The Particle Size of Wheat Bran Dietary Fiber Influences Its Improvement Effects on Constipation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. WBDF Preparation
2.3. Physicochemical Properties of WBDF
2.3.1. Particle Size Distribution
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.3. Scanning Electron Microscope (SEM)
2.3.4. Water-Holding Capacity (WHC)
2.3.5. Oil-Holding Capacity (OHC)
2.3.6. Swelling Capacity (SC)
2.4. Animal Experiments
2.5. Measurement of Constipation Indices
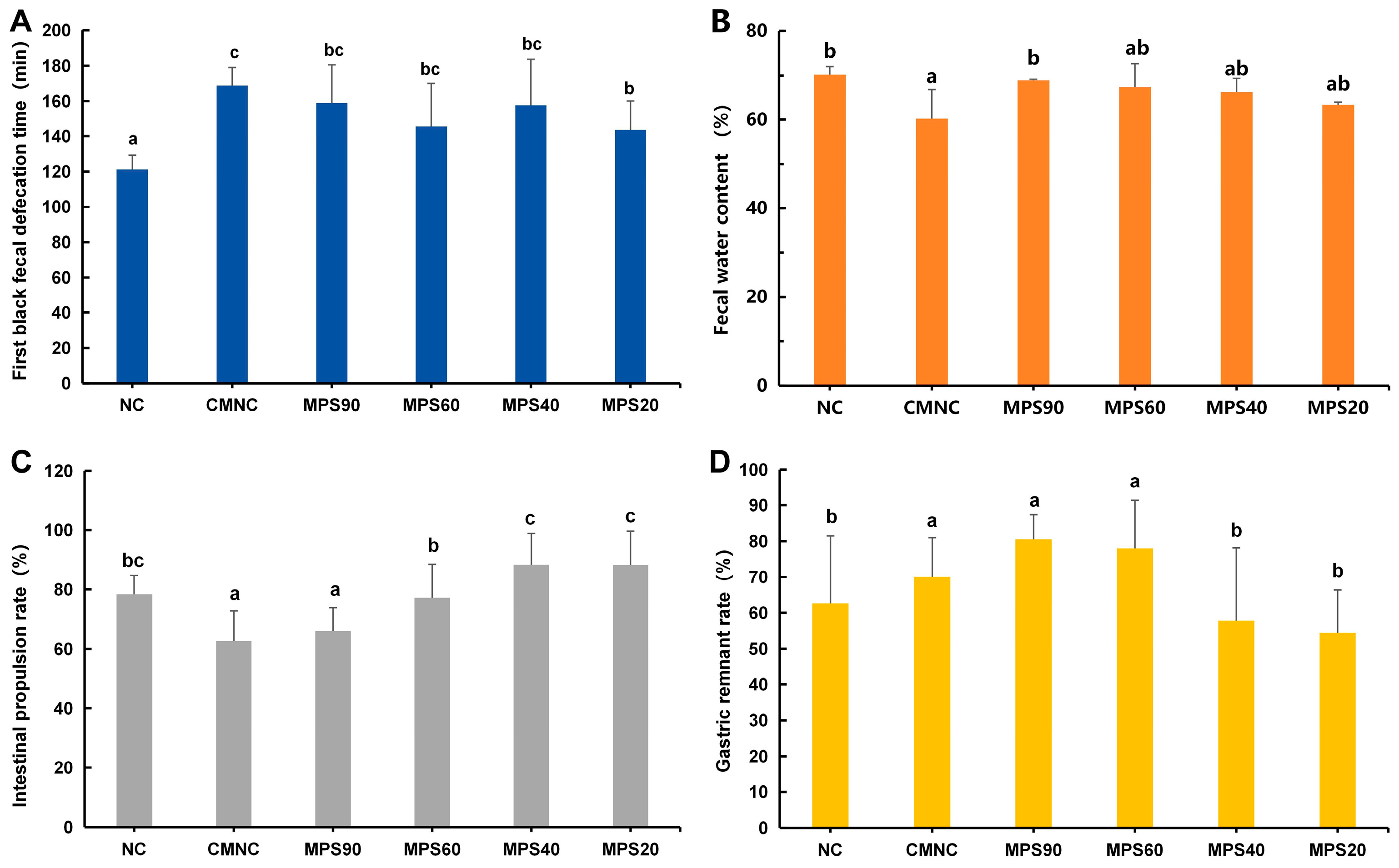
2.5.1. Fecal Moisture Content and Defecation Time of First Dark Stool
2.5.2. Gastric Residual Rate
2.5.3. Intestinal Propulsive Rate
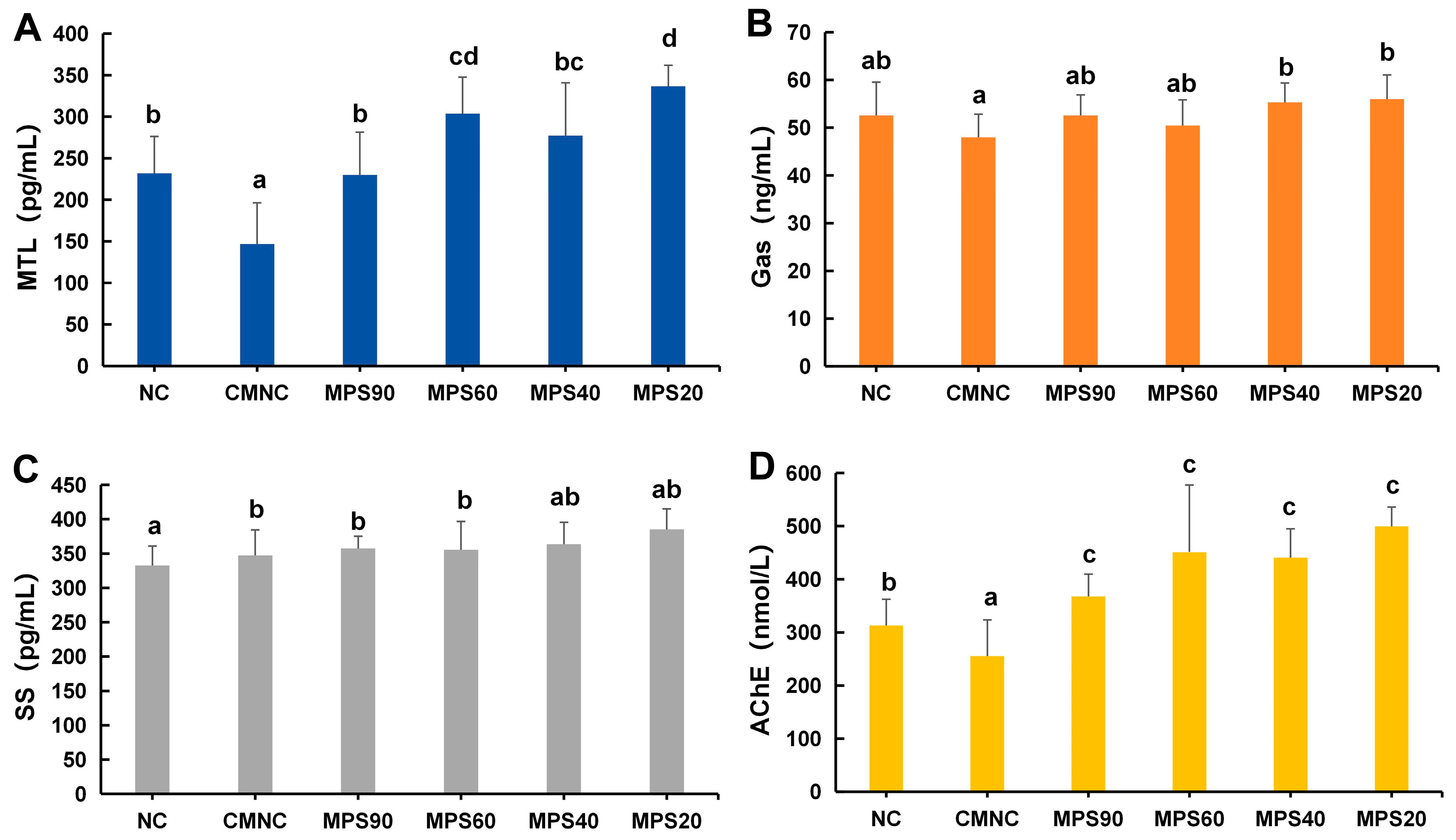
2.6. Biochemical Assays
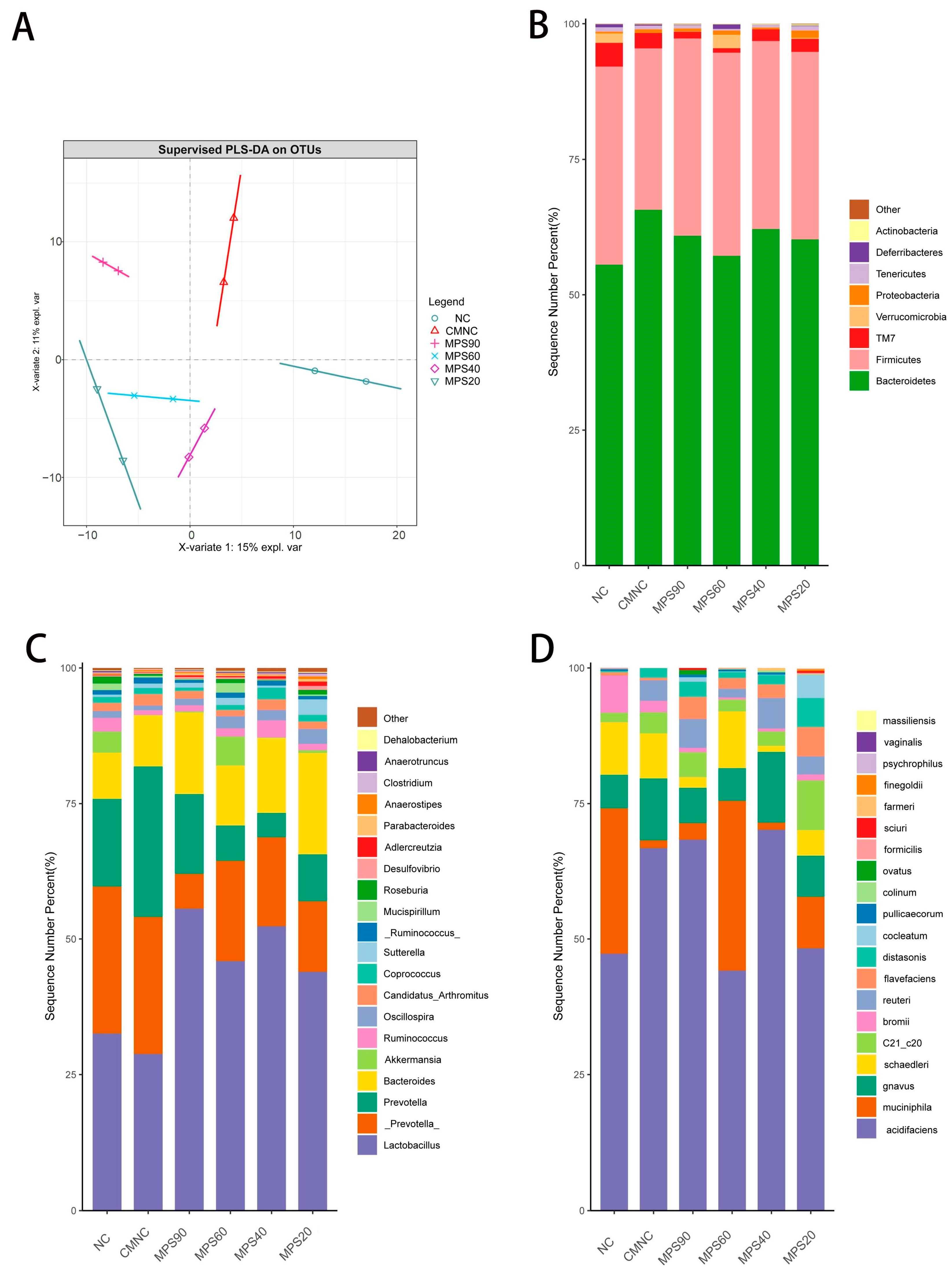
2.7. Fecal Microbiome Profiles by 16S rRNA Gene Sequencing
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.1.1. Particle Size Distribution
3.1.2. FT-IR
3.1.3. SEM
3.1.4. WHC, OHC, and SC
3.2. Effect of WBDF on Excretion Indices in Constipated Mice
3.3. Effect of WBDF on Contents of GI Hormones in Mice
3.4. Effect of WBDF with Different Particle Sizes on Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| DF | dietary fiber |
| FT-IR | Fourier-transform infrared |
| Gas | gastrin |
| IDF | insoluble dietary fiber |
| MPS | mean particle size |
| MTL | motilin |
| OHC | oil-holding capacity |
| OTU | operational taxonomic unit |
| SC | swelling capacity |
| SDF | soluble dietary fiber |
| SEM | scanning electron microscope |
| SS | somatostatin |
| WBDF | wheat bran dietary fiber |
| WHC | water-holding capacity |
References
- Shi, Y.; Chen, F.; Wang, Z.; Cao, J.; Li, C. Effect and mechanism of functional compound fruit drink on gut microbiota in constipation mice. Food Chem. 2023, 401, 134210. [Google Scholar] [CrossRef]
- Hu, M.; Fang, C.; Liu, Y.; Gao, M.; Zhang, D.; Shi, G.; Yin, Z.; Zheng, R.; Zhang, J. Comparative study of the laxative effects of konjac oligosaccharides and konjac glucomannan on loperamide-induced constipation in rats. Food Funct. 2021, 12, 7709–7717. [Google Scholar] [CrossRef] [PubMed]
- Koppen, I.J.N.; Vriesman, M.H.; Saps, M.; Rajindrajith, S.; Shi, X.; van Etten-Jamaludin, F.S.; Di Lorenzo, C.; Benninga, M.A.; Tabbers, M.M. Prevalence of functional defecation disorders in children: A systematic review and meta-analysis. J. Pediatr. 2018, 198, 121–130.e6. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Jiang, Y.; Wu, B.; Yu, Y. Aqueous Extract of Phyllanthus emblica L. Alleviates functional dyspepsia through regulating gastrointestinal hormones and gut microbiome in vivo. Foods 2022, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zou, H.; Qiao, X.; Wang, Y.; Lv, M.; Zhang, K.; Wang, F. The relationship between oxidative balance scores and chronic diarrhea and constipation: A population-based study. BMC Public Health 2024, 24, 1366. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Sun, B.; Ma, S. Interaction of wheat bran dietary fiber-gluten protein affects dough product: A critical review. Int. J. Biol. Macromol. 2024, 255, 128199. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Sun, Y.; Fan, M.; Li, Y.; Wang, L.; Qian, H. Wheat bran, as the resource of dietary fiber: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7269–7281. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 63, 8752–8767. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Peng, Z.; Ahmat, A.; Wang, M.; Hai, W. Effects of insoluble dietary fiber from wild apricot flesh on intestinal function and intestinal flora of mice. Sci. Technol. Food Ind. 2020, 41, 307–310. [Google Scholar]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Vuksan, V.; Augustin, L.S.A.; Li, Y.M.; Lee, B.; Mehling, C.C.; Parker, T.; Faulkner, D.; Seyler, H.; et al. The effect of wheat bran particle size on laxation and colonic fermentation. J. Am. Coll. Nutr. 1999, 18, 339–345. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, F.; Gao, W. Effects of superfine grinding on asparagus pomace. Part II: Changes on intestinal function and health. LWT-Food Sci. Technol. 2021, 140, 110799. [Google Scholar] [CrossRef]
- Yang, B.; Li, K.; Niu, M.; Wei, J.; Zhao, S.; Jia, C.; Xu, Y. Structural characteristics of wheat bran insoluble dietary fiber with various particle size distributions and their influences on the kinetics of gastrointestinal emptying in mice. Int. J. Biol. Macromol. 2024, 272, 132905. [Google Scholar] [CrossRef]
- van der Schoot, A.; Drysdale, C.; Whelan, K.; Dimidi, E. The effect of fiber supplementation on chronic constipation in adults: An updated systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Li, Y.; He, Y.; Chen, F.; Mi, B.; Li, J.; Xie, J.; Ma, G.; Yang, J.; Xu, K.; et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: A double-blinded randomized placebo trial. Gut Microbes 2023, 15, 2197837. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, J.; Ruan, Z.; Wang, Z. Fermentation of dietary fibers modified by an enzymatic-ultrasonic treatment and evaluation of their impact on gut microbiota in mice. J. Food Process. Preserv. 2021, 45, e15337. [Google Scholar] [CrossRef]
- Dapa, T.; Xavier, K.B. Effect of diet on the evolution of gut commensal bacteria. Gut Microbes 2024, 16, 2369337. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Bao, Q.; Yan, J.; Ma, S. Effect of heat treatment on conformation and aggregation properties of wheat bran dietary fiber-gluten protein. Int. J. Biol. Macromol. 2023, 253, 127164. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Shao, H.; Zheng, X.; Liu, J.; Liu, J.; Huang, J.; Zhang, C.; Liu, Z.; Wang, J.; Guan, W. Modification of sweet potato (Ipomoea batatas Lam.) residues soluble dietary fiber following twin-screw extrusion. Food Chem. 2021, 335, 127522. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Al-Nuaimi, A.K.; Al-Mahadin, S.; Liu, S.-Q. In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chem. 2018, 239, 588–597. [Google Scholar] [CrossRef]
- Lu, Z.; Ye, F.; Zhou, G.; Gao, R.; Qin, D.; Zhao, G. Micronized apple pomace as a novel emulsifier for food O/W Pickering emulsion. Food Chem. 2020, 330, 127325. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J. Cereal Sci. 2011, 54, 98–103. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation Through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef]
- Huang, J.; Lin, B.; Zhang, Y.; Xie, Z.; Zheng, Y.; Wang, Q.; Xiao, H. Bamboo shavings derived O-acetylated xylan alleviates loperamide-induced constipation in mice. Carbohydr. Polym. 2022, 276, 118761. [Google Scholar] [CrossRef]
- Hu, T.G.; Wen, P.; Fu, H.Z.; Lin, G.Y.; Liao, S.T.; Zou, Y.X. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, H.; Sun, M.; Jiang, C.; Jiang, S.; Mu, G.; Tuo, Y.; Gao, P. Milk fermented by combined starter cultures comprising three Lactobacillus strains exerts an alleviating effect on loperamide-induced constipation in BALB/c mice. Food Funct. 2023, 14, 5264–5276. [Google Scholar] [CrossRef]
- Cao, J.; Wang, K.; Li, N.; Zhang, L.; Qin, L.; He, Y.; Wang, J.; Qu, C.; Miao, J. Soluble dietary fiber and cellulose from Saccharina japonica by-product ameliorate Loperamide-induced constipation via modulating enteric neurotransmitters, short-chain fatty acids and gut microbiota. Int. J. Biol. Macromol. 2023, 226, 1319–1331. [Google Scholar] [CrossRef]
- Davis, L.D.R.; Spencer, W.J.; Mack, D.R.; Altosaar, I. Maternal separation and gastrointestinal transit time in neonate rats. Lab. Anim. 2011, 45, 280–282. [Google Scholar] [CrossRef]
- Jia, F.; Yang, S.; Ma, Y.; Gong, Z.; Cui, W.; Wang, Y.; Wang, W. Extraction optimization and constipation-relieving activity of dietary fiber from Auricularia polytricha. Food Biosci. 2020, 33, 100506. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, S.; Xu, Z.; Su, H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary synbiotic ameliorates constipation Through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, M.; Huang, S.; Deng, G.; Chen, H.; Lu, M.; Zhang, G.; Chen, L. Effects of tris (2-chloroethyl) phosphate exposure on gut microbiome using the simulator of the human intestinal microbial ecosystem (SHIME). Chemosphere 2023, 340, 139969. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Z.; Li, C.; Luo, Y.; Zhang, G. Diethyl ethylphosphonate retardants disturbed the gut microbiome and metabolite SCFAs in vitro based on simulator of the human intestinal microbial ecosystem. Environ. Pollut. 2024, 363, 125064. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, J.; Liu, D.; Zhang, T.; Wu, Y.; Xie, M. Gut microbiota and metabolite profiles associated with functional constipation severity. Microbiol. Immunol. 2025, 69, 85–95. [Google Scholar] [CrossRef]
- Yue, Y.; Hovey, K.M.; Wactawski-Wende, J.; Lamonte, M.J.; Andrews, C.A.; Diaz, P.I.; McSkimming, D.I.; Buck, M.; Sun, Y.; Millen, A.E. Association between healthy eating index-2020 and oral microbiome among postmenopausal women. J. Nutr. 2025, 155, 66–77. [Google Scholar] [CrossRef]
- Chau, C.F.; Wen, Y.L.; Wang, Y.T. Effects of micronisation on the characteristics and physicochemical properties of insoluble fibres. J. Sci. Food Agric. 2006, 86, 2380–2386. [Google Scholar] [CrossRef]
- Yang, R.; Song, X.; Wang, T.; Liang, B.; Li, X.; Ji, C.; Sun, C.; Zhang, X. Superfine grinding combined with enzymatic modification of pea dietary fibre: Structures, physicochemical properties, hypoglycaemic functional properties, and antioxidant activities. LWT-Food Sci. Technol. 2024, 207, 116651. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of modification methods on physicochemical and structural properties of soluble dietary fiber from corn bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Cao, X.; Liu, T. The structural characteristics of dietary fibers from Tremella fuciformis and their hypolipidemic effects in mice. Food Sci. Hum. Wellness 2023, 12, 503–511. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Fu, X.; Huang, Q.; Chen, Q. Physicochemical, functional and biological properties of soluble dietary fibers obtained from Rosa roxburghii Tratt pomace using different extraction methods. Process Biochem. 2023, 128, 40–48. [Google Scholar] [CrossRef]
- Yan, T.; Liu, R.; Shi, L.; Wang, Y.; Meng, X.; Shen, Y. Superfine grinding improves the physicochemical, sensory and functional characteristics of Hanfu apple pomace. Int. J. Food Sci. Technol. 2023, 58, 2077–2084. [Google Scholar] [CrossRef]
- Liu, T.; Lei, H.; Zhen, X.; Liu, J.; Xie, W.; Tang, Q.; Gou, D.; Zhao, J. Advancements in modifying insoluble dietary fiber: Exploring the microstructure, physicochemical properties, biological activity, and applications in food industry—A review. Food Chem. 2024, 458, 140154. [Google Scholar] [CrossRef] [PubMed]
- Qiu-ping, Y.; Xin-ping, Z.; Xiao-qian, Z. Research progress on preparation technology and physicochemical properties of dietary fiber. Food Res. Dev. 2019, 40, 212–217. [Google Scholar] [CrossRef]
- Huo, Y.; Niu, Y.; Feng, S.; Zhang, L.; Xu, J.; Hu, Q. Effect of three modification methods on physical and functional properties of flaxseed cake dietary fiber. LWT-Food Sci. Technol. 2024, 198, 116076. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Liao, A.-M.; Thakur, K.; Huang, J.-H.; Zhang, J.-G.; Wei, Z.-J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019, 297, 124983. [Google Scholar] [CrossRef]
- Cao, P.Q.; Li, X.P.; Jian, O.Y.; Jiang, R.G.; Huang, F.F.; Wen, B.B.; Zhang, X.N.; Huang, J.A.; Liu, Z.H. The protective effects of yellow tea extract against loperamide-induced constipation in mice. Food Funct. 2021, 12, 5621–5636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhong, D.; Sun, R.; Zhang, Y.; Pegg, R.B.; Zhong, G. Prevention of loperamide induced constipation in mice by KGM and the mechanisms of different gastrointestinal tract microbiota regulation. Carbohydr. Polym. 2021, 256, 117418. [Google Scholar] [CrossRef]
- Zhang, S.; Okuhara, Y.; Iijima, M.; Takemi, S.; Sakata, I.; Kaiya, H.; Teraoka, H.; Kitazawa, T. Identification of pheasant ghrelin and motilin and their actions on contractility of the isolated gastrointestinal tract. Gen. Comp. Endocrinol. 2020, 285, 113294. [Google Scholar] [CrossRef]
- Asano, H.; Tomita, T.; Nakamura, K.; Yamasaki, T.; Okugawa, T.; Kondo, T.; Kono, T.; Tozawa, K.; Ohda, Y.; Oshima, T.; et al. Prevalence of gastric motility disorders in patients with functional dyspepsia. J. Neurogastroenterol. Motil. 2017, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Yan, Q.; Li, Y.; Jiang, Z. Effect of Konjac mannan oligosaccharides on diphenoxylate-induced constipation in mice. J. Funct. Foods 2019, 57, 399–407. [Google Scholar] [CrossRef]
- Zhang, H.; Zu, Q.; Zhang, J.; Liu, S.; Zhang, G.; Chang, X.; Li, X. Soluble dietary fiber of hawthorn relieves constipation induced by loperamide hydrochloride by improving intestinal flora and inflammation, thereby regulating the aquaporin ion pathway in mice. Foods 2024, 13, 2220. [Google Scholar] [CrossRef]
- Wang, A.; Wang, X.; Zhuang, M.; Ke, S.; Ning, M.; Yu, P.; Zhou, Z. Size-dependent physicochemical property and functionality of insoluble dietary fiber derived from wheat bran. LWT-Food Sci. Technol. 2024, 193, 115747. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Lu, L.; Yang, W.; Huang, T.; Lin, Z.; Lin, C.; Kwan, H.; Wong, H.L.X.; Chen, Y.; et al. Saturated long-chain fatty acid-producing bacteria contribute to enhanced colonic motility in rats. Microbiome 2018, 6, 107. [Google Scholar] [CrossRef]
- Tang, S.; Dong, X.; Ma, Y.; Zhou, H.; He, Y.; Ren, D.; Li, X.; Cai, Y.; Wang, Q.; Wu, L. Highly crystalline cellulose microparticles from dealginated seaweed waste ameliorate high fat-sugar diet-induced hyperlipidemia in mice by modulating gut microbiota. Int. J. Biol. Macromol. 2024, 263, 130485. [Google Scholar] [CrossRef]
- Han, W.; Zhuang, X. Research progress on the next-generation probiotic Akkermansia muciniphila in the intestine. Food Front. 2021, 2, 443–448. [Google Scholar] [CrossRef]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of dietary fiber on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, 14, 356–369. [Google Scholar] [CrossRef]
- Liu, D.; Lin, L.; Lin, Y.; Zhong, Y.; Zhang, S.; Liu, W.; Zou, B.; Liao, Q.; Xie, Z. Zengye decoction induces alterations to metabolically active gut microbiota in aged constipated rats. Biomed. Pharmacother. 2019, 109, 1361–1371. [Google Scholar] [CrossRef]



| Method | Disintegrator | Colloid Mill | Ultra-High Pressure Homogenizer | Ball Mill |
|---|---|---|---|---|
| Median particle size | 101.72 ± 0.99 a | 72.80 ± 2.53 b | 41.98 ± 3.34 c | 20.55 ± 1.34 d |
| Modal particle size | 119.46 ± 14.53 a | 115.54 ± 5.79 a | 53.34 ± 5.78 b | 12.53 ± 5.63 b |
| Mean particle size | 84.14 ± 2.29 a | 61.74 ± 1.61 b | 37.39 ± 2.66 c | 22.33 ± 1.99 d |
| Samples | MPS90 | MPS60 | MPS40 | MPS20 |
| Group | NC | CMNC | MPS90 | MPS60 | MPS40 | MPS20 |
|---|---|---|---|---|---|---|
| Chao1 | 260.88 ± 2.53 ab | 181.04 ± 63.70 a | 315.65 ± 8.63 b | 352.11 ± 10.40 b | 288.78 ± 5.26 b | 327.70 ± 104.23 b |
| Faith’s pd | 13.66 ± 2.46 a | 13.94 ± 7.03 a | 17.57 ± 2.33 a | 17.16 ± 0.63 a | 15.97 ± 0.40 a | 16.68 ± 2.97 a |
| Shannon | 5.99 ± 0.31 b | 5.30 ± 0.26 a | 6.08 ± 0.20 b | 6.18 ± 0.05 b | 6.12 ± 0.22 b | 6.35 ± 0.59 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Hu, L.; Chen, R.; Yang, R.; Gong, L.; Wang, J. The Particle Size of Wheat Bran Dietary Fiber Influences Its Improvement Effects on Constipation. Foods 2025, 14, 1001. https://doi.org/10.3390/foods14061001
Li L, Hu L, Chen R, Yang R, Gong L, Wang J. The Particle Size of Wheat Bran Dietary Fiber Influences Its Improvement Effects on Constipation. Foods. 2025; 14(6):1001. https://doi.org/10.3390/foods14061001
Chicago/Turabian StyleLi, Luyao, Linlin Hu, Rui Chen, Ruoyan Yang, Lingxiao Gong, and Jing Wang. 2025. "The Particle Size of Wheat Bran Dietary Fiber Influences Its Improvement Effects on Constipation" Foods 14, no. 6: 1001. https://doi.org/10.3390/foods14061001
APA StyleLi, L., Hu, L., Chen, R., Yang, R., Gong, L., & Wang, J. (2025). The Particle Size of Wheat Bran Dietary Fiber Influences Its Improvement Effects on Constipation. Foods, 14(6), 1001. https://doi.org/10.3390/foods14061001








