Role of Medium-Chain Triglycerides on the Emulsifying Properties and Interfacial Adsorption Characteristics of Pork Myofibrillar Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Myofibrillar (MP) Proteins
2.3. Preparation of MP Emulsion
2.4. Emulsifying Activity Index (EAI) and Emulsion Stability Index (ESI)
2.5. Particle Size and ζ-Potential
2.6. Oxidative Stability
2.7. Rheological Properties of Emulsion
2.8. Emulsion Microstructure
2.9. Chemical Forces
2.10. Secondary Structure of MP
2.11. Reactive Sulfhydryl (SH) Group of MP
2.12. Surface Hydrophobicity
2.13. Determination of Interfacial Proteins and Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.14. Statistical Analysis
3. Results and Discussion
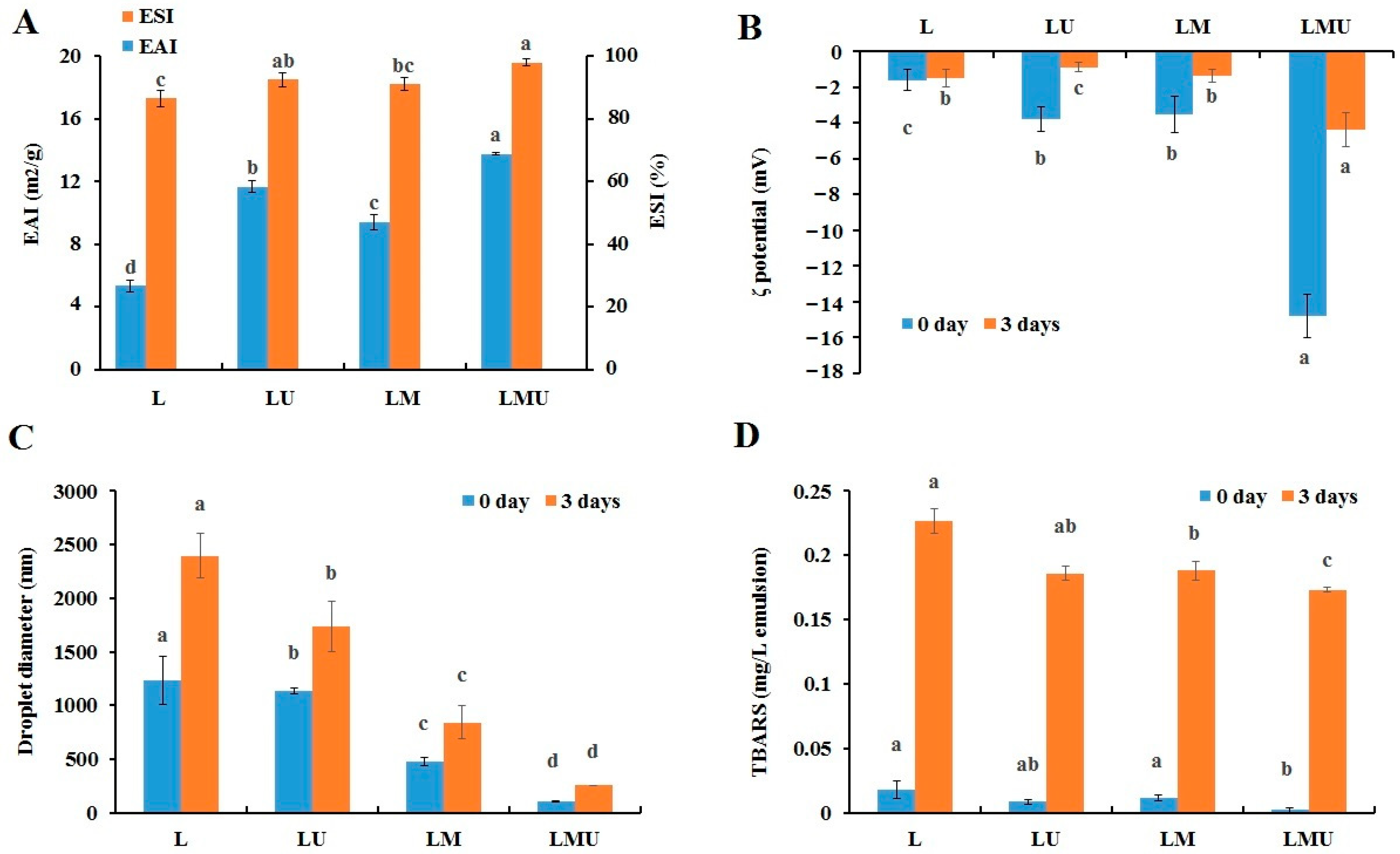
3.1. Characteristics of the Emulsions
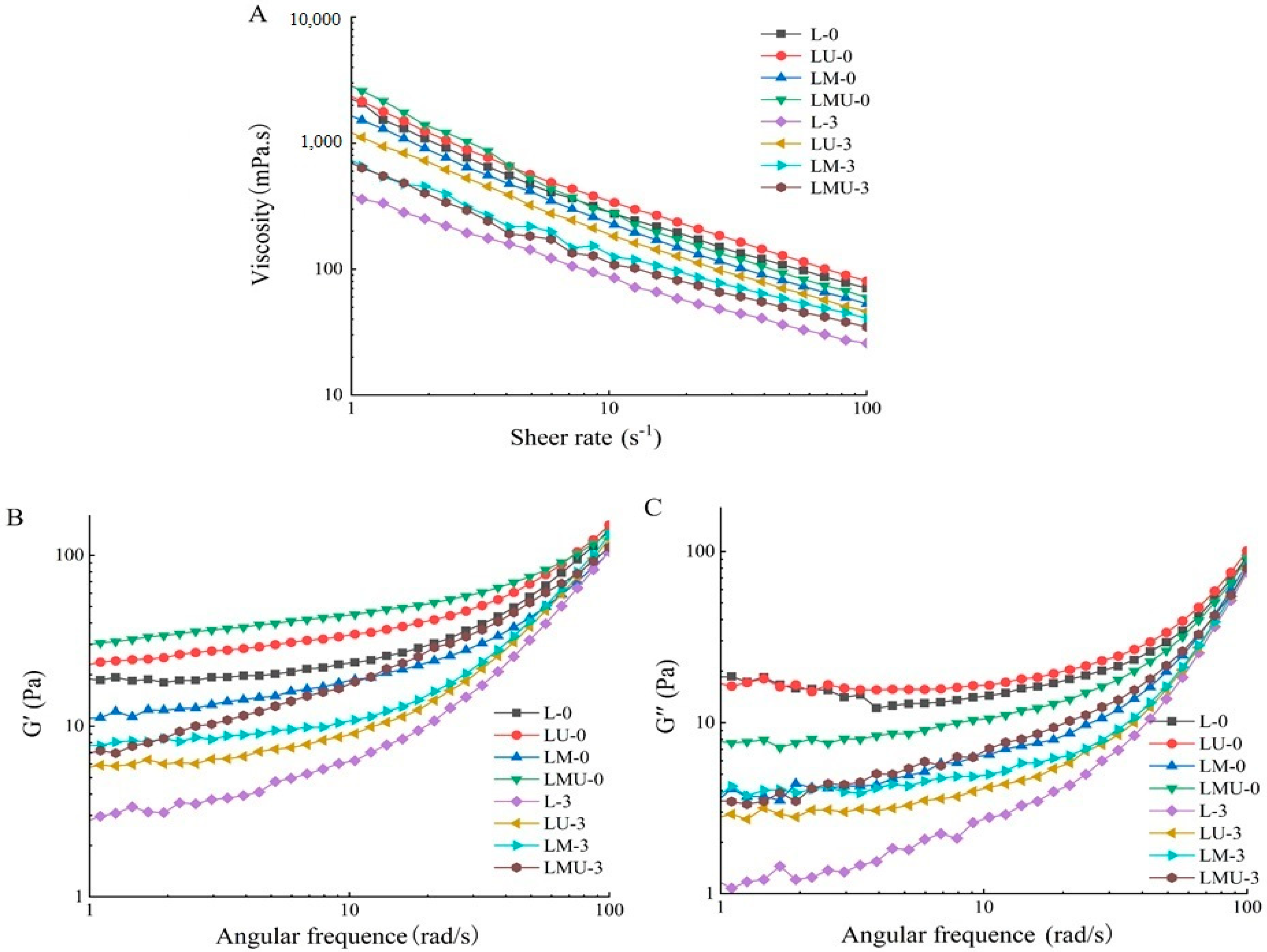
3.2. Rheological Properties of Emulsion
3.3. Microstructure and Macroscopic View of Emulsions
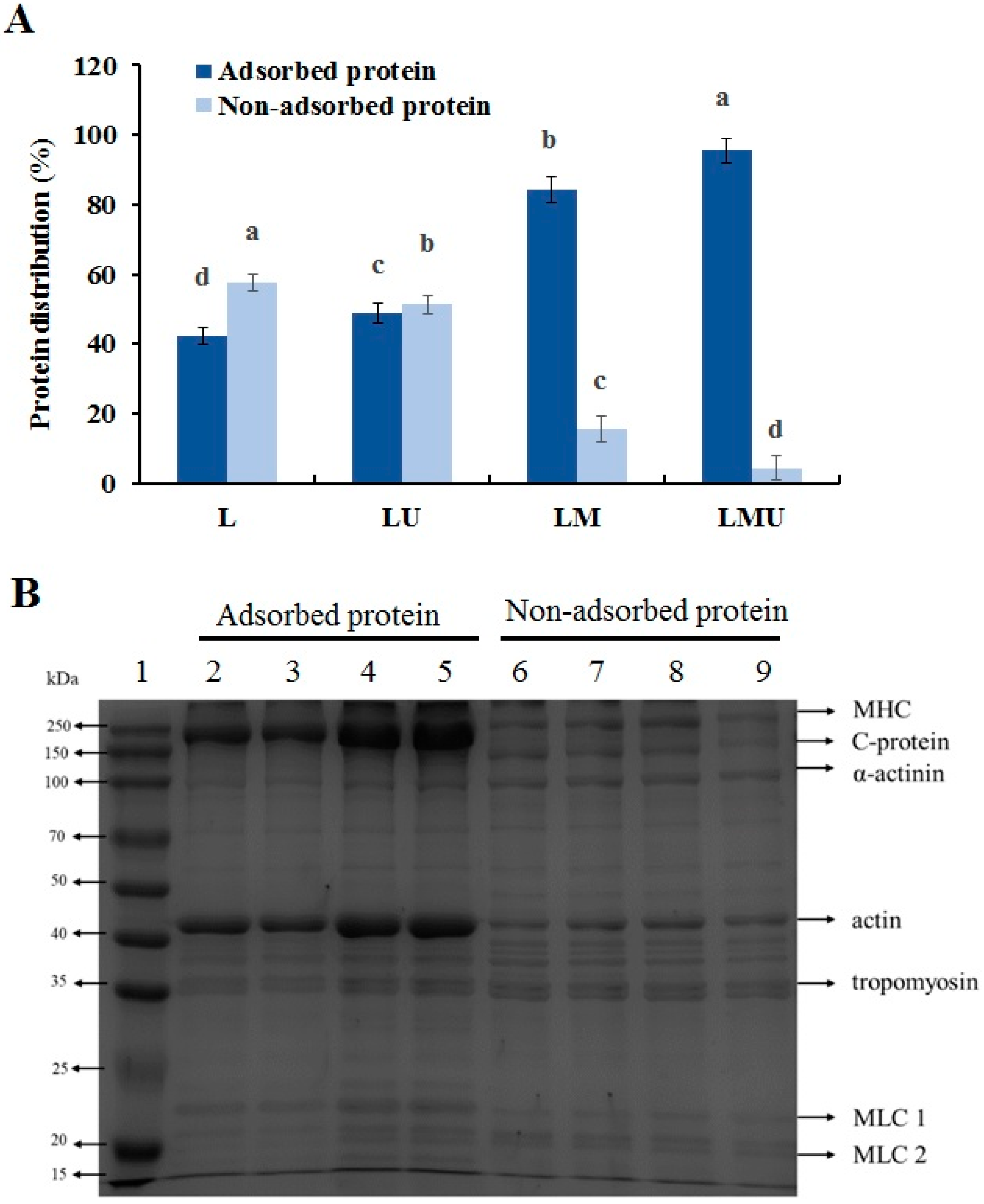
3.4. Characteristics of Interfacial Proteins
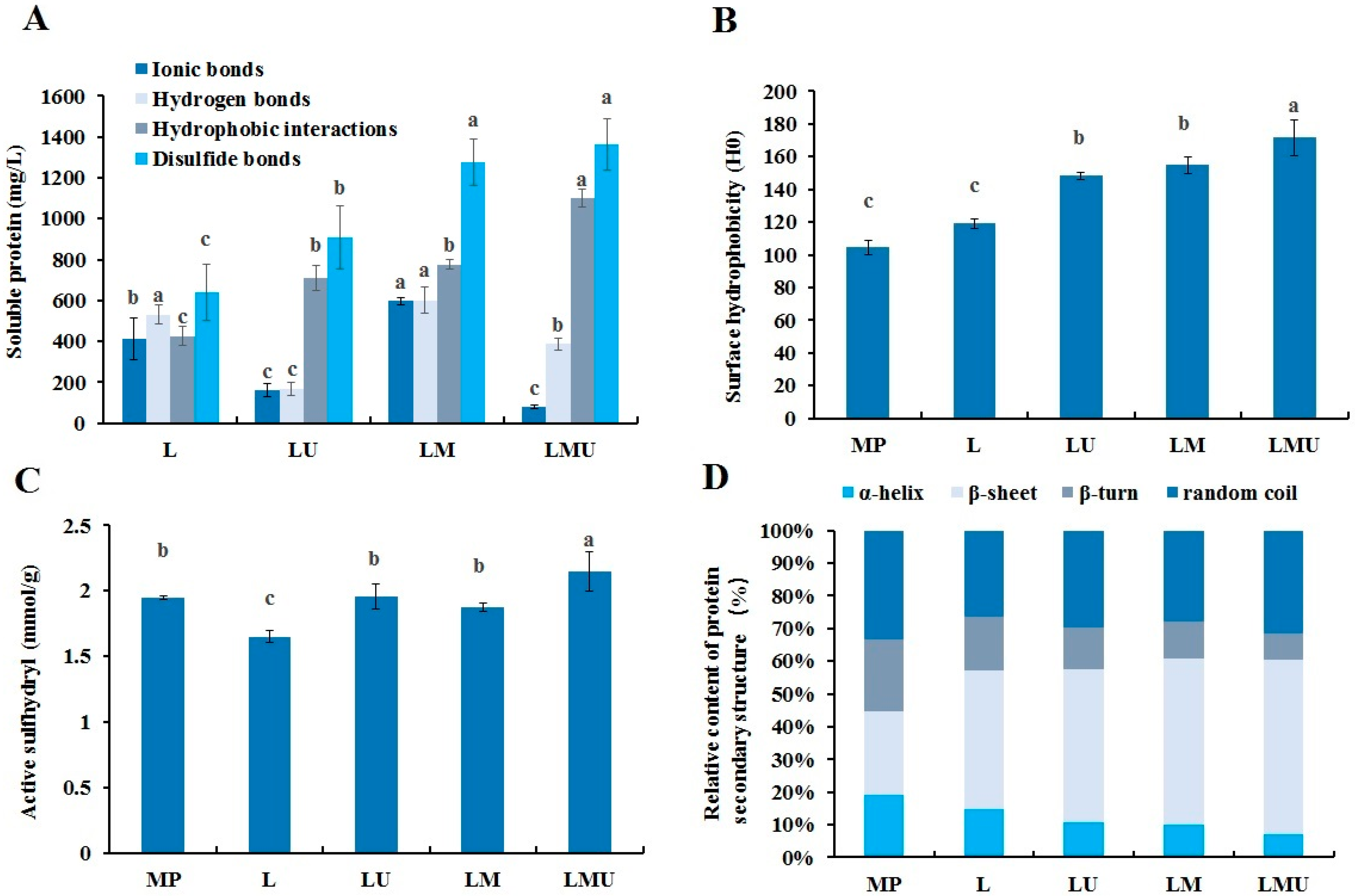
3.5. Physicochemical Properties of MP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fan, L.; Liu, Y.; Huang, S.; Li, J. Effects of proteins on emulsion stability: The role of proteins at the oil-water interface. Food Chem. 2022, 397, 133726. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Guan, H.; Zhao, X.; Chen, Q.; Kong, B. Properties and oxidative stability of emulsions prepared with myofibrillar protein and lard diacylglycerols. Meat Sci. 2016, 115, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cui, L.; Meng, Z. Oleogels/emulsion gels as novel saturated fat replacers in meat products: A review. Food Hydrocoll. 2023, 137, 108313. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.; Zhao, X.; Zhou, G. Insight into the oil polarity impact on interfacial properties of myofibrillar protein. Food Hydrocoll. 2022, 128, 107563. [Google Scholar] [CrossRef]
- Han, Z.; Xu, S.; Sun, J.; Yue, X.; Wu, Z.; Shao, J. Effects of fatty acid saturation degree on salt-soluble pork protein conformation and interfacial adsorption characteristics at the oil/water interface. Food Hydrocoll. 2021, 113, 106472. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, D.; Li, X.; Liu, D.; Li, C.; Zheng, Y.; Yue, X.; Shao, J. The effect of fatty acid chain length and saturation on the emulsification properties of pork myofibrillar proteins. Food Sci. Technol. 2021, 139, 110242. [Google Scholar] [CrossRef]
- Nimbkar, S.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Medium chain triglycerides (MCT): State-of-the-art on chemistry, synthesis, health benefits and applications in food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 843–867. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2022, 60, 2143–2152. [Google Scholar] [CrossRef]
- Dziza, K.; Santini, E.; Liggieri, L.; Jarek, E.; Krzan, M.; Fischer, T.; Ravera, F. Interfacial properties and emulsification of biocompatible liquid-liquid systems. Coatings 2020, 10, 397. [Google Scholar] [CrossRef]
- Liu, J.; Han, Y.; Chen, J.; Zhang, Z.; Miao, S.; Zheng, B.; Zhang, L. MCT/LCT mixed oil phase enhances the rheological property and freeze-thawing stability of emulsion. Foods 2022, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Henao-Ardila, A.; Quintanilla-Carvajal, M.X.; Moreno, F.L. Emulsification and stabilisation technologies used for the inclusion of lipophilic functional ingredients in food systems. Heliyon 2024, 10, e32150. [Google Scholar] [PubMed]
- Guida, C.; Aguiar, A.C.; Magalhaes, A.E.R.; Soares, M.G.; Cunha, R.L. Impact of ultrasound process on cassava starch nanoparticles and pickering emulsions stability. Food Res. Int. 2024, 192, 114810. [Google Scholar] [CrossRef]
- Tao, Y.; Cai, J.; Wang, P.; Chen, J.; Zhou, L.; Yang, Z.; Xu, X. Exploring the relationship between the interfacial propertis and emulsion properties of ultrasound-assisted cross-linked myofibrillar protein. Food Hydrocoll. 2024, 146, 109287. [Google Scholar]
- Ai, M.; Zhang, Z.; Fan, H.; Cao, Y.; Jiang, A. High-intensity ultrasound together with heat treatment improves the oil-in-water emulsion stability of egg white protein peptides. Food Hydrocoll. 2021, 111, 106256. [Google Scholar]
- Li, K.; Fu, L.; Zhao, Y.; Xue, S.; Wang, P.; Xu, X.; Bai, Y. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Park, D.; Xiong, Y.L. Oxidative modification of amino acids in porcine myofibrillar protein isolates exposed to three oxidizing systems. Food Chem. 2007, 103, 607–616. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar]
- Cheng, J.J.; Dudu, O.E.; Li, X.D.; Yan, T.S. Effect of emulsifier-fat interactions and interfacial competitive adsorption of emulsifiers with proteins on fat crystallization and stability of whipped-frozen emulsions. Food Hydrocoll. 2020, 101, 105491. [Google Scholar] [CrossRef]
- Hu, Z.; Wei, X.; Liu, X.; Bai, W.; Zeng, X. Effect of starch categories and mass ratio of TA/starch on the emulsifying performance and stability of emulsions stabilized by tannic acid-starch complexes. Int. J. Biol. Macromol. 2024, 280, 136345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Han, G.; Li, B.; Xu, Z.; Pan, S.; Liu, F. Effect of oil phase type and lutein loading on emulsifying properties and in vitro digestion of mandarin peel pectin emulsions. Food Sci. Technol. 2024, 194, 115799. [Google Scholar]
- Kim, T.; Lee, M.H.; Yong, H.I.; Jang, H.W.; Jung, S.; Choi, Y. Impacts of fat types and myofibrillar protein on the rheological properties and thermal stability of meat emulsion systems. Food Chem. 2021, 346, 128930. [Google Scholar] [PubMed]
- Fan, H.; Zhu, P.; Hui, G.; Shen, Y.; Yong, Z.; Xie, Q.; Wang, M. Mechanism of synergistic stabilization of emulsions by amorphous taro starch and protein and emulsion stability. Food Chem. 2023, 424, 136342. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, H.; Emadzadeh, B.; Kadkhodaee, R.; Fang, Y. Effect of Persian gum on whey protein concentrate cold-set emulsion gel: Structure and rheology study. Int. J. Biol. Macromol. 2019, 125, 17–26. [Google Scholar] [CrossRef]
- Boutin, C.; Giroux, H.J.; Paquin, P.; Britten, M. Characterization and acid-induced gelation of butter oil emulsions produced from heated whey protein dispersions. Int. Dairy J. 2007, 17, 696–703. [Google Scholar] [CrossRef]
- Chen, E.; Wu, S.; McClements, D.J.; Li, B.; Li, Y. Influence of pH and cinnamaldehyde on the physical stability and lipolysis of whey protein isolate-stabilized emulsions. Food Hydrocoll. 2017, 69, 103–110. [Google Scholar]
- Zhang, W.; Lu, J.; Zhao, X.; Xu, X. An optimized approach to recovering O/W interfacial myofibrillar protein: Emphasizing on interface-induced structural changes. Food Hydrocoll. 2022, 124, 107194. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J.; Xu, X.; Qin, L.; Wu, C.; Du, M. Ultrasound treatment improved the physicochemical characteristics of cod protein and enhanced the stability of oil-in-water emulsion. Food Res. Int. 2019, 121, 247–256. [Google Scholar] [CrossRef]
- Bergfreund, J.; Bertsch, P.; Fischer, P. Adsorption of proteins to fluid interfaces: Role of the hydrophobic subphase. J. Colloid Interf. Sci. 2021, 584, 411–417. [Google Scholar]
- Yang, J.; Xiong, Y. Inhibition of lipid oxidation in oil-in-water emulsions by interface-adsorbed myofibrillar protein. J. Agric. Food Chem. 2015, 63, 8896–8904. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, X.; Zhou, K.; Xie, Y.; Wang, Y.; Zhou, H.; Bai, Y.; Xu, B. Differentiating the effects of hydrophobic interaction and disulfide bond on the myofibrillar protein emulsion gels at the high temperature and the protein interfacial properties. Food Chem. 2023, 412, 135472. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interf. Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H. Effects of calcium ion on gel properties and gelation of tilapia (Oreochromis niloticus) protein isolates processed with pH shift method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef]
- Gu, J.; Pan, M.; Chiou, Y.; Wei, S.; Ding, B. Enhanced stability of Pickering emulsions through co-stabilization with nanoliposomes and thermally denatured ovalbumin. Int. J. Biol. Macromol. 2024, 278, 134561. [Google Scholar] [CrossRef]
- Kong, D.; Quan, C.; Xi, Q.; Han, R.; Koseke, S.; Li, P.; Du, Q.; Yang, Y.; Forghani, F.; Wang, J. Study on the quality and myofibrillar protein structure of chicken breasts during thawing of ultrasound-assisted slightly acidic electrolyzed water (SAEW). Ultrason. Sonochem. 2022, 88, 106105. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Sani, M.A.; McClements, D.J.; Mehr, H.M. Ultrasound-modified protein-based colloidal particles: Interfacial activity, gelation properties, and encapsulation efficiency. Adv. Colloid Interf. Sci. 2022, 309, 102768. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Zhang, M.; Bian, H.; Wang, D.; Xu, W.; Wei, S.; Guo, R. Role of Medium-Chain Triglycerides on the Emulsifying Properties and Interfacial Adsorption Characteristics of Pork Myofibrillar Protein. Foods 2025, 14, 796. https://doi.org/10.3390/foods14050796
Shi M, Zhang M, Bian H, Wang D, Xu W, Wei S, Guo R. Role of Medium-Chain Triglycerides on the Emulsifying Properties and Interfacial Adsorption Characteristics of Pork Myofibrillar Protein. Foods. 2025; 14(5):796. https://doi.org/10.3390/foods14050796
Chicago/Turabian StyleShi, Miaomiao, Muhan Zhang, Huan Bian, Daoying Wang, Weimin Xu, Suhuan Wei, and Ruirui Guo. 2025. "Role of Medium-Chain Triglycerides on the Emulsifying Properties and Interfacial Adsorption Characteristics of Pork Myofibrillar Protein" Foods 14, no. 5: 796. https://doi.org/10.3390/foods14050796
APA StyleShi, M., Zhang, M., Bian, H., Wang, D., Xu, W., Wei, S., & Guo, R. (2025). Role of Medium-Chain Triglycerides on the Emulsifying Properties and Interfacial Adsorption Characteristics of Pork Myofibrillar Protein. Foods, 14(5), 796. https://doi.org/10.3390/foods14050796



