynology and Raman spectroscopy techniques coupled with multivariate analysis. Food Chem. 2015, 169, 297–304. [Google Scholar] [CrossRef]
- Gok, S.; Severcan, M.; Goormaghtigh, E.; Kandemir, I.; Severcan, F. Differentiation of Anatolian honey samples from different botanical origins by ATR-FTIR spectroscopy using multivariate analysis. Food Chem. 2015, 170, 234–240. [Google Scholar] [CrossRef]
- Frausto-Reyes, C.; Casillas-Peñuelas, R.; Quintanar-Stephano, J.L.; Macías-López, E.; Bujdud-Pérez, J.M.; Medina-Ramírez, I. Spectroscopic study of honey from Apis mellifera from different regions in Mexico. Spectrochim. Acta Part A 2017, 178, 212–217. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Berghian-Grosan, C.; Molnar, C.M. Challenges and a step forward in honey classification based on Raman spectroscopy. Food Control 2021, 123, 107769. [Google Scholar] [CrossRef]
- Svečnjak, L.; Bubalo, D.; Baranović, G.; Novosel, H. Optimization of FTIR-ATR spectroscopy for botanical authentication of unifloral honey types and melissopalynological data prediction. Eur. Food Res. Technol. 2015, 240, 1101–1115. [Google Scholar] [CrossRef]
- David, M.; Hategan, A.R.; Magdas, D.A.; Berghian-Grosan, C.; Simionescu, B. Botanical Origin Assessment of Honey Based on ATR-IR Spectroscopy: A Comparison between the Efficiency of Supervised Statistical Methods and Artificial Intelligence. Appl. Sci. 2022, 12, 9645. [Google Scholar] [CrossRef]
- Ruoff, K.; Luginbühl, W.; Bogdanov, S.; Bosset, J.O.; Estermann, B.; Ziolko, T.; Amadò, R. Authentication of the botanical origin of honey by near-infrared spectroscopy. J. Agric. Food Chem. 2006, 54, 6867–6872. [Google Scholar] [CrossRef]
- David, M.; Magdas, D.A. Authentication of honey origin and harvesting year based on Raman spectroscopy and chemometrics. Talanta Open 2024, 10, 100342. [Google Scholar] [CrossRef]
- Woodcock, T.; Downey, G.; O’Donnell, C.P. Near infrared spectral fingerprinting for confirmation of claimed PDO provenance of honey. Food Chem. 2009, 114, 742–746. [Google Scholar] [CrossRef]
- David, M.; Hategan, A.R.; Berghian-Grosan, C.; Magdas, D.A. The development of honey recognition models based on the association between ATR-IR spectroscopy and advanced statistical tools. Int. J. Mol. Sci. 2022, 23, 9977. [Google Scholar] [CrossRef]
- Gan, Z.; Yang, Y.; Li, J.; Wen, X.; Zhu, M.; Jiang, Y.; Ni, Y. Using sensor and spectral analysis to classify botanical origin and determine adulteration of raw honey. J. Food Eng. 2016, 178, 151–158. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data fusion methodologies for food and beverage authentication and quality assessment–A review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef]
- Márquez, C.; López, M.I.; Ruisánchez, I.; Callao, M.P. FT-Raman and NIR spectroscopy data fusion strategy for multivariate qualitative analysis of food fraud. Talanta 2016, 161, 80–86. [Google Scholar] [CrossRef]
- Jandrić, Z.; Haughey, S.A.; Frew, R.D.; McComb, K.; Galvin-King, P.; Elliott, C.T.; Cannavan, A. Discrimination of honey of different floral origins by a combination of various chemical parameters. Food Chem. 2015, 189, 52–59. [Google Scholar] [CrossRef]
- Ballabio, D.; Robotti, E.; Grisoni, F.; Quasso, F.; Bobba, M.; Vercelli, S.; Marengo, E. Chemical profiling and multivariate data fusion methods for the identification of the botanical origin of honey. Food Chem. 2018, 266, 79–89. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Zhihua, L.; Jiyong, S.; Zhai, X.; Wang, S.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Xia, J.; Xiong, Y.; Min, S. Quantitative analysis of honey adulteration by spectrum analysis combined with several high-level data fusion strategies. Vib. Spectrosc. 2020, 108, 103060. [Google Scholar] [CrossRef]
- Liu, N.; Chen, L.; Liu, C.; Sun, X.; Zhang, S. Non-destructive discrimination of honey origin based on multispectral information fusion technology. Infrared Phys. Technol. 2024, 139, 105327. [Google Scholar] [CrossRef]
- European Commission. Honey Market Presentation. (23 November 2023). Available online: https://agriculture.ec.europa.eu/document/download/c04a9774-5ba3-41f5-b256-08396b2888ec_en?filename=market-presentation-honey_spring2024_en.pdf (accessed on 2 February 2025).
- Magdas, D.A.; Berghian-Grosan, C. Botanical honey recognition and quantitative mixture detection based on Raman spectroscopy and machine learning. Spectrochim. Acta Part A 2023, 293, 122433. [Google Scholar] [CrossRef]
- de Sousa, J.M.B.; de Souza, E.L.; Marques, G.; de Toledo Benassi, M.; Gullón, B.; Pintado, M.M.; Magnani, M. Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT-Food Sci. Technol. 2016, 65, 645–651. [Google Scholar] [CrossRef]
- Manzanares, A.B.; García, Z.H.; Galdón, B.R.; Rodríguez, E.R.; Romero, C.D. Physicochemical characteristics of minor monofloral honeys from Tenerife, Spain. LWT-Food Sci. Technol. 2014, 55, 572–578. [Google Scholar] [CrossRef]
- White, J.W. Composition of honey. In Honey: A Comprehensive Survey; Crane, E., Ed.; Heinemann: London, UK, 1979; pp. 157–158. [Google Scholar]
- Tewari, J.C.; Irudayaraj, J.M. Floral classification of honey using mid-infrared spectroscopy and surface acoustic wave based z-Nose sensor. J. Agric. Food Chem. 2005, 53, 6955–6966. [Google Scholar] [CrossRef]
- Kelly, J.D.; Petisco, C.; Downey, G. Application of Fourier transform midinfrared spectroscopy to the discrimination between Irish artisanal honey and such honey adulterated with various sugar syrups. J. Agric. Food Chem. 2006, 54, 6166–6171. [Google Scholar] [CrossRef]
- Wang, J.; Kliks, M.M.; Jun, S.; Jackson, M.; Li, Q.X. Rapid analysis of glucose, fructose, sucrose, and maltose in honeys from different geographic regions using Fourier transform infrared spectroscopy and multivariate analysis. J. Food Sci. 2010, 75, C208–C214. [Google Scholar] [CrossRef]
- Benković, M.; Jurina, T.; Longin, L.; Grbeš, F.; Valinger, D.; Jurinjak Tušek, A.; Gajdoš Kljusurić, J. Qualitative and Quantitative Detection of Acacia Honey Adulteration with Glucose Syrup Using Near-Infrared Spectroscopy. Separations 2022, 9, 312. [Google Scholar] [CrossRef]
- Sun, D.W. Infrared Spectroscopy for Food Quality Analysis and Control; Academic Press: Cambridge, MA, USA; Elsevier: San Diego, CA, USA, 2009. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies-Tables and Charts, 3rd ed.; J. Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Cai, S.; Singh, B.R. A distinct utility of the amide III infrared band for secondary structure estimation of aqueous protein solutions using partial least squares methods. Biochemistry 2004, 43, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M.; Koenig, J.L. Vibrational spectra of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987, 44, 7–89. [Google Scholar] [CrossRef]
- Subari, N.; Saleh, J.M.; Shakaff, A.Y.M.; Zakaria, A. A hybrid sensing approach for pure and adulterated honey classification. Sensors 2012, 12, 14022–14040. [Google Scholar] [CrossRef]
- Gallardo-Velázquez, T.; Osorio-Revilla, G.; Zuñiga-de Loa, M.; Rivera-Espinoza, Y. Application of FTIR-HATR spectroscopy and multivariate analysis to the quantification of adulterants in Mexican honeys. Food Res. Int. 2009, 42, 313–318. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Luu, C.; Meffroy-Biget, A.M.; Luu, D.V. Laser-Raman study of solute-solvent interactions in aqueous solutions of D-fructose, D-glucose, and sucrose. Carbohydr. Res. 1980, 81, 213–223. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Feher, I.; Pinzaru, S.C. Wine discrimination based on chemometric analysis of untargeted markers using FT-Raman spectroscopy. Food Control 2018, 85, 385–391. [Google Scholar] [CrossRef]
- Pierna, J.A.F.; Abbas, O.; Dardenne, P.; Baeten, V. Discrimination of Corsican Honey by FT-Raman Spectroscopy and Chemometrics. Base. 2011. Available online: https://popups.uliege.be/1780-4507/index.php?id=6895Honey (accessed on 2 February 2025).
- De Oliveira, L.F.C.; Colombara, R.; Edwards, H.G. Fourier transform Raman spectroscopy of honey. Appl. Spectrosc. 2002, 56, 306–311. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Söderholm, S.; Roos, Y.H.; Meinander, N.; Hotokka, M. Raman spectra of fructose and glucose in the amorphous and crystalline states. J. Raman Spectrosc. 1999, 30, 1009–1018. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, M.; Berghian-Grosan, C.; Magdas, D.A. Honey Differentiation Using Infrared and Raman Spectroscopy Analysis and the Employment of Machine-Learning-Based Authentication Models. Foods 2025, 14, 1032. https://doi.org/10.3390/foods14061032
David M, Berghian-Grosan C, Magdas DA. Honey Differentiation Using Infrared and Raman Spectroscopy Analysis and the Employment of Machine-Learning-Based Authentication Models. Foods. 2025; 14(6):1032. https://doi.org/10.3390/foods14061032
Chicago/Turabian StyleDavid, Maria, Camelia Berghian-Grosan, and Dana Alina Magdas. 2025. "Honey Differentiation Using Infrared and Raman Spectroscopy Analysis and the Employment of Machine-Learning-Based Authentication Models" Foods 14, no. 6: 1032. https://doi.org/10.3390/foods14061032
APA StyleDavid, M., Berghian-Grosan, C., & Magdas, D. A. (2025). Honey Differentiation Using Infrared and Raman Spectroscopy Analysis and the Employment of Machine-Learning-Based Authentication Models. Foods, 14(6), 1032. https://doi.org/10.3390/foods14061032







