The Antioxidant Capacity and Flavor Diversity of Strawberry Wine Are Improved Through Fermentation with the Indigenous Non-Saccharomyces Yeasts Hanseniaspora uvarum and Kurtzmaniella quercitrusa
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strawberry Wine Fermentation
2.3. Basic Parameters Analysis of the Strawberry Wine
2.4. Determination of Active Substances Content and Antioxidant Capacity
2.5. Profiling of the Volatile Organic Compounds
2.6. Electronic Nose Analysis
2.7. Statistical Analysis
3. Results and Discussion
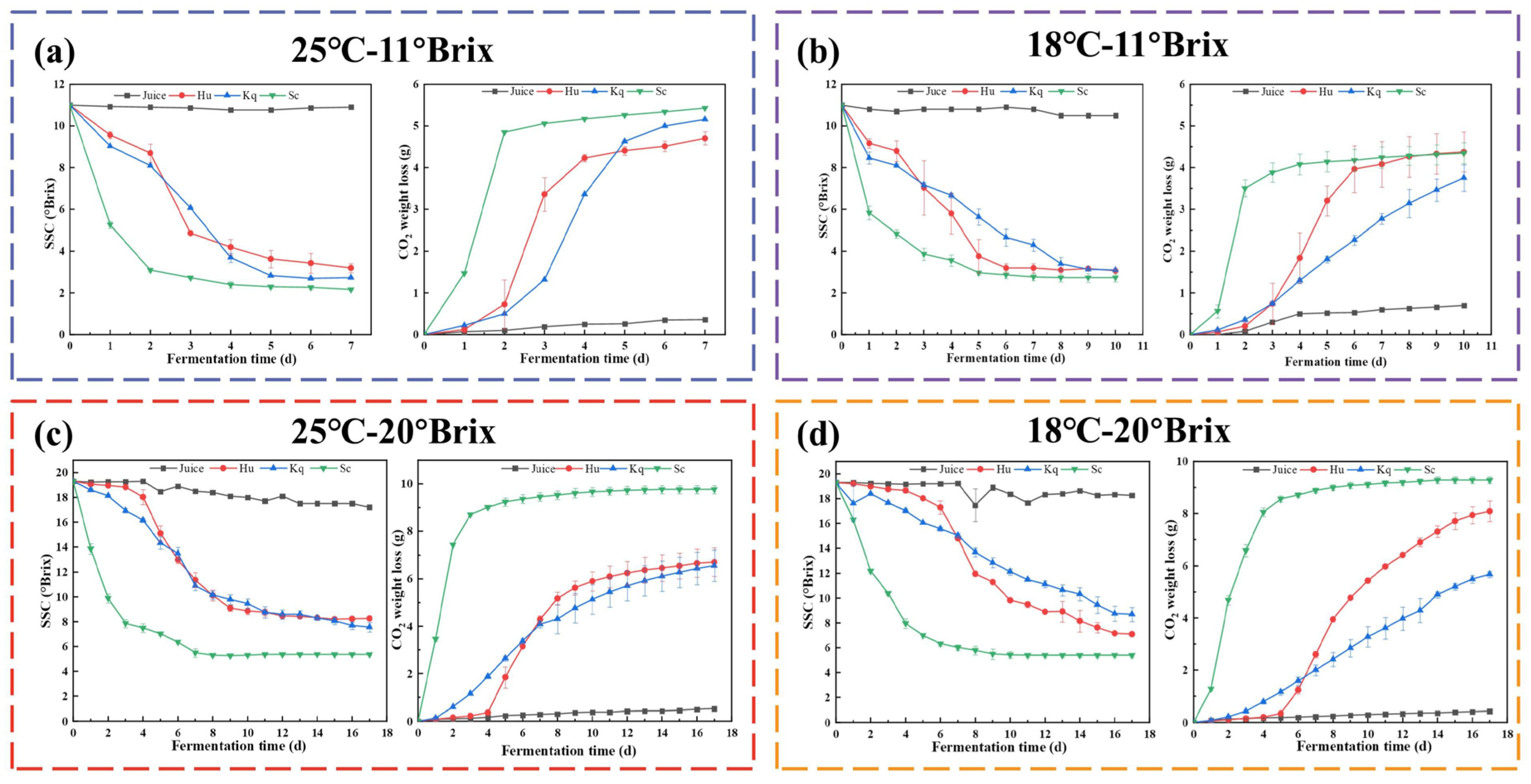
3.1. Fermentation Kinetics
3.2. Physicochemical Parameters
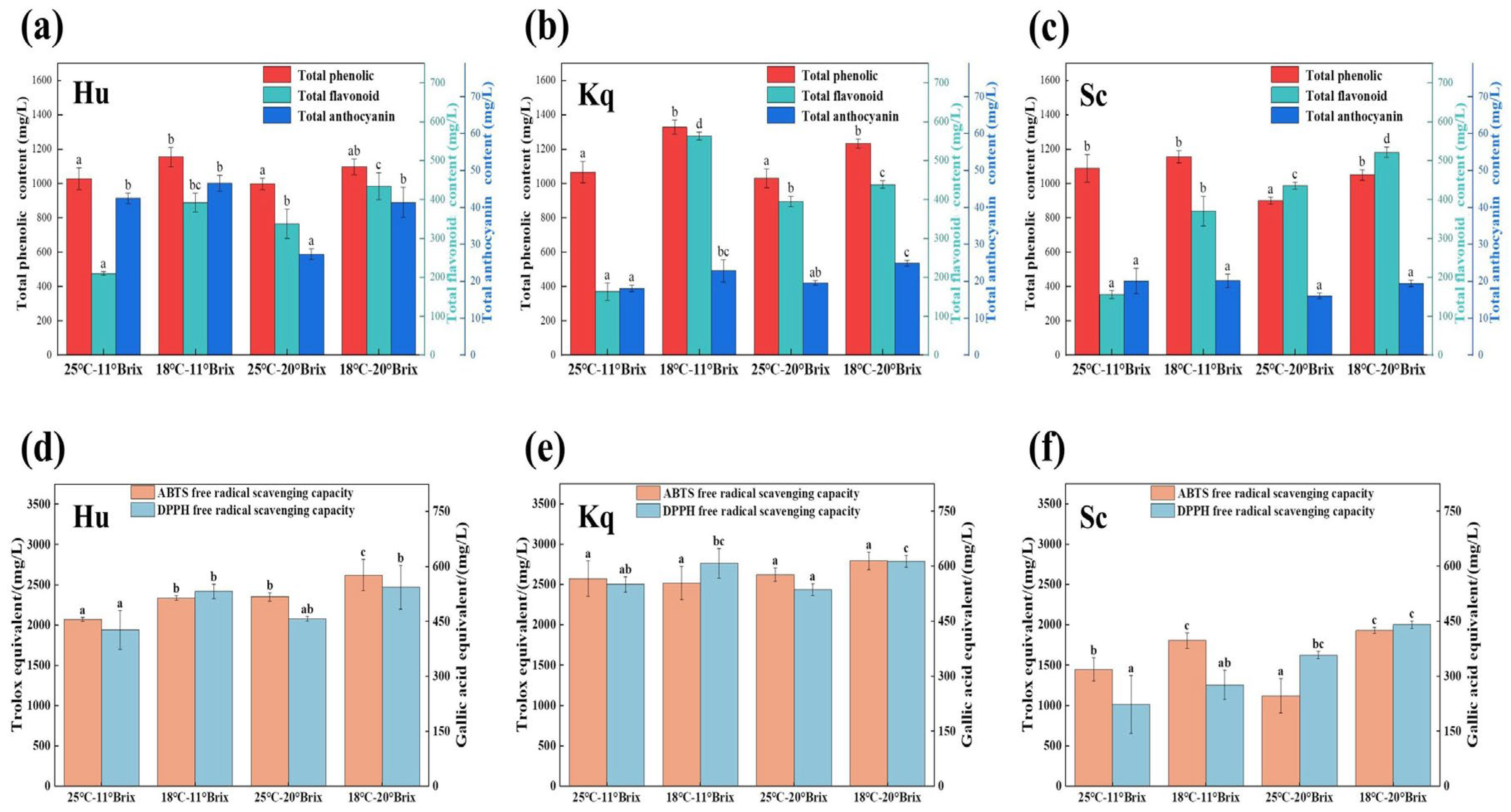
3.3. Active Substances Content and Antioxidant Capacity
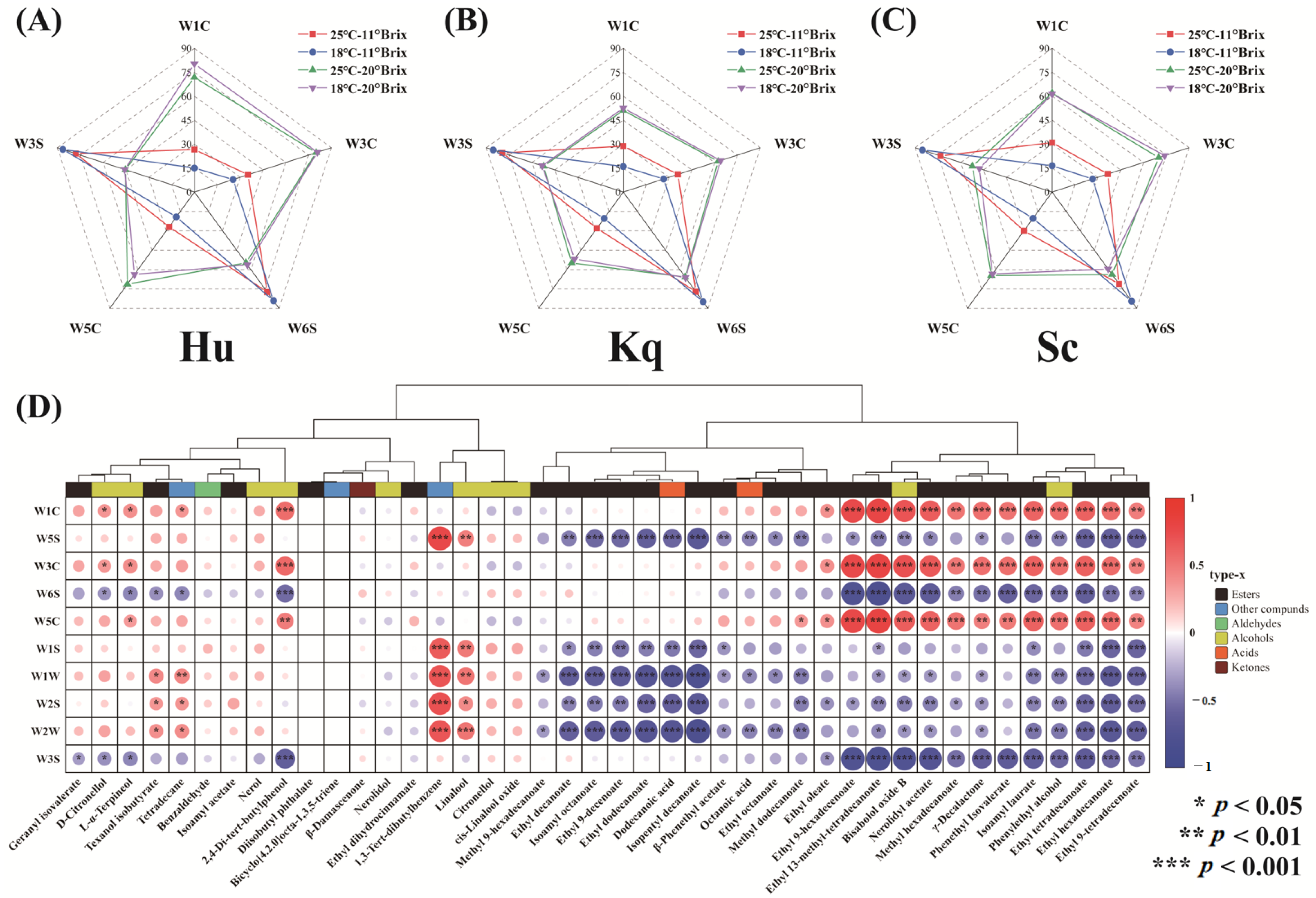
3.4. Volatile Organic Compounds
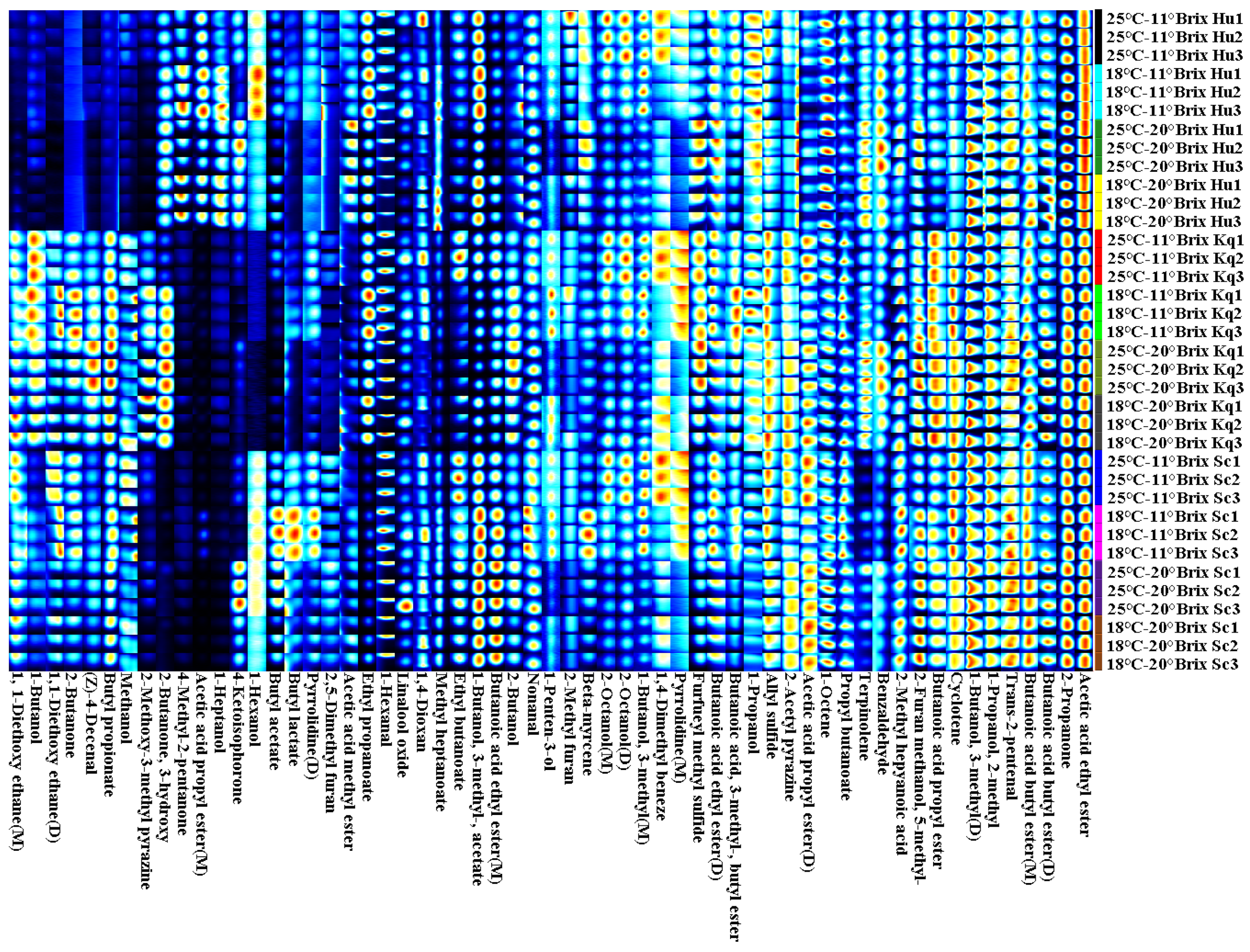
3.4.1. GC-IMS Analysis
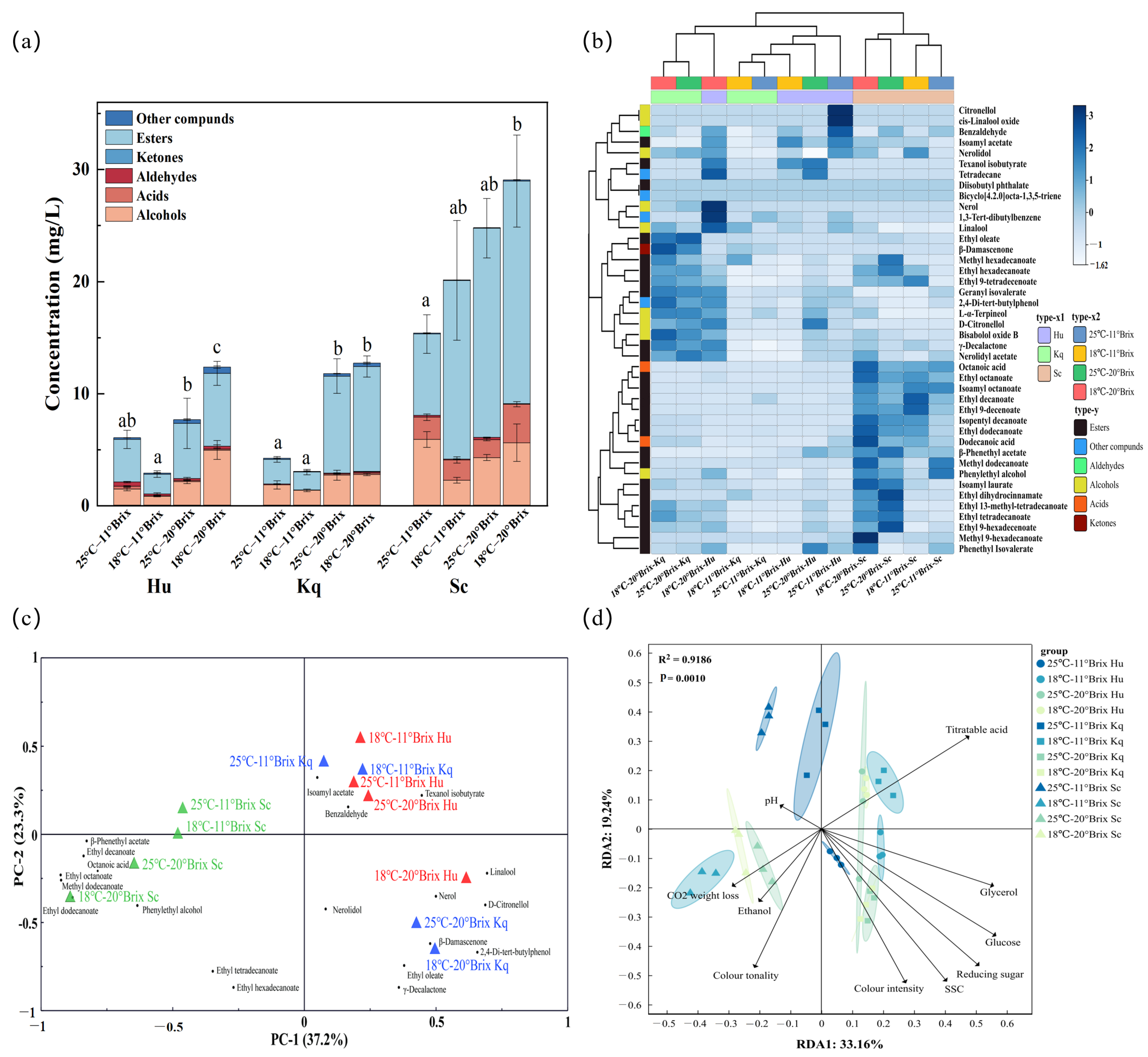
3.4.2. GC-MS Analysis
3.4.3. Principal Component Analysis
3.4.4. Redundancy Analysis
3.5. Electronic Nose Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bebek Markovinović, A.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Skendrović Babojelić, M.; Duralija, B.; Šic Žlabur, J.; Putnik, P.; Bursać Kovačević, D. Strawberry Tree Fruits and Leaves (Arbutus unedo L.) as Raw Material for Sustainable Functional Food Processing: A Review. Horticulturae 2022, 8, 881. [Google Scholar] [CrossRef]
- Qaderi, R.; Mezzetti, B.; Capocasa, F.; Mazzoni, L. Stability of Strawberry Fruit (Fragaria x ananassa Duch.) Nutritional Quality at Different Storage Conditions. Appl. Sci. 2023, 13, 313. [Google Scholar] [CrossRef]
- Bezerra, M.; Ribeiro, M.; Cosme, F.; Nunes, F.M. Overview of the Distinctive Characteristics of Strawberry, Raspberry, and Blueberry in Berries, Berry Wines, and Berry Spirits. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13354. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Sun, W.; Li, S.; Liu, X.; Tian, Y.; Long, F.; Zhang, Z.; Guo, J. Characterization of the Effect of Non-Saccharomyces cerevisiaes on the Non-Volatile Constituents and Volatile Profiles of Low-Alcoholic Pomegranate Beverages. Food Biosci. 2024, 59, 103870. [Google Scholar] [CrossRef]
- Klimczak, K.; Cioch-Skoneczny, M.; Ciosek, A.; Poreda, A. Application of Non-Saccharomyces Yeast for the Production of Low-Alcohol Beer. Foods 2024, 13, 3214. [Google Scholar] [CrossRef]
- Wang, X.; Fan, G.; Peng, Y.; Xu, N.; Xie, Y.; Zhou, H.; Liang, H.; Zhan, J.; Huang, W.; You, Y. Mechanisms and Effects of Non-Saccharomyces Yeast Fermentation on the Aromatic Profile of Wine. J. Food Compos. Anal. 2023, 124, 105660. [Google Scholar] [CrossRef]
- Yang, W.; Liu, S.; Marsol-Vall, A.; Tähti, R.; Laaksonen, O.; Karhu, S.; Yang, B.; Ma, X. Chemical Composition, Sensory Profile and Antioxidant Capacity of Low-Alcohol Strawberry Beverages Fermented with Saccharomyces cerevisiae and Torulaspora delbrueckii. LWT 2021, 149, 111910. [Google Scholar] [CrossRef]
- Alonso-del-Real, J.; Lairon-Peris, M.; Barrio, E.; Querol, A. Effect of temperature on the prevalence of Saccharomyces non cerevisiae species against a S. cerevisiae wine strain in wine fermentation: Competition, physiological fitness, and influence in final wine composition. Front. Microbiol. 2017, 8, 150. [Google Scholar] [CrossRef]
- Gschaedler, A.; Iñiguez-Muñoz, L.E.; Flores-Flores, N.Y.; Kirchmayr, M.; Arellano-Plaza, M. Use of Non-Saccharomyces Yeasts in Cider Fermentation: Importance of the Nutrients Addition to Obtain an Efficient Fermentation. Int. J. Food Microbiol. 2021, 347, 109169. [Google Scholar] [CrossRef]
- Yang, B.; Liu, S.; Zang, H.; Dai, Y.; Zhang, S.; Lin, X.; Liang, H.; Chen, Y. Flavor Profile and Quality of Strawberry Wine Are Improved through Sequential Fermentation with Indigenous Non-Saccharomyces Yeasts and Saccharomyces cerevisiae. Food Biosci. 2024, 59, 104021. [Google Scholar] [CrossRef]
- Deed, R.C.; Fedrizzi, B.; Gardner, R.C. Influence of Fermentation Temperature, Yeast Strain, and Grape Juice on the Aroma Chemistry and Sensory Profile of Sauvignon Blanc Wines. J. Agric. Food Chem. 2017, 65, 8902–8912. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of Chemical Composition and Sensorial Properties of Ciders Fermented with Different Non-Saccharomyces Yeasts in Pure and Mixed Fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Z.; Zou, S.; Dong, L.; Lin, X.; Chen, Y.; Zhang, S.; Ji, C.; Liang, H. Chemical Composition and Flavor Characteristics of Cider Fermented with Saccharomyces cerevisiae and Non-Saccharomyces cerevisiae. Foods 2023, 12, 3565. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, F.; Yang, L.; Li, J.; Zhu, X. Enhancement of the Aroma in Low-Alcohol Apple-Blended Pear Wine Mixed Fermented with Saccharomyces cerevisiae and Non-Saccharomyces Yeasts. LWT 2022, 155, 112994. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, K.; Liu, C.; Wang, Y.; Chen, T.; Yan, G.; Li, J. Non-Saccharomyces Yeasts Highly Contribute to Characterisation of Flavour Profiles in Greengage Fermentation. Food Res. Int. 2022, 157, 111391. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Zhao, Y.; Ma, T.; Zhao, F.; Huang, W.; Zhan, J. Effect of Copper Stress on Growth Characteristics and Fermentation Properties of Saccharomyces cerevisiae and the Pathway of Copper Adsorption during Wine Fermentation. Food Chem. 2016, 192, 43–52. [Google Scholar] [CrossRef]
- Jennings, P.A.; Mullen, C.A.; Roy, M. Titration and Measurement. In Encyclopedia of Life Sciences; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Veeranki, V.D.; Sastri, C.V. Pitfalls in the 3, 5-Dinitrosalicylic Acid (DNS) Assay for the Reducing Sugars: Interference of Furfural and 5-Hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Kortesniemi, M.; Kalpio, M.; Yang, B. Chemical Composition of Bilberry Wine Fermented with Non-Saccharomyces Yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in Pure, Sequential and Mixed Fermentations. Food Chem. 2018, 266, 262–274. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Morales-Pérez, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship of Quality Parameters, Antioxidant Capacity and Total Phenolic Content of EVOO with Ripening State and Olive Variety. Food Chem. 2020, 325, 126926. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, L.; Liu, X.; Hasan, K.M.F.; Li, H.; Zhou, S.; Zhang, Q.; Zhou, Y. Effect of Thermosonication Treatment on Blueberry Juice Quality: Total Phenolics, Flavonoids, Anthocyanin, and Antioxidant capacity. LWT 2021, 150, 112021. [Google Scholar] [CrossRef]
- Nogueira, D.P.; Jiménez-Moreno, N.; Esparza, I.; Moler, J.A.; Ferreira-Santos, P.; Sagües, A.; Teixeira, J.A.; Ancín-Azpilicueta, C. Evaluation of Grape Stems and Grape Stem Extracts for Sulfur Dioxide Replacement during Grape Wine Production. Curr. Res. Food Sci. 2023, 6, 100453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chi, H.; Ma, S.; Zhao, L.; Cai, S. Identification of Novel α-Glucosidase Inhibitory Peptides in Rice Wine and Their Antioxidant capacities Using in Silico and in Vitro Analyses. LWT 2023, 178, 114629. [Google Scholar] [CrossRef]
- Sun, Z.; Cong, Y.; Li, T.; Meng, X.; Zhang, F. Enhancement of Nutritional, Sensory and Storage Stability by Lactic Fermentation of Auricularia Auricula. J. Sci. Food Agric. 2022, 102, 5172–5180. [Google Scholar] [CrossRef]
- Wang, X.-C.; Li, A.-H.; Dizy, M.; Ullah, N.; Sun, W.-X.; Tao, Y.-S. Evaluation of Aroma Enhancement for “Ecolly” Dry White Wines by Mixed Inoculation of Selected Rhodotorula Mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhu, Y.; Kortesniemi, M.; Zhu, B.; Li, H. Aromatic Characteristics of Passion Fruit Wines Measured by E-Nose, GC-Quadrupole MS, GC-Orbitrap-MS and Sensory Evaluation. Foods 2022, 11, 3789. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Y.; Liu, S.Q. Effects of Pectinase Treatment on the Physicochemical and Oenological Properties of Red Dragon Fruit Wine Fermented with Torulaspora delbrueckii. LWT 2020, 132, 109929. [Google Scholar] [CrossRef]
- Maksim, Z.; Matthias, R. Cell size and morphological properties of yeast Saccharomyces cerevisiae in relation to growth temperature. FEMS Yeast Res. 2018, 18, foy052. [Google Scholar] [CrossRef]
- Sadras, V.O.; Petrie, P.R.; Moran, M.A. Effects of Elevated Temperature in Grapevine. II Juice PH, Titratable Acidity and Wine Sensory Attributes. Aust. J. Grape Wine Res. 2013, 19, 107–115. [Google Scholar] [CrossRef]
- Torija, M. Effects of Fermentation Temperature on the Strain Population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003, 80, 47–53. [Google Scholar] [CrossRef]
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Chemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef]
- Du, Q.; Ye, D.; Zang, X.; Nan, H.; Liu, Y. Effect of Low Temperature on the Shaping of Yeast-Derived Metabolite Compositions during Wine Fermentation. Food Res. Int. 2022, 162, 112016. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Bosman, G.; du Toit, W.; Aleixandre-Tudo, J.L. White Wine Phenolics: Current Methods of Analysis. J. Sci. Food Agric. 2023, 103, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, M.; Chen, W.; Chen, H.; Chen, W.; Zhong, Q. Screening and Evaluation of Suitable Non-Saccharomyces Yeast for Aroma Improvement of Fermented Mango Juice. Food Biosci. 2021, 44, 101414. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.; Feng, S.; Bi, P.; Han, J.; Li, S.; Liu, X.; Zhang, Z.; Long, F.; Guo, J. Effect of Sequential Fermentation with Indigenous Non-Saccharomyces cerevisiae Combinations and Saccharomyces cerevisiae on the Chemical Composition and Aroma Compounds Evolution of Kiwifruit Wine. Food Chem. 2024, 460, 140758. [Google Scholar] [CrossRef]
- Massera, A.; Assof, M.; Sari, S.; Ciklic, I.; Mercado, L.; Jofré, V.; Combina, M. Effect of Low Temperature Fermentation on the Yeast-Derived Volatile Aroma Composition and Sensory Profile in Merlot Wines. LWT 2021, 142, 111069. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Nofer, J. Effect of Different Yeast Strains and Temperature of Fermentation on Basic Enological Parameters, Polyphenols and Volatile Compounds of Aurore White Wine. Foods 2019, 8, 599. [Google Scholar] [CrossRef]
- Zeinali, S.; Natalia Wieczorek, M.; Pawliszyn, J. Free versus Droplet-Bound Aroma Compounds in Sparkling Beverages. Food Chem. 2022, 378, 131985. [Google Scholar] [CrossRef]
- Synos, K.; Reynolds, A.G.; Bowen, A.J. Effect of Yeast Strain on Aroma Compounds in Cabernet Franc Icewines. LWT Food Sci. Technol. 2015, 64, 227–235. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, M.; Ouyang, Y.; Zhao, X.; Ju, Y.; Fang, Y. Comparative Study of Aromatic Compounds in Fruit Wines from Raspberry, Strawberry, and Mulberry in Central Shaanxi Area. Food Nutr. Res. 2015, 59, 29290. [Google Scholar] [CrossRef]
- Aubert, C.; Bruaut, M.; Chalot, G.; Cottet, V. Impact of maturity stage at harvest on the main physicochemical characteristics, the levels of vitamin C, polyphenols and volatiles and the sensory quality of Gariguette strawberry. Eur. Food Res. Technol. 2021, 247, 37–49. [Google Scholar] [CrossRef]
- Cozzolino, R.; Pace, B.; Palumbo, M.; Laurino, C.; Picariello, G.; Siano, F.; De Giulio, B.; Pelosi, S.; Cefola, M. Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries. Foods 2021, 10, 3102. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Hao, J.; Zhu, N.; Wang, M. Geographical Indication Characteristics of Aroma and Phenolic Acids of the Changping Strawberry. Foods 2023, 12, 3889. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Cappellin, L.; Spadoni, A.; Cameldi, I.; Algarra Alarcon, A.; Aprea, E.; Romano, A.; Gasperi, F.; Biasioli, F. Role of Strawberry Volatile Organic Compounds in the Development of Botrytis Cinerea Infection. Plant Pathol. 2015, 64, 709–717. [Google Scholar] [CrossRef]
- Prat, L.; Espinoza, M.I.; Agosin, E.; Silva, H. Identification of Volatile Compounds Associated with the Aroma of White Strawberries (Fragaria chiloensis). J. Sci. Food Agric. 2014, 94, 752–759. [Google Scholar] [CrossRef]
- Guo, C.; Deng, H.; Li, E. Removal of Acetic Acid in Mulberry Wine by Co-Inoculating Saccharomyces cerevisiae with Indigenous Non-Saccharomyces Yeast. Food Biosci. 2024, 58, 103658. [Google Scholar] [CrossRef]
- Peng, B.; Li, F.; Cui, L.; Guo, Y. Effects of Fermentation Temperature on Key Aroma Compounds and Sensory Properties of Apple Wine. J. Food Sci. 2015, 80, 13111. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Yang, F.; Wang, H.; Wang, L. Yeasts and Their Importance to the Flavour of Traditional Chinese Liquor: A Review. J. Inst. Brew. 2019, 125, 214–221. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Effects of Sugar Concentration Processes in Grapes and Wine Aging on Aroma Compounds of Sweet Wines—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1053–1073. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, C.; Yang, D.; Liu, H.; Xue, J.; Duan, C.; Yan, G. Effects of Three Indigenous Non-Saccharomyces Yeasts and Their Pairwise Combinations in Co-Fermentation with Saccharomyces cerevisiae on Volatile Compounds of Petit Manseng Wines. Food Chem. 2022, 368, 130807. [Google Scholar] [CrossRef]
- Tian, M.; Lin, K.; Yang, L.; Jiang, B.; Zhang, B.; Zhu, X.; Ren, D.; Yu, H. Characterization of Key Aroma Compounds in Gray Sufu Fermented Using Leuconostoc Mesenteroides Subsp. Mesenteroides F24 as a Starter Culture. Food Chem. X 2023, 20, 100881. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Liu, Y.; Wang, D. Analyzing Volatile Compounds of Young and Mature Docynia Delavayi Fruit by HS-SPME-GC-MS and ROAV. Foods 2022, 12, 59. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, Y.; Liu, Y.; Shi, Y.; Lin, X.; Wang, L.; Wen, P.; Hu, X.; Li, J. Comprehensive Evaluation of Aroma and Taste Properties of Different Parts from the Wampee Fruit. Food Chem. X 2023, 19, 100835. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-J.; Zhang, J.-R.; Zhang, R.-X.; Qin, Y.; Yang, X.-B.; Jin, G.-J.; Tao, Y.-S. Oxidation-Reduction Potential Affects Medium-Chain Fatty Acid Ethyl Ester Production during Wine Alcohol Fermentation. Food Res. Int. 2022, 157, 111369. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, K.; Song, N.; Yao, W.; Xia, H.; Yang, Q.; Zhang, X.; Li, X.; Wang, Z.; Yao, L.; et al. Zygosaccharomyces rouxii, an Aromatic Yeast Isolated from Chili Sauce, Is Able to Biosynthesize 2-Phenylethanol via the Shikimate or Ehrlich Pathways. Front. Microbiol. 2020, 11, 597454. [Google Scholar] [CrossRef]
- Tian, T.; Sun, J.; Wu, D.; Xiao, J.; Lu, J. Objective Measures of Greengage Wine Quality: From Taste-Active Compound and Aroma-Active Compound to Sensory Profiles. Food Chem. 2021, 340, 128179. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, R.; Sirisena, S.; Gan, R.; Fang, Z. Beta-Glucosidase capacity of Wine Yeasts and Its Impacts on Wine Volatiles and Phenolics: A Mini-Review. Food Microbiol. 2021, 100, 103859. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, X.; Li, X.; Sheng, X.; Li, T.; Tang, W.; Yu, Z.; Wang, Y. Effect of Hanseniaspora uvarum–Saccharomyces cerevisiae Mixed Fermentation on Aroma Characteristics of Rosa Roxburghii Tratt, Blueberry, and Plum Wines. Molecules 2022, 27, 8097. [Google Scholar] [CrossRef]
- Chaumont-Olive, P.; Sánchez-Quesada, J.; Collado Pérez, A.M.; Cossy, J. Synthetic Approaches to the Damascone and Damascenone Isomers. Tetrahedron 2021, 82, 131932. [Google Scholar] [CrossRef]
- Cândido da Silva, M.C.; de Brito Araújo Carvalho, A.J.; Cardoso Viana, A.; Almeida dos Anjos, V.H.; Prudêncio Dutra, M.d.C.; Pimentel, T.C.; Magnani, M.; dos Santos Lima, M. Effects of Unconventional Non-Saccharomyces Yeast Fermentation on the Chemical Profile and Bioaccessibility of Watermelon Wine. Food Biosci. 2024, 61, 104961. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Guo, Z.; Guo, X.; Zhang, Q.; Zhao, X.; Ning, M.; Shan, C. Effects of Pretreatment Methods and Leaching Methods on Jujube Wine Quality Detected by Electronic Senses and HS-SPME–GC–MS. Food Chem. 2020, 330, 127330. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Feng, X.; Capozzi, V.; Laaksonen, O.; Yang, B.; Li, P.; Gu, Q. Comparison of Anthocyanin and Volatile Organic Compounds in Juices and Fruit Wines Made from Blood Oranges (Citrus sinensis L. Osbeck) at Different Maturity Stages. Food Biosci. 2023, 56, 103194. [Google Scholar] [CrossRef]
- Jiang, J.; Yin, R.; Xie, Y.; Ma, X.; Cui, M.; Chen, Y.; Li, Y.; Hu, Y.; Niu, J.; Cheng, W.; et al. Effects of Cofermentation of Saccharomyces cerevisiae and Different Lactic Acid Bacteria on the Organic Acid Content, Soluble Sugar Content, Biogenic Amines, Phenol Content, Antioxidant capacity and Aroma of Prune Wine. Food Chem. X 2024, 22, 101502. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, Q.; Zhao, Y.; Yue, T.; Yuan, Y. Untargeted Metabolomics Combined with Chemometrics Reveals Distinct Metabolic Profiles across Two Sparkling Cider Fermentation Stages. Food Res. Int. 2024, 195, 114946. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Yang, C.; Peng, L.; Xie, Q.; Zheng, R.; Dai, Y.; Liu, S.; Peng, X. Effects of Lactobacillus Plantarum C7 and Staphylococcus Warneri S6 on Flavor Quality and Bacterial Diversity of Fermented Meat Rice, a Traditional Chinese Food. Food Res. Int. 2021, 150, 110745. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Arroyo, T. Non-Saccharomyces Yeasts: Biotechnological Role for Wine Production. In Grape and Wine Biotechnology; InTech: London, UK, 2016. [Google Scholar]
- Ge, X.; Liu, Y.; Wang, X.; Gao, C.; Mu, J.; Wang, W.; Wang, J. Correlations between Microbes with Volatile Compounds and Physicochemical Indicators of Cabernet Sauvignon Wines Fermented with Different Starters. LWT 2024, 198, 115918. [Google Scholar] [CrossRef]
- Wang, J.; Wei, B.; Zhai, Y.; Li, K.; Wang, C. Non-volatile and Volatile Compound Changes in Blueberry Juice Inoculated with Different Lactic Acid Bacteria Strains. J. Sci. Food Agric. 2024, 104, 2587–2596. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; ISBN 979-90-810894-0-1. [Google Scholar]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Effect of sequential fermentation with four non-Saccharomyces and Saccharomyces cerevisiae on nutritional characteristics and flavor profiles of kiwi wines. J. Food Compos. Anal. 2022, 109, 104480. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Gao, Z.; Zhang, Z.; Bi, P.; Guo, J. Enhancing antioxidant activity and fragrant profile of low-ethanol kiwi wine via sequential culture of indigenous Zygosaccharomyces rouxii and Saccharomyces cerevisiae. Food Biosci. 2023, 51, 102210. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Ge, X.; Fu, X.; Dang, C.; Wang, J.; Liu, Y. Sequential fermentation with indigenous non-Saccharomyces yeasts and Saccharomyces cerevisiae for flavor and quality enhancement of Longyan dry white wine. Food Biosci. 2023, 55, 102952. [Google Scholar] [CrossRef]
- Available online: http://www.flavornet.org/flavornet.html (accessed on 17 August 2024).
- Available online: https://china.guidechem.com/dict/ (accessed on 17 August 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yang, B.; Jia, S.; Dai, Y.; Lin, X.; Ji, C.; Chen, Y. The Antioxidant Capacity and Flavor Diversity of Strawberry Wine Are Improved Through Fermentation with the Indigenous Non-Saccharomyces Yeasts Hanseniaspora uvarum and Kurtzmaniella quercitrusa. Foods 2025, 14, 886. https://doi.org/10.3390/foods14050886
Wang R, Yang B, Jia S, Dai Y, Lin X, Ji C, Chen Y. The Antioxidant Capacity and Flavor Diversity of Strawberry Wine Are Improved Through Fermentation with the Indigenous Non-Saccharomyces Yeasts Hanseniaspora uvarum and Kurtzmaniella quercitrusa. Foods. 2025; 14(5):886. https://doi.org/10.3390/foods14050886
Chicago/Turabian StyleWang, Ruipeng, Bo Yang, Saihong Jia, Yiwei Dai, Xinping Lin, Chaofan Ji, and Yingxi Chen. 2025. "The Antioxidant Capacity and Flavor Diversity of Strawberry Wine Are Improved Through Fermentation with the Indigenous Non-Saccharomyces Yeasts Hanseniaspora uvarum and Kurtzmaniella quercitrusa" Foods 14, no. 5: 886. https://doi.org/10.3390/foods14050886
APA StyleWang, R., Yang, B., Jia, S., Dai, Y., Lin, X., Ji, C., & Chen, Y. (2025). The Antioxidant Capacity and Flavor Diversity of Strawberry Wine Are Improved Through Fermentation with the Indigenous Non-Saccharomyces Yeasts Hanseniaspora uvarum and Kurtzmaniella quercitrusa. Foods, 14(5), 886. https://doi.org/10.3390/foods14050886





