Simultaneous Determination of Six Acidic Pesticides, Including 2,4-DB and 2,4,5-T with No Established MRL in Korea Using LC-MS/MS and QuEChERS for the Safety of Imported Agricultural Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Agricultural Products
2.2. Reagents and Chemicals
2.3. Instrumental Conditions for Analysis
2.4. Optimization of Quantitation Method
2.5. Validation of Analytical Method
2.6. Pesticide Monitoring of Imported Agricultural Products Using the Validated Method
3. Results and Discussion
3.1. Optimization and Verification of the QuEChERS Method
3.2. Method Validation
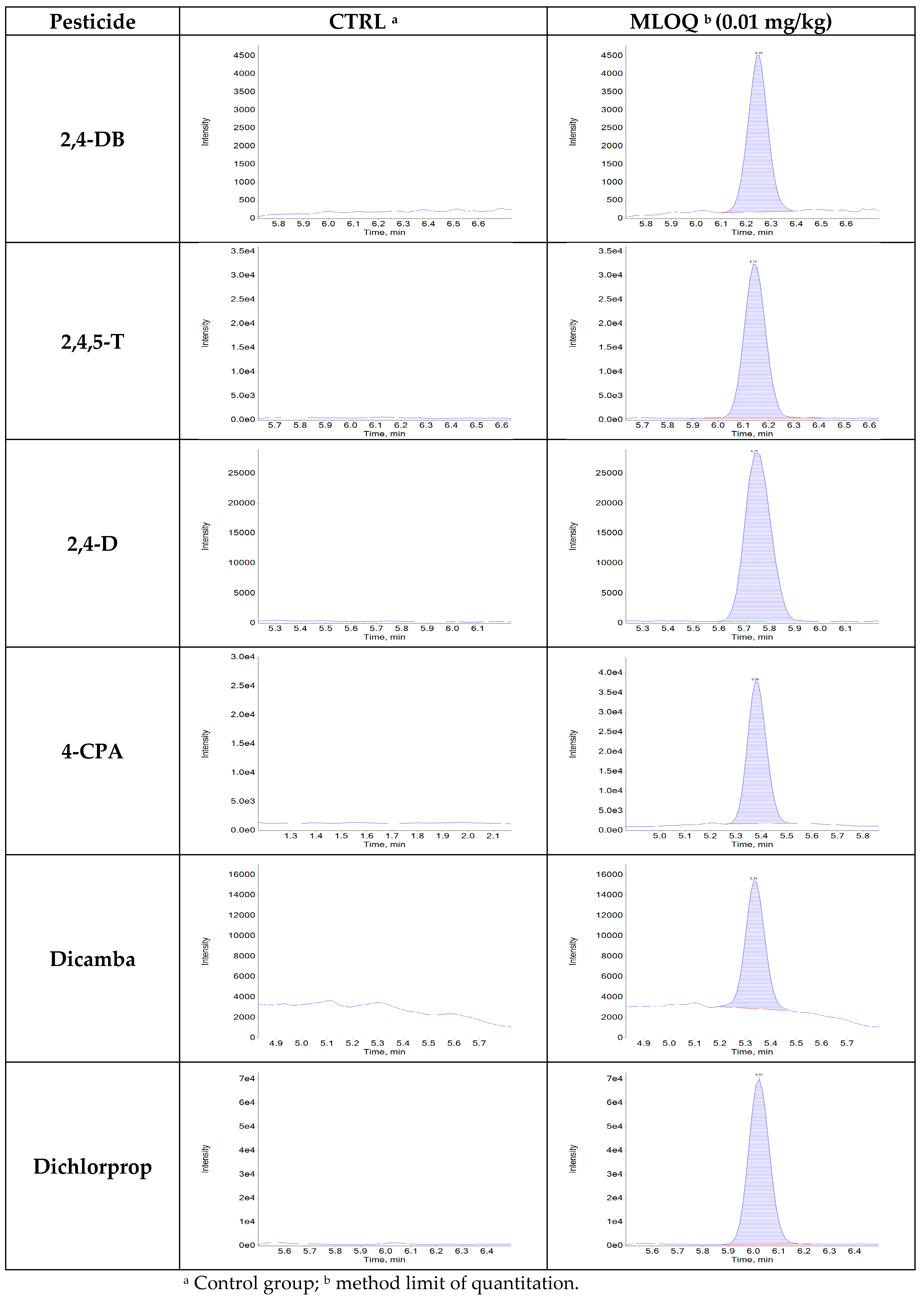
3.2.1. Selectivity, LOD, and LOQ
3.2.2. Linearity
3.2.3. Accuracy and Precision
3.2.4. Matrix Effect
3.2.5. Ion Ratio
3.2.6. Pesticide Monitoring of Imported Agricultural Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Properties | Acidic Pesticides | |||||
|---|---|---|---|---|---|---|
| 2,4-DB | 2,4,5-T | 2,4-D | 4-CPA | Dicamba | Dichlorprop | |
| Chemical Structure |  | 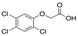 |  | 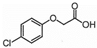 |  | 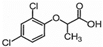 |
| Classification | Herbicide | Herbicide | Herbicide | PGR d | Herbicide | PGR, Herbicide |
| Group | PCA e | PCA | PCA | PCA | Benzoic acid | PCA |
| M.f. a | C10H10Cl2O3 | C8H5Cl3O3 | C8H6Cl2O3 | C8H7ClO3 | C8H6Cl2O3 | C9H8Cl2O3 |
| Mol. Wt. b | 249.1 | 255.5 | 221.0 | 186.6 | 221.0 | 235.1 |
| Log Kow | −0.25 (pH 9) 1.35 (pH 7) 2.94 (pH 5) | 4.0 | −1.07 (pH 10) −0.82 (pH 7) 1.54 (pH 4) | 2.52 | −1.9 (pH 8.9) −1.88 (pH 6.8) −0.55 (pH 5.0) | <1.0 (pH 7, 9) 1.11 (pH 5) |
| pKa (20−25 °C) | 4.1 | 2.88 | 3.4 | 3.18 | 1.87 | 3.0 |
| V.p. c | 0.0944 (23.6 °C) | 1 × 10−7 (25 °C) | 0.0099 (20 °C) 0.023 (25 °C) | 0.024 (25 °C) | 1.67 (25 °C, calc.) | <0.01 (20 °C) |
| Water solubility (mg/l, 20−25 °C) | 62.0 (pH 5) 4385.0 (pH 7) 4.548 × 105 (pH 9) | 268 (25 °C) | 3390.0 (pH 4) 2.0031 × 104 (pH 5) 2.43 × 104 (pH 7) 2.65 × 104 (pH 10) | Insoluble | 6600.0 (pH 1.8) >2.5 × 105 (pH 4.1, 6.8, 8.2) | 350.0 |
| Pesticide | Exact Mass | Ionization Mode | MRM Transitions | TR e | |||||
|---|---|---|---|---|---|---|---|---|---|
| Precursor Ion, m/z | Product Ion, m/z | DP a | EP b | CE c | CXP d | ||||
| 2,4-DB * | 248.0 | [M−H]− | 247.0 | 160.8 | −64 | −10 | −12 | −10 | 6.25 |
| 2,4-DB-1 | 248.6 | 162.9 | −35 | −10 | −18 | −18 | |||
| 2,4,5-T * | 253.9 | [M−H]− | 253.0 | 159.0 | −50 | −10 | −40 | −22 | 6.15 |
| 2,4,5-T-1 | 253.0 | 195.0 | −50 | −10 | −40 | −22 | |||
| 2,4-D * | 220.0 | [M−H]− | 219.0 | 160.9 | −19 | −10 | −14 | −10 | 5.75 |
| 2,4-D-1 | 219.0 | 124.9 | −10 | −10 | −34 | −26 | |||
| 4-CPA * | 186.0 | [M−H]− | 185.0 | 126.8 | −71 | −10 | −18 | −10 | 5.38 |
| 4-CPA-1 | 186.7 | 128.9 | −40 | −10 | −20 | −18 | |||
| Dicamba * | 220.0 | [M−H]− | 219.0 | 175.0 | −21 | −10 | −6 | −12 | 5.34 |
| Dicamba-1 | 218.9 | 35.0 | −30 | −10 | −35 | −18 | |||
| Dichlorprop * | 234.0 | [M−H]− | 233.0 | 161.0 | −26 | −10 | −14 | −12 | 6.03 |
| Dichlorprop-1 | 233.0 | 125.1 | −26 | −10 | −34 | −26 | |||
Appendix B
References
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and health effects of pesticide residues. In Sustainable Agriculture Reviews, 48th ed.; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Springer: Cham, Switzerland; New York, NY, USA, 2021; Volume 8, pp. 311–336. [Google Scholar]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Kim, G.H.; Ahn, K.G.; Kim, G.P.; Hwang, Y.S.; Chang, M.I.; Kang, I.K.; Lee, Y.D.; Choung, M.G. Development of an Official Method for Measurement of Fluazinam Residues for Quarantine of Imported and Exported Horticultural Products. Korean J. Hortic. Sci. Technol. 2016, 34, 183–194. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, H.J.; Lee, J.H.; Gwak, I.S.; Moon, H.S.; Song, M.H.; Jang, M.N.; Lee, M.S.; Park, J.S.; Lee, K.H. Monitoring of residual pesticides in commercial agricultural products in Korea. Korean J. Food Sci. Technol. 2007, 39, 237–245. [Google Scholar]
- Lakatos, C.; Nilsson, L. The EU-Korea FTA: Anticipation, trade policy uncertainty and impact. Rev. World Econ. 2017, 153, 179–198. [Google Scholar] [CrossRef]
- Cheong, I.K. Analysis of the FTA Negotiation between China and Korea. Asian Econ. Pap. 2016, 15, 170–187. [Google Scholar] [CrossRef]
- Konduru, S.; Kim, T.H.; Paggi, M. Implications of the US -South Korea Free Trade Agreement on Agricultural Exports from the US. J. Econ. Financ. Stud. 2014, 2, 46–56. [Google Scholar] [CrossRef][Green Version]
- Lee, G.S.; Lim, S.S. FTA Effects on Agricultural Trade with Matching Approaches. Economics 2015, 9, 201543. [Google Scholar] [CrossRef]
- Korea Agro-Fisheries & Food Trade Corporation (KATI). Agriculture and Forestry Export & Import Statistics. 2022, p. 225. Available online: https://www.kati.net/board/pubilshedMaterialsView.do?board_seq=97859&menu_dept2=48 (accessed on 3 July 2024).
- Akiyama, H.; Iwasaki, Y.; Ito, R. Basic Principles for Setting MRLs for Pesticides in Food Commodities in Japan. Food Saf. 2024, 12, 34–51. [Google Scholar] [CrossRef]
- Chung, S.J.; Kim, H.Y.; Kim, J.H.; Yeom, M.S.; Cho, J.H.; Lee, S.Y. Monitoring of Pesticide Residues and Risk Assessment in Some Fruits on the Market in Incheon, Korea. Korean J. Environ. Agric. 2014, 33, 111–120. [Google Scholar] [CrossRef]
- Yun, D.Y.; Bae, J.Y.; Park, C.W.; Jang, G.H.; Choe, W.J. Determination of Modified QuEChERS Method for Chlorothalonil Analysis in Agricultural Products Using Gas Chromatography-Mass Spectrometry (GC-MS/MS). Foods 2023, 12, 3793. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.; Lee, D.U.; Kim, W.S.; Park, C.W.; Choe, W.J.; Moon, M.J. Simultaneous Screening of 322 Residual Pesticides in Fruits and Vegetables Using GC-MS/MS and Deterministic Health Risk Assessments. Foods 2023, 12, 3001. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, K.Y.; Kim, Y.S.; Ryu, K.S.; Kang, M.S.; Lim, J.H.; Yoo, N.Y.; Han, Y.L.; Choi, H.J.; Kang, C.W.; et al. Comparative Analysis of Pesticide Residues in Agricultural Products in Circulation in Gyeonggi-do Before and After Positive List System Enforcement. J. Food Hyg. Saf. 2021, 36, 239–247. [Google Scholar] [CrossRef]
- Kuang, H.; Chu, X.-G.; Hou, Y.-X.; Xu, C.-L. Simultaneous Determination of 13 Phenoxy Acid Herbicide Residues in Soybean by GC-ECD. Anal. Lett. 2006, 39, 2617–2627. [Google Scholar]
- Zhang, L.; Wang, M.; Wang, C.; Hu, X.; Wang, G. Label-free impedimetric immunosensor for sensitive detection of 2,4-dichlorophenoxybutyric acid (2,4-DB) in soybean. Talanta 2012, 101, 226–232. [Google Scholar] [CrossRef]
- Crespo-Corral, E.; Santos-Delgadoa, M.J.; Polo-Díez, L.M.; Soria, A.C. Determination of carbamate, phenylurea and phenoxy acid herbicide residues by gas chromatography after potassium tert-butoxide/dimethyl sulphoxide/ethyliodide derivatization reaction. J. Chromatogr. A 2008, 1209, 22–28. [Google Scholar] [CrossRef]
- Geng, H.; Xu, G.; Liu, L.; Wang, X.; Zhao, R. Determination of trace phenoxy carboxylic acid herbicides in environmental water samples by covalent organic frameworks based solid phase extraction coupled with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2022, 1682, 463516. [Google Scholar] [CrossRef]
- Wells, M.J.M.; Yu, L.Z. Solid-phase extraction of acidic herbicides. J. Chromatogr. A 2000, 885, 237–250. [Google Scholar] [CrossRef]
- Tölgyesi, Á.; Korozs, G.; Tóth, E.; Bálint, M.; Ma, X.; Sharma, V.K. Automation in quantifying phenoxy herbicides and bentazon in surface water and groundwater using novel solid phase extraction and liquid chromatography tandem mass spectrometry. Chemosphere 2022, 286, 131927. [Google Scholar] [CrossRef]
- Mol, H.J.; Zomer, P.; de Koning, M. Qualitative aspects and validation of a screening method for pesticides in vegetables and fruits based on liquid chromatography coupled to full scan high resolution (Orbitrap) mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Sack, C.; Vonderbrink John Smoker, M.; Smith, R.E. Determination of acid herbicides using modified QuEChERS with fast switching ESI+/ESI− LC–MS/MS. J. Agric. Food Chem. 2015, 63, 9657–9665. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Sample treatment and determination of pesticide residues in fatty vegetable matrices: A review. Talanta 2009, 79, 109–128. [Google Scholar] [CrossRef]
- Bibi, A.; Rafique, N.; Khalid, S.; Samad, A.; Ahad, K.; Mehboob, F. Method optimization and validation for the routine analysis of multi-class pesticide residues in Kinnow Mandarin and fruit quality evaluation. Food Chem. 2022, 369, 130914. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission (CAC). Guidelines on Good Laboratory Practice in Pesticide Residue Analysis, CAC/GL 40-1993; Rev.1-2003; CAC: Rome, Italy, 2003. [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). Guideline of Standard Procedures of Test Methods for Foods and Other Substances; Ministry of Food and Drug Safety (MFDS): Cheongju, Republic of Korea, 2016. [Google Scholar]
- Main Changes Introduced in Document N SANTE/11312/2021 with Respect to the Previous Version (Document N SANTE 12682/2019). Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 26 February 2025).
- Schürmann, A.; Dvorak, V.; Crüzer, C.; Butcher, P.; Kaufmann, A. False-positive liquid chromatography/tandem mass spectrometric confirmation of sebuthylazine residues using the identification points system according to EU directive 2002/657/EC due to a biogenic insecticide in tarragon. Rapid Commun. Mass Spectrom. 2009, 23, 1196–1200. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, S.Y.; Sarker, A.; Moon, J.K.; Kim, J.E. Optimization of a Multi-Residue Analytical Method during Determination of Pesticides in Meat Products by GC-MS/MS. Foods 2022, 11, 2930. [Google Scholar] [CrossRef]
- Steiner, D.; Krska, R.; Malachová, A.; Taschl, I.; Sulyok, M. Evaluation of Matrix Effects and Extraction Efficiencies of LC−MS/MS Methods as the Essential Part for Proper Validation of Multiclass Contaminants in Complex Feed. J. Agric. Food Chem. 2020, 68, 3868–3880. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Zampieri, D.; GEnnaro, M.C. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3929–3937. [Google Scholar] [CrossRef]
- Bozena, L.; Magdalena, J. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar]
- European Commission. Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. 2002/657/EC. Off. J. Eur. Union 2002, L 221, 8–36.
- Kim, H.C.; Kim, M.R. Analysis of Online Food Purchase Behavior and Factors Determining Online Purchases by Adult Consumers. J. Korean Soc. Food Sci. Nutr. 2019, 48, 97–108. [Google Scholar] [CrossRef]
- EURL-SRM. Analytical Observations Report: Analysis of Pesticides Entailing Conjugates or Esters in Their Residue Definitions, Version 2. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlSRM/EURL-SRM_Anal_Observ_Report_hydrol_of_Esters&Conj_of_Pesticides_V2.pdf (accessed on 26 February 2025).
- Koesukwiwat, U.; Sanguankaew, K.; Leepipatpiboon, N. Rapid determination of phenoxy acid residues in rice by modified QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2008, 626, 10–20. [Google Scholar] [CrossRef]
- Laganà, A.; Bacaloni, A.; Leva, I.D.; Faberi, A.; Fago, G.; Marino, A. Occurrence and determination of herbicides and their major transformation products in environmental waters. Anal. Chim. Acta 2002, 462, 187–198. [Google Scholar] [CrossRef]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. The QuEChERS approach in a novel application for the identification of antifungal compounds produced by lactic acid bacteria cultures. Talanta 2014, 129, 364–373. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.H.; Jeong, M.J.; Gwon, D.Y.; Lee, J.H.; Shin, Y.; Choi, H. LC-MS/MS Method Minimizing Matrix Effect for the Analysis of Bifenthrin and Butachlor in Chinese Chives and Its Application for Residual Study. Foods 2023, 12, 1683. [Google Scholar] [CrossRef]
- da Silva, L.P.; Madureira, F.; de Azevedo Vargas, E.; Faria, A.F.; Augusti, R. Development and validation of a multianalyte method for quantification of mycotoxins and pesticides in rice using a simple dilute and shoot procedure and UHPLC-MS/MS. Food Chem. 2019, 270, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Kaczyn’ski, P. Clean-up and matrix effect in LC-MS/MS analysis of food of plant origin for high polar herbicides. Food Chem. 2017, 230, 524–531. [Google Scholar] [CrossRef]
- Roa, A.R.; García-Luís, A.; Barcena, J.L.G.; Huguet, C.M. Effect of 2,4-D on fruit sugar accumulation and invertase activity in sweet orange cv. Salustiana. Aust. J. Crop Sci. 2015, 9, 105–111. [Google Scholar]
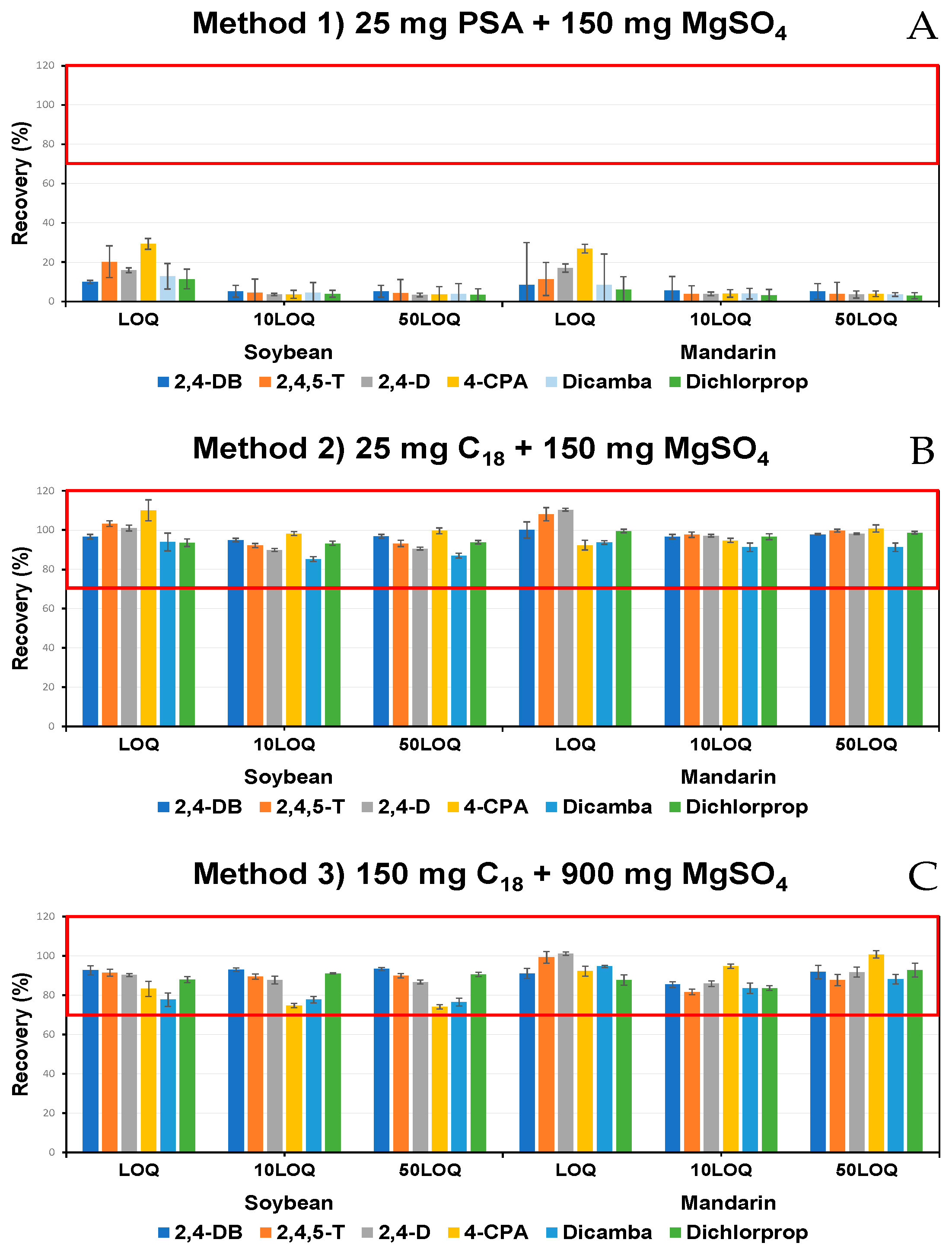

| Pesticide | Linear Regression | LOD a (mg/kg) | LOQ b (mg/kg) | |
|---|---|---|---|---|
| Equation | r2 | |||
| 2,4-DB | y = 7990819.42x − 9504.70 | 0.9958 | 0.0011 | 0.0037 |
| 2,4,5-T | y = 7748012.3914x − 8054.70 | 0.9989 | 0.0005 | 0.0016 |
| 2,4-D | y = 38900297.02x − 38136.35 | 0.9984 | 0.0004 | 0.0015 |
| 4-CPA | y = 42920484.90x − 45643.87 | 0.9987 | 0.0008 | 0.0025 |
| Dicamba | y = 14478819.18x − 1441.53 | 0.9997 | 0.0002 | 0.0007 |
| Dichlorprop | y = 55988246.52x + 6841.23 | 0.9987 | 0.0011 | 0.0037 |
| Pesticide | r2 (Coefficient of Determination) | ||||
|---|---|---|---|---|---|
| Soybean | Mandarin | Hulled Rice | Green Pepper | Potato | |
| 2,4-DB | 0.9989 | 0.9989 | 0.9994 | 0.9991 | 0.9994 |
| 2,4,5-T | 0.9997 | 0.9993 | 0.9984 | 0.9994 | 0.9983 |
| 2,4-D | 0.9994 | 0.9990 | 0.9995 | 0.9990 | 0.9990 |
| 4-CPA | 0.9978 | 0.9984 | 0.9956 | 0.9956 | 0.9989 |
| Dicamba | 0.9996 | 0.9996 | 0.9998 | 0.9991 | 0.9992 |
| Dichlorprop | 0.9997 | 0.9994 | 0.9999 | 0.9998 | 0.9996 |
| Analyte | MLOQ a (mg/kg) | Fortified Level (mg/kg) | Recovery (%), n = 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soybean | Mandarin | Hulled Rice | Green Pepper | Potato | ||||||||
| Mean ± SD b | %CV c | Mean ± SD | %CV | Mean ± SD | %CV | Mean ± SD | %CV | Mean ± SD | %CV | |||
| 2,4-DB | 0.01 | 0.01 | 92.7 ± 2.2 | 2.4 | 91.1 ± 2.4 | 2.6 | 85.4 ± 3.0 | 3.5 | 91.8 ± 2.3 | 2.5 | 102.8 ± 3.4 | 3.3 |
| 0.1 | 93.0 ± 0.8 | 0.9 | 85.4 ± 1.3 | 1.5 | 87.1 ± 2.0 | 2.3 | 92.9 ± 0.4 | 0.4 | 98.6 ± 0.9 | 0.9 | ||
| 0.5 | 93.3 ± 0.7 | 0.8 | 91.8 ± 3.2 | 3.5 | 80.9 ± 1.8 | 2.2 | 94.2 ± 1.4 | 1.5 | 100.3 ± 0.2 | 0.2 | ||
| 2,4,5-T | 0.01 | 91.4 ± 1.6 | 1.7 | 99.3 ± 3.0 | 3.0 | 94.1 ± 2.0 | 2.1 | 104.6 ± 1.4 | 1.3 | 109.4 ± 4.5 | 4.1 | |
| 0.1 | 88.8 ± 1.2 | 1.3 | 82.3 ± 1.2 | 1.4 | 83.1 ± 1.3 | 1.6 | 92.3 ± 0.6 | 0.7 | 98.4 ± 0.7 | 0.7 | ||
| 0.5 | 89.9 ± 1.0 | 1.1 | 87.7 ± 2.5 | 2.9 | 80.2 ± 0.8 | 1.0 | 93.3 ± 1.8 | 1.9 | 99.0 ± 1.0 | 1.0 | ||
| 2,4-D | 0.01 | 90.3 ± 0.7 | 0.8 | 101.1 ± 0.9 | 0.9 | 88.5 ± 1.3 | 1.4 | 106.5 ± 0.8 | 0.7 | 113.6 ± 3.2 | 2.8 | |
| 0.1 | 86.9 ± 1.7 | 2.0 | 86.7 ± 1.3 | 1.5 | 82.1 ± 1.4 | 1.7 | 94.4 ± 0.6 | 0.6 | 100.7 ± 1.0 | 1.0 | ||
| 0.5 | 86.7 ± 0.8 | 0.9 | 91.7 ± 2.3 | 2.5 | 79.8 ± 1.1 | 1.3 | 94.8 ± 1.3 | 1.3 | 102.6 ± 0.6 | 0.6 | ||
| 4-CPA | 0.01 | 83.2 ± 3.1 | 3.8 | 92.3 ± 2.3 | 2.5 | 95.7 ± 2.1 | 2.2 | 97.4 ± 2.5 | 2.5 | 114.5 ± 4.4 | 3.9 | |
| 0.1 | 74.1 ± 0.8 | 1.1 | 95.3 ± 1.0 | 1.1 | 78.7 ± 1.5 | 1.9 | 94.3 ± 0.5 | 0.5 | 96.8 ± 2.0 | 2.1 | ||
| 0.5 | 74.0 ± 0.8 | 1.1 | 100.8 ± 1.8 | 1.8 | 75.6 ± 1.1 | 1.4 | 94.8 ± 1.6 | 1.7 | 99.4 ± 1.7 | 1.7 | ||
| Dicamba | 0.01 | 77.7 ± 2.7 | 3.4 | 94.7 ± 0.6 | 0.6 | 79.1 ± 3.2 | 4.0 | 97.8 ± 2.7 | 2.7 | 104.8 ± 1.6 | 1.5 | |
| 0.1 | 78.2 ± 1.2 | 1.6 | 84.5 ± 2.2 | 2.6 | 86.1 ± 1.5 | 1.8 | 94.2 ± 1.3 | 1.4 | 102.5 ± 1.5 | 1.5 | ||
| 0.5 | 76.5 ± 1.5 | 2.0 | 88.2 ± 2.1 | 2.4 | 89.3 ± 0.9 | 1.0 | 94.0 ± 0.9 | 1.0 | 101.3 ± 2.4 | 2.4 | ||
| Dichlorprop | 0.01 | 88.0 ± 1.4 | 1.6 | 87.7 ± 2.2 | 2.6 | 87.3 ± 1.7 | 2.0 | 95.6 ± 1.4 | 1.5 | 108.3 ± 2.9 | 2.6 | |
| 0.1 | 91.1 ± 0.3 | 0.4 | 84.2 ± 1.1 | 1.3 | 83.7 ± 1.7 | 2.0 | 94.3 ± 0.4 | 0.4 | 98.4 ± 0.9 | 0.9 | ||
| 0.5 | 90.6 ± 0.9 | 1.0 | 92.8 ± 3.2 | 3.5 | 81.0 ± 0.7 | 0.9 | 95.1 ± 1.0 | 1.0 | 99.1 ± 0.9 | 0.9 | ||
| Analyte | Soybean | Mandarin | Hulled Rice | Green Pepper | Potato | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ion Ratio (%) a | Relative Tolerance (%) b | Ion Ratio (%) | Relative Tolerance (%) | Ion Ratio (%) | Relative Tolerance (%) | Ion Ratio (%) | Relative Tolerance (%) | Ion Ratio (%) | Relative Tolerance (%) | |
| 2,4-DB | 14.3 | −8.7 | 13.1 | −1.9 | 16.0 | 0.6 | 16.0 | −7.0 | 11.6 | −11.2 |
| 2,4,5-T | 63.5 | 1.4 | 58.5 | −11.1 | 62.0 | −3.2 | 61.2 | 14.4 | 61.4 | 3.0 |
| 2,4-D | 8.7 | 5.2 | 8.8 | −1.0 | 8.6 | 0.4 | 8.6 | 2.2 | 8.8 | 4.8 |
| 4-CPA | 13.4 | −1.7 | 12.6 | 0.5 | 13.3 | 0.9 | 13.7 | −11.7 | 13.9 | -1.8 |
| Dicamba | 33.3 | −1.0 | 33.4 | 0.0 | 33.2 | 0.6 | 33.4 | −0.2 | 33.4 | 0.4 |
| Dichlorprop | 7.7 | 6.2 | 7.9 | −10.6 | 7.3 | 2.7 | 7.5 | 0.9 | 7.8 | −7.6 |
| Commodity Class | Sample | Origin | Residue Amount (μg/g) (Mean/%CV a) | |||||
|---|---|---|---|---|---|---|---|---|
| 2,4-DB | 2,4,5-T | 2,4-D | 4-CPA | Dicamba | Dichlorprop | |||
| Legumes | Soybean | China | ND b | ND | ND | ND | ND | ND |
| Soybean | China | <0.01 | ND | ND | ND | ND | ND | |
| Soybean | China | ND | ND | ND | ND | ND | ND | |
| Chickpea | Canada | ND | ND | ND | ND | ND | ND | |
| Kidney bean | China | ND | ND | ND | ND | ND | ND | |
| Lentil | Canada | ND | ND | ND | ND | ND | ND | |
| White kidney bean | China | ND | ND | ND | ND | ND | ND | |
| Adzuki bean | China | ND | ND | ND | ND | ND | ND | |
| Lentil | Canada | ND | ND | ND | ND | ND | ND | |
| Chickpea | Canada | ND | ND | ND | ND | ND | ND | |
| Fruits | Orange | USA | <0.01 | ND | <0.01 | ND | ND | ND |
| Orange | USA | ND | ND | 0.014/3.6 | ND | ND | ND | |
| Orange | Australia | <0.01 | ND | <0.01 | ND | ND | <0.01 | |
| Cherry | USA | <0.01 | ND | <0.01 | ND | ND | ND | |
| Cherry | USA | <0.01 | ND | <0.01 | ND | <0.01 | ND | |
| Cherry | USA | ND | ND | <0.01 | ND | <0.01 | ND | |
| Rainbow mango | Thailand | ND | ND | ND | ND | ND | ND | |
| Lemon | Chile | ND | ND | ND | ND | ND | ND | |
| Pineapple | The Philippines | ND | ND | ND | ND | ND | ND | |
| Blueberry | USA | ND | ND | ND | ND | ND | ND | |
| Black sapphire grape | USA | ND | ND | ND | ND | ND | ND | |
| Melon | USA | ND | ND | ND | ND | ND | ND | |
| Lemon | USA | ND | ND | ND | ND | ND | ND | |
| Lime | Vietnam | ND | ND | ND | ND | ND | ND | |
| Red grape | USA | ND | ND | ND | ND | ND | ND | |
| Green grape | USA | ND | ND | ND | ND | ND | ND | |
| Raspberry | Chile | ND | ND | ND | ND | ND | ND | |
| Kiwi | New Zealand | ND | ND | ND | ND | ND | ND | |
| Banana | The Philippines | ND | ND | ND | ND | ND | ND | |
| Grains | Quinoa | USA | ND | ND | <0.01 | ND | ND | <0.01 |
| Quinoa | USA | ND | ND | <0.01 | ND | ND | <0.01 | |
| Quinoa | Peru | ND | ND | <0.01 | ND | ND | <0.01 | |
| Calrose rice | USA | <0.01 | ND | ND | ND | ND | <0.01 | |
| Corn | USA | ND | ND | ND | ND | ND | <0.01 | |
| Kamut | Canada | ND | ND | ND | ND | ND | ND | |
| Millet | China | ND | ND | ND | ND | ND | ND | |
| Farro | Italy | ND | ND | ND | ND | ND | ND | |
| Oat groat | Canada | ND | ND | ND | ND | ND | ND | |
| Kamut | Canada | ND | ND | ND | ND | ND | ND | |
| Tuber Vegetables | Potato | USA | ND | ND | ND | ND | ND | ND |
| Potato | USA | ND | ND | ND | ND | ND | ND | |
| Fruiting Vegetables | Green Pepper c | China | ND | ND | ND | ND | ND | ND |
| Green Pepper c | China | ND | ND | <0.01 | ND | ND | ND | |
| Green Pepper c | China | ND | ND | ND | ND | ND | ND | |
| Red Pepper c | Vietnam | ND | ND | ND | ND | ND | ND | |
| Garlic scape | China | ND | ND | ND | ND | ND | ND | |
| Spinach | China | ND | ND | ND | ND | ND | ND | |
| Green onion | China | ND | ND | ND | ND | ND | ND | |
| Red bell pepper | China | ND | ND | ND | ND | ND | ND | |
| Okra | China | ND | ND | ND | ND | ND | ND | |
| Leek | Belgium | ND | ND | ND | ND | ND | ND | |
| Broccolini | Spain | ND | ND | ND | ND | ND | ND | |
| Shallot | Australia | ND | ND | ND | ND | ND | ND | |
| Asparagus | New Zealand | ND | ND | ND | ND | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-K.; Kim, J.-H.; An, G.-E.-H.; Chang, H.-R. Simultaneous Determination of Six Acidic Pesticides, Including 2,4-DB and 2,4,5-T with No Established MRL in Korea Using LC-MS/MS and QuEChERS for the Safety of Imported Agricultural Products. Foods 2025, 14, 904. https://doi.org/10.3390/foods14050904
Oh J-K, Kim J-H, An G-E-H, Chang H-R. Simultaneous Determination of Six Acidic Pesticides, Including 2,4-DB and 2,4,5-T with No Established MRL in Korea Using LC-MS/MS and QuEChERS for the Safety of Imported Agricultural Products. Foods. 2025; 14(5):904. https://doi.org/10.3390/foods14050904
Chicago/Turabian StyleOh, Joon-Kyung, Jae-Hyeong Kim, Ga-Eul-Hae An, and Hee-Ra Chang. 2025. "Simultaneous Determination of Six Acidic Pesticides, Including 2,4-DB and 2,4,5-T with No Established MRL in Korea Using LC-MS/MS and QuEChERS for the Safety of Imported Agricultural Products" Foods 14, no. 5: 904. https://doi.org/10.3390/foods14050904
APA StyleOh, J.-K., Kim, J.-H., An, G.-E.-H., & Chang, H.-R. (2025). Simultaneous Determination of Six Acidic Pesticides, Including 2,4-DB and 2,4,5-T with No Established MRL in Korea Using LC-MS/MS and QuEChERS for the Safety of Imported Agricultural Products. Foods, 14(5), 904. https://doi.org/10.3390/foods14050904






