Progress in Research on the Mechanism of GABA in Improving Sleep
Abstract
1. Introduction
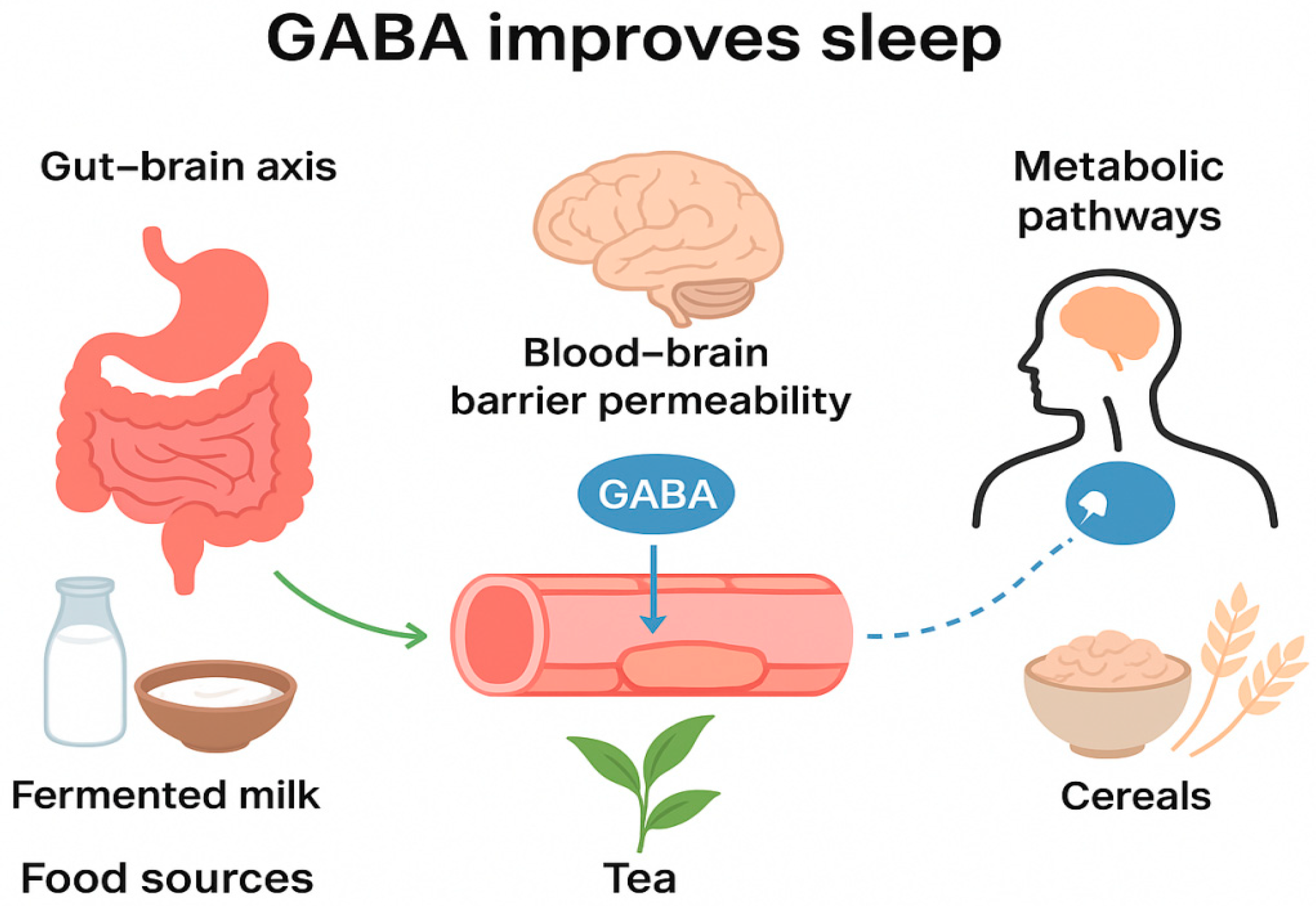
2. Mechanisms of GABA in Sleep Improvement via the Gut–Brain Axis
2.1. Vagus Nerve Pathway
2.2. Neuroendocrine Pathway
2.3. Immune Pathway
3. Mechanisms of GABA Penetration Through the Blood–Brain Barrier
3.1. Dynamic Regulation of BBB Permeability
3.2. GABA Transport Pathways Across the BBB
3.3. Innovative Delivery Technologies for Enhancing GABA Brain Permeability
4. Mechanisms of GABA in Sleep Improvement via Metabolic Pathways
4.1. Dual Regulatory Mechanisms of GHB
4.2. New Discoveries in GABA Metabolic Pathways
4.3. Association Between Metabolites and Sleep–Wake Regulation
5. Application and Future Research Directions
5.1. Summary
5.2. Application
5.3. Future Research Directions
- Dynamic and Precise Mechanistic Investigations. Future efforts can focus on elucidating the dynamic processes of GABA-mediated sleep regulation as current studies are largely static and descriptive. This includes integrating multi-omics technologies with neuroimaging techniques (e.g., fMRI, PET) to uncover the dynamic changes in GABA across the gut–brain axis, blood–brain barrier, and metabolic networks during different sleep–wake stages (e.g., NREM vs. REM); and employing single-cell sequencing to meticulously map the distribution of GABA receptors, transporters, and metabolic enzymes in key sleep-regulating brain regions (e.g., the suprachiasmatic nucleus, amygdala, thalamic reticular nucleus), thereby clarifying region-specific regulatory mechanisms. It is recommended to do some work to answer the few specific and testable research questions such as: Does the transport efficiency of GABA across the BBB and its subsequent metabolic flux in the brain undergo cyclical fluctuations in accordance with circadian rhythms and sleep–wake states? How specific probiotics (e.g., high-GABA-yielding lactobacilli strains) modulate the overall structure of the gut microbiota to indirectly enhance GABAergic signaling along the gut–brain axis?
- Rational Design and Safety Validation of Next-Generation Probiotics for Food Applications and Fermentation Processes. Regarding strain and process optimization, future work should concentrate on employing synthetic biology to construct next-generation probiotics (NGPs) with high GABA yields, for instance, by increasing the copy number of glutamate decarboxylase (GAD) genes or utilizing gut-specific inducible promoters. It is a prerequisite for the application translation to systematically evaluate the intestinal colonization stability, immunogenicity, and long-term consumption safety of these engineered strains.
- Standardization and Personalization of Clinical Research. To advance the precise application of GABA-based functional foods or drugs, there is an urgent need for multicenter, large-sample randomized controlled trials employing standardized sleep assessment metrics (e.g., polysomnography). Research should integrate population genomics, metabolomics, and microbiomics data to deeply analyze the impact of individual differences (e.g., GABA receptor gene polymorphisms, baseline gut microbiota composition) on intervention efficacy, thereby providing a basis for developing personalized nutritional strategies for sleep. For example, define the synergistic effects of GABA in combination with natural active compounds (e.g., apigenin, L-theanine) and establish optimal dosages and intervention durations for specific populations (e.g., insomnia patients with comorbid anxiety).
- Future research should integrate pharmacokinetic methods to systematically elucidate the relationship between the plasma concentration-time profiles of different oral GABA doses (e.g., 50, 100, 300 mg) and corresponding sleep improvement outcomes (such as PSQI scores and polysomnographic parameters). This is crucial for determining the minimum effective dose and the optimal dosage range for GABA-based functional foods, ultimately bridging the gap between mechanistic understanding and practical application.
- Innovation in Food-Grade Delivery Technologies and Industrialization of Functional Foods. To improve the central bioavailability of GABA, further optimization of food-grade nano-delivery systems (e.g., whey protein nanoparticles and chitosan microspheres) is necessary, potentially through surface modification with targeting ligands (e.g., transferrin receptor antibodies) to enhance brain targeting efficiency. Simultaneously, systematic in vitro and in vivo experiments, coupled with long-term human studies, are mandatory to evaluate the intestinal absorption efficiency, biosafety, and long-term consumption risks of these nanocarriers, thereby clearing obstacles for their industrialization.
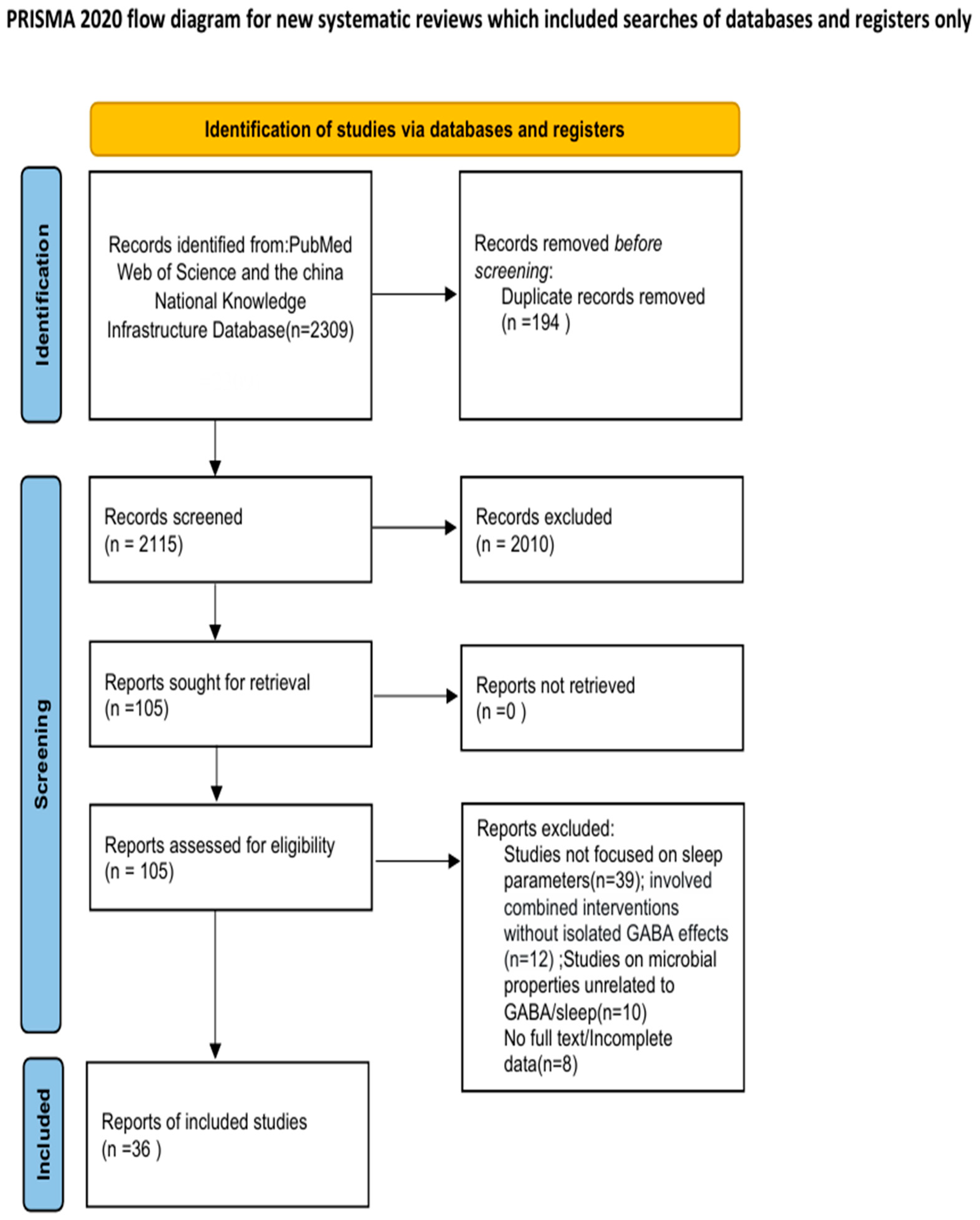
6. Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2019, 7, 1. [Google Scholar] [CrossRef]
- Rahmani, M.; Rahmani, F.; Rezaei, N. The Brain-Derived Neurotrophic Factor: Missing Link Between Sleep Deprivation, Insomnia, and Depression. Neurochem. Res. 2020, 45, 221–231. [Google Scholar] [CrossRef]
- Milosevic, L.; Gramer, R.; Kim, T.H.; Algarni, M.; Fasano, A.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Popovic, M.R.; Hutchison, W.D. Modulation of inhibitory plasticity in basal ganglia output nuclei of patients with Parkinson’s disease. Neurobiol. Dis. 2019, 124, 46–56. [Google Scholar] [CrossRef]
- Li, K.; Yu, L.; Xu, L. Research Progress on Improving Sleep Mechanism of γ-aminobutyric Acid. Sci. Technol. Food Ind. 2019, 40, 353–358. [Google Scholar] [CrossRef]
- Grant, A.D.; McBride, E. GABA Probiotic Lactiplantibacillus plantarum Lp815 Improves Sleep, Anxiety and Increases Urinary GABA: A Randomized, Double-Blind, Placebo-Controlled Study. J. Sleep Res. 2025. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Yu, L.; Han, X.; Cen, S.; Duan, H.; Feng, S.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef]
- Almutairi, S.; Sivadas, A.; Kwakowsky, A. The Effect of Oral GABA on the Nervous System: Potential for Therapeutic Intervention. Nutraceuticals 2024, 4, 241–259. [Google Scholar] [CrossRef]
- Aburto, M.R.; Cryan, J.F. Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota–gut–brain axis. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 222–247. [Google Scholar] [CrossRef]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J.; Wan, M.L.Y.; Monteiro, A.R.; Nutt, D.J.; Swinny, J.D. From bugs to brain: Unravelling the GABA signalling networks in the brain–gut–microbiome axis. Brain 2025, 148, 1479–1506. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Zou, Q.; Han, S.; Liang, J.; Yan, G.; Wang, Q.; Wang, Y.; Zhang, Z.; Hu, J.; Li, J.; Yuan, T.; et al. Alleviating effect of vagus nerve cutting in Salmonella-induced gut infections and anxiety-like behavior via enhancing microbiota-derived GABA. Brain Behav. Immun. 2024, 119, 607–620. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, C.; Du, X.; Zhu, Y.; Wang, L.; Hu, J.; Sun, Y. Vagus Nerve and Gut-Brain Communication. Neuroscientist 2025, 31, 262–278. [Google Scholar] [CrossRef]
- Nakamura, U.; Nohmi, T.; Sagane, R.; Hai, J.; Ohbayashi, K.; Miyazaki, M.; Yamatsu, A.; Kim, M.; Iwasaki, Y. Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves. Nutrients 2022, 14, 2492. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, N.; Tang, C.; Long, H.; Wang, J. Microglia in Microbiota-Gut-Brain Axis: A Hub in Epilepsy. Mol. Neurobiol. 2024, 61, 7109–7126. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Reed, F.; Foldi, C.J. Do the therapeutic effects of psilocybin involve actions in the gut? Trends Pharmacol. Sci. 2024, 45, 107–117. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, S.; Zhang, R.; Zhuang, Y.; Li, L.; Yi, S.; Li, Y.; Wu, L.; Ding, Y.; Zhang, J.; et al. Supplementation with high-GABA-producing Lactobacillus plantarum L5 ameliorates essential tremor triggered by decreased gut bacteria-derived GABA. Transl. Neurodegener. 2023, 12, 19–58. [Google Scholar] [CrossRef]
- Khan, M.T.; Zohair, M.; Khan, A.; Kashif, A.; Mumtaz, S.; Muskan, F. From Gut to Brain: The roles of intestinal microbiota, immune system, and hormones in intestinal physiology and gut–brain–axis. Mol. Cell Endocrinol. 2025, 607, 112599. [Google Scholar] [CrossRef]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut–brain axis. npj Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef]
- Samtiya, M.; Dhewa, T.; Puniya, A.K. Probiotic Mechanism to Modulate the Gut-Brain Axis (GBA); Sayyed, R.Z., Khan, M., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 237–259. [Google Scholar]
- Yan, F.; Polk, D.B. Probiotics and Probiotic-Derived Functional Factors—Mechanistic Insights into Applications for Intestinal Homeostasis. Front. Immunol. 2020, 11, 1428. [Google Scholar] [CrossRef]
- Olshansky, B. Vagus nerve modulation of inflammation: Cardiovascular implications. Trends Cardiovasc. Med. 2016, 26, 1–11. [Google Scholar] [CrossRef]
- Wang, M.; Song, Z.; Lai, S.; Tang, F.; Dou, L.; Yang, F. Depression-associated gut microbes, metabolites and clinical trials. Front. Microbiol. 2024, 15, 1292004. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Oh, J.S. Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis. Int. J. Mol. Sci. 2025, 26, 1160. [Google Scholar] [CrossRef]
- Jeong, A.; Hwang, J.; Jo, K.; Kim, S.; Ahn, Y.; Suh, H.J.; Choi, H. Fermented Gamma Aminobutyric Acid Improves Sleep Behaviors in Fruit Flies and Rodent Models. Int. J. Mol. Sci. 2021, 22, 3537. [Google Scholar] [CrossRef]
- Nazir, M.M.; Ghaffar, W.; Mustafa, G.; Saeed, S.; Ijaz, M.U.; Ashraf, A. Modulating depression through the gut–brain axis: The role of gut microbiota in therapeutic interventions. Naunyn Schmiedeberg’s Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Fan, X.X.; Zhang, B.; Zhang, Q. Research progress of gut microbiota in pathogenesis of insomnia disorder. Chin. J. New Clin. Med. 2021, 14, 1246–1249. [Google Scholar] [CrossRef]
- Topan, R.; Scott, S.M. Sleep: An Overlooked Lifestyle Factor in Disorders of Gut-Brain Interaction. Curr. Treat. Options Gastroenterol. 2023, 21, 435–446. [Google Scholar] [CrossRef]
- Xie, F.; Yang, W.; Xing, M.; Zhang, H.; Ai, L. Natural polyphenols-gut microbiota interactions and effects on glycolipid metabolism via polyphenols-gut-brain axis: A state-of-the-art review. Trends Food Sci. Tech. 2023, 140, 104171. [Google Scholar] [CrossRef]
- Kore, M.S.; Mamsa, R.; Patil, D.; Bhatt, L.K. Ghrelin in Depression: A Promising Therapeutic Target. Mol. Neurobiol. 2025, 62, 4237–4249. [Google Scholar] [CrossRef] [PubMed]
- Asarnow, L.D. Depression and sleep: What has the treatment research revealed and could the HPA axis be a potential mechanism? Curr. Opin. Psychol. 2020, 34, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, J.; Rao, X.; Zhang, R.; Li, Q.; Zhang, K.; Ma, S.; Zhao, J.; Ji, C. Exploring the pathogenesis of insomnia and acupuncture intervention strategies based on the microbiota-gut-brain axis. Front. Microbiol. 2024, 15, 1456848. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Sen, P.; Molinero-Perez, A.; ORiordan, K.J.; McCafferty, C.P.; OHalloran, K.D.; Cryan, J.F. Microbiota and sleep: Awakening the gut feeling. Trends Mol. Med. 2021, 27, 935–945. [Google Scholar] [CrossRef]
- Riazi, K.; Galic, M.A.; Kuzmiski, J.B.; Ho, W.; Sharkey, K.A.; Pittman, Q.J. Microglial activation and TNFα production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 17151–17156. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Li, L.; Yu, L.L.; Jiang, Y. Effect of compound probiotics with GABA production for sleep improvement. Chin. J. Microecol. 2022, 34, 1006–1012. [Google Scholar] [CrossRef]
- Ajibola, O.O.; Thomas, R.; Bakare, B.F. Selected fermented indigenous vegetables and fruits from Malaysia as potential sources of natural probiotics for improving gut health. Food Sci. Hum. Well 2023, 12, 1493–1509. [Google Scholar] [CrossRef]
- Ngo, D.H.; Tran, Q.T.; Kim, Y.S.; Hang, N.T.N.; Ngo, D.N.; Vo, T.S. GABA -enriched rice bran inhibits inflammation inLPS -stimulated macrophages via suppression ofTLR4-MAPK /NF-κB signaling cascades. J. Food Biochem. 2022, 46, e14421. [Google Scholar] [CrossRef]
- Umeyama, L.; Kasahara, S.; Sugawara, M.; Yokoyama, S.; Saiki, I.; Hayakawa, Y. Anti-inflammatory effect of fermented brown rice and rice bran with Aspergillus oryzae on mice. Tradit. Kamp Med. 2021, 8, 60–65. [Google Scholar] [CrossRef]
- Piper, K.; Kumar, J.I.; Domino, J.; Tuchek, C.; Vogelbaum, M.A. Consensus review on strategies to improve delivery across the blood-brain barrier including focused ultrasound. Neuro Oncol. 2024, 26, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef]
- Mhaske, A.; Shukla, S.; Ahirwar, K.; Singh, K.K.; Shukla, R. Receptor-Assisted Nanotherapeutics for Overcoming the Blood–Brain Barrier. Mol. Neurobiol. 2024, 61, 8702–8738. [Google Scholar] [CrossRef]
- Constans, C.; Ahnine, H.; Santin, M.; Lehericy, S.; Tanter, M.; Pouget, P.; Aubry, J. Non-invasive ultrasonic modulation of visual evoked response by GABA delivery through the blood brain barrier. J. Control. Release 2020, 318, 223–231. [Google Scholar] [CrossRef]
- Akande, A.O.; Carter, Z.A.; Stokes, K.Y.; Nam, H.W. Endothelial Neurogranin Regulates Blood–Brain Barrier Permeability via Modulation of the AKT Pathway. Mol. Neurobiol. 2025, 62, 3991–4007. [Google Scholar] [CrossRef]
- Balaji, P.G.; Bhimrao, L.S.; Yadav, A.K. Revolutionizing Stroke Care: Nanotechnology-Based Brain Delivery as a Novel Paradigm for Treatment and Diagnosis. Mol. Neurobiol. 2025, 62, 184–220. [Google Scholar] [CrossRef]
- Gallardo-Fernandez, M.; Garcia, A.R.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Brito, M.A. In vitro study of the blood–brain barrier transport of bioactives from Mediterranean foods. Food Funct. 2024, 15, 3420–3432. [Google Scholar] [CrossRef]
- Wang, Q.; Xin, X.; Dai, Q.; Sun, M.; Chen, J.; Mostafavi, E.; Shen, Y.; Li, X. Medulloblastoma targeted therapy: From signaling pathways heterogeneity and current treatment dilemma to the recent advances in development of therapeutic strategies. Pharmacol Ther. 2023, 250, 108527. [Google Scholar] [CrossRef]
- de Rus Jacquet, A.; Layé, S.; Calon, F. How nutrients and natural products act on the brain: Beyond pharmacology. Cell Rep. Med. 2023, 4, 101243. [Google Scholar] [CrossRef]
- Amaral, A.U.; Wajner, M. Pathophysiology of maple syrup urine disease: Focus on the neurotoxic role of the accumulated branched-chain amino acids and branched-chain α-keto acids. Neurochem. Int. 2022, 157, 105360. [Google Scholar] [CrossRef]
- Yadava, S.; Reddy, D.H.; Nakka, V.P.; Anusha, V.L.; Dumala, N.; Viswanadh, M.K.; Chakravarthi, G.; Nalluri, B.N.; Ramakrishna, K. Unravelling neuroregenerative and neuroprotective roles of Wnt/β-catenin pathway in ischemic stroke: Insights into molecular mechanisms. Neuroscience 2025, 565, 527–547. [Google Scholar] [CrossRef]
- Gu, X.; Dong, M.; Xia, S.; Li, H.; Bao, X.; Cao, X.; Xu, Y. γ-Glutamylcysteine ameliorates blood-brain barrier permeability and neutrophil extracellular traps formation after ischemic stroke by modulating Wnt/β-catenin signalling in mice. Eur. J. Pharmacol. 2024, 969, 176409. [Google Scholar] [CrossRef]
- Nance, E.; Pun, S.H.; Saigal, R.; Sellers, D.L. Drug delivery to the central nervous system. Nat. Rev. Mater. 2022, 7, 314–331. [Google Scholar] [CrossRef]
- Liu, L.; McClements, D.J.; Liu, X.; Liu, F. Overcoming Biopotency Barriers: Advanced Oral Delivery Strategies for Enhancing the Efficacy of Bioactive Food Ingredients. Adv. Sci. 2024, 11, 2401172. [Google Scholar] [CrossRef]
- Abdoullateef, B.M.T.; El-Din Al-Mofty, S.; Azzazy, H.M.E. Nanoencapsulation of general anaesthetics. Nanoscale Adv. 2024, 6, 1361–1373. [Google Scholar] [CrossRef]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGA Nanoparticle-Based Formulations to Cross the Blood–Brain Barrier for Drug Delivery: From R&D to cGMP. Pharmaceutics 2021, 13, 500. [Google Scholar] [CrossRef]
- Barbosa-Nuñez, J.A.; Espinosa-Andrews, H.; Cardona, A.A.V.; Haro-González, J.N. Polymer-based encapsulation in food products: A comprehensive review of applications and advancements. J. Future Foods 2025, 5, 36–49. [Google Scholar] [CrossRef]
- Zelikina, D.; Chebotarev, S.; Antipova, A.; Martirosova, E.; Anokhina, M.; Palmina, N.; Bogdanova, N.; Semenova, M. Efficacy of a Maillard-type conjugate of whey protein isolate with chitosan as a carrier for a liposomal form of a combination of curcumin and balanced amounts of n-3 and n-6 PUFAs. Part II. Carrier behaviour under simulated in vitro digestion. Int. Dairy J. 2024, 154, 105924. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef]
- Oishi, Y.; Saito, Y.C.; Sakurai, T. GABAergic modulation of sleep-wake states. Pharmacol Ther. 2023, 249, 108505. [Google Scholar] [CrossRef]
- Kim, M.; Oh, S.; Kim, S.; Ji, M.; Choi, B.; Bae, J.; Lee, Y.S.; Paik, M.; Lee, S. Alcohol perturbed locomotor behavior, metabolism, and pharmacokinetics of gamma-hydroxybutyric acid in rats. Biomed. Pharmacother. 2023, 164, 114992. [Google Scholar] [CrossRef]
- Dufayet, L.; Bargel, S.; Bonnet, A.; Boukerma, A.K.; Chevallier, C.; Evrard, M.; Guillotin, S.; Loeuillet, E.; Paradis, C.; Pouget, A.M.; et al. Gamma-hydroxybutyrate (GHB), 1,4-butanediol (1,4BD), and gamma-butyrolactone (GBL) intoxication: A state-of-the-art review. Regul. Toxicol. Pharmcol. 2023, 142, 105435. [Google Scholar] [CrossRef]
- Dornbierer, D.A.; Baur, D.M.; Stucky, B.; Quednow, B.B.; Kraemer, T.; Seifritz, E.; Bosch, O.G.; Landolt, H. Neurophysiological signature of gamma-hydroxybutyrate augmented sleep in male healthy volunteers may reflect biomimetic sleep enhancement: A randomized controlled trial. Neuropsychopharmacology 2019, 44, 1985–1993. [Google Scholar] [CrossRef]
- Bavato, F.; Schnider, L.K.; Dornbierer, D.A.; Di Floriano, J.R.; Stucky, B.; Friedli, N.; Janki, M.; Quednow, B.B.; Landolt, H.; Bosch, O.G.; et al. Gamma-hydroxybutyrate to promote slow-wave sleep in major depressive disorder: A randomized crossover trial. Neuropsychopharmacology 2025, 50, 1237–1244. [Google Scholar] [CrossRef]
- Frisoni, P.; Corli, G.; Bilel, S.; Tirri, M.; Gasparini, L.C.; Alfieri, L.; Neri, M.; De-Giorgio, F.; Marti, M. Effect of Repeated Administration of ɣ-Valerolactone (GVL) and GHB in the Mouse: Neuroadaptive Changes of the GHB and GABAergic System. Pharmaceuticals 2023, 16, 1225. [Google Scholar] [CrossRef]
- Maitre, M.; Klein, C.; Mensah-Nyagan, A.G. Mechanisms for the Specific Properties of γ-Hydroxybutyrate in Brain. Med. Res. Rev. 2016, 36, 363–388. [Google Scholar] [CrossRef]
- Watanabe, T.; Kan, S.; Koike, T.; Misaki, M.; Konishi, S.; Miyauchi, S.; Miyahsita, Y.; Masuda, N. Network-dependent modulation of brain activity during sleep. Neuroimage 2014, 98, 1–10. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Madden, E.F.; Roe, A.L.; Betz, J.M. United States Pharmacopeia (USP) Safety Review of Gamma-Aminobutyric Acid (GABA). Nutrients 2021, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Vignoli, T.; Maremmani, I.; Bernardi, M.; Zoli, G. Gamma Hydroxybutyric Acid (GHB) for the Treatment of Alcohol Dependence: A Review. Int. J. Environ. Res. Public Health 2009, 6, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Hayat, F.; Khan, U.; Li, J.; Ahmed, N.; Khanum, F.; Iqbal, S.; Altaf, M.A.; Ahmad, J.; Javed, H.U.; Peng, Y.; et al. γ Aminobutyric Acid (GABA): A Key Player in Alleviating Abiotic Stress Resistance in Horticultural Crops: Current Insights and Future Directions. Horticulturae 2023, 9, 647. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ghosh, R.; Biswas, P.; Das, S.; Sengupta, S.; Dubey, S.; Ray, B.K.; Pandit, A.; Benito-León, J.; Bhattacharjee, R. Diabetic striatopathy and other acute onset de novo movement disorders in hyperglycemia. Diabetes Metab. Syndr. Clin. Res. Rev. 2024, 18, 102997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, L.; Li, H.; Chen, Q.; Li, N.; Li, J.; Zhao, Z.; Xiao, D.; Tang, T.; Bi, C.; et al. Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): A review. PeerJ 2024, 12, e18712. [Google Scholar] [CrossRef]
- Tokatly Latzer, I.; Pearl, P.L. Update on inherited disorders of GABA metabolism. Eur. J. Paediatr. Neurol 2025, 56, 10–16. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.; Han, S.H.; Suh, H.J. GABA andl -theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 64–72. [Google Scholar] [CrossRef]
- Ono, D.; Honma, K.I.; Honma, S. GABAergic mechanisms in the suprachiasmatic nucleus that influence circadian rhythm. J. Neurochem. 2021, 157, 31–41. [Google Scholar] [CrossRef]
- Kjaerby, C.; Andersen, M.; Hauglund, N.; Untiet, V.; Dall, C.; Sigurdsson, B.; Ding, F.; Feng, J.; Li, Y.; Weikop, P.; et al. Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nat. Neurosci. 2022, 25, 1059–1070. [Google Scholar] [CrossRef]
- Greene, R. Biological Mechanisms of Sleep/Wake-Related Function. Biol. Psychiatry 2025, 98, 875–882. [Google Scholar] [CrossRef]
- Shelp, B.J.; Mullen, R.T.; Waller, J.C. Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci. 2012, 17, 57–59. [Google Scholar] [CrossRef]
- Tokatly Latzer, I.; Yang, E.; Afacan, O.; Arning, E.; Rotenberg, A.; Lee, H.H.C.; Roullet, J.B.; Pearl, P.L. Glymphatic dysfunction coincides with lowerGABA levels and sleep disturbances in succinic semialdehyde dehydrogenase deficiency. J. Sleep Res. 2024, 33, e14105. [Google Scholar] [CrossRef]
- Ravasz, D.; Kacso, G.; Fodor, V.; Horvath, K.; Adam-Vizi, V.; Chinopoulos, C. Catabolism of GABA, succinic semialdehyde or gamma-hydroxybutyrate through the GABA shunt impair mitochondrial substrate-level phosphorylation. Neurochem. Int. 2017, 109, 41–53. [Google Scholar] [CrossRef]
- Kitamura, T.; Miyazaki, S.; Sulaiman, H.B.; Akaike, R.; Ito, Y.; Suzuki, H. Insomnia and obstructive sleep apnea as potential triggers of dementia: Is personalized prediction and prevention of the pathological cascade applicable? EPMA J. 2020, 11, 355–365. [Google Scholar] [CrossRef]
- Calafate, S.; Özturan, G.; Thrupp, N.; Vanderlinden, J.; Santa-Marinha, L.; Morais-Ribeiro, R.; Ruggiero, A.; Bozic, I.; Rusterholz, T.; Lorente-Echeverría, B.; et al. Early alterations in the MCH system link aberrant neuronal activity and sleep disturbances in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2023, 26, 1021–1031. [Google Scholar] [CrossRef]
- Qian, J.; Zheng, L.; Huang, M.; Zhao, M. Potential Mechanisms of Casein Hexapeptide YPVEPF on Stress-Induced Anxiety and Insomnia Mice and Its Molecular Effects and Key Active Structure. J. Agric. Food Chem. 2024, 72, 6189–6202. [Google Scholar] [CrossRef]
- Kim, H.D.; Suh, H.J.; Chung, S.H.; Byun, J.; Yoo, Y.; Lee, H. Sleep-enhancing activity of fermented pea protein hydrolysate with enhancedGABA content by Lactobacillus brevis SYLB 0016 fermentation. J. Sci. Food Agric. 2025, 105, 4297–4305. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, S.; Yu, Z.; Zhang, J.; Mei, Z. Mechanisms for the biological activity of Gastrodia elata Blume and its constituents: A comprehensive review on sedative-hypnotic, and antidepressant properties. Phytomedicine 2024, 123, 155251. [Google Scholar] [CrossRef]
- Lebovich, M.; Lora, M.A.; Gracia-David, J.; Andrews, L.B. Genetic Circuits for Feedback Control of Gamma-Aminobutyric Acid Biosynthesis in Probiotic Escherichia coli Nissle 1917. Metabolites 2024, 14, 44. [Google Scholar] [CrossRef]
- Seo, M.; Nam, Y.; Park, S.; Lee, S.; Yi, S.; Lim, S. γ-aminobutyric acid production in skim milk co-fermented with Lactobacillus brevis 877G and Lactobacillus sakei 795. Food Sci. Biotechnol. 2013, 22, 751–755. [Google Scholar] [CrossRef]
- Wang, J.; Tang, F.; Zheng, X.; Zhao, J.; Zhang, Y.; Shi, Y. Research on Production of γ-Aminobutyric Acid by Lactobacillus Fermentation of Brown Rice. Proc. Int. Conf. Meter. 2016, 107, 103–108. [Google Scholar] [CrossRef]
- Park, S.; Kim, K.; Lee, M.; Lim, S. Physiological Characteristics and GABA Production of Lactobacillus plantarum K255 Isolated from Kimchi. Korean J. Food Sci. Anim. Resour. 2013, 33, 595–602. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, J.; Lee, S. Functional Kimchi Beverage Enhanced with γ-Aminobutyric Acid (GABA) Through Serial Co-Fermentation Using Leuconostoc citreum S5 and Lactiplantibacillus plantarum KS2020. Fermentation 2025, 11, 44. [Google Scholar] [CrossRef]
- Yamatsu, A.; Yamashita, Y.; Maru, I.; Yang, J.; Tatsuzaki, J.; Kim, M. The Improvement of Sleep by Oral Intake of GABA and Apocynum venetum Leaf Extract. J. Nutr. Sci. Vitaminol. 2015, 61, 182–187. [Google Scholar] [CrossRef]
- Yamatsu, A.; Yamashita, Y.; Pandharipande, T.; Maru, I.; Kim, M. Effect of oral γ-aminobutyric acid (GABA) administration on sleep and its absorption in humans. Food Sci. Biotechnol. 2016, 25, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Xiao, H. Oral Nutritional Dietary Supplement Containing GABA and Asparagus Powder Improves Sleep. Int. J. Biol. Life Sci. 2024, 6, 20–23. [Google Scholar] [CrossRef]
- Hao, Y.; Song, W.; Qu, L. Effects of a combination of Poria Cocos, Ziziphus spinose, and gamma-aminobutyric acid (GABA) on sleep quality and skin health: A randomized double-blind placebo-controlled clinical trial. Food Sci. Nutr. 2024, 12, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Zhang, Q.; Zhang, Y.; Xu, F. A double blinded randomized placebo trial of Bifidobacterium animalis subsp. lactis BLa80 on sleep quality and gut microbiota in healthy adults. Sci. Rep. 2025, 15, 11095. [Google Scholar] [CrossRef]

| Food Matrix | Strain/Method Used | Optimal Parameters | GABA Yield/Enrichment Effect | Reference |
|---|---|---|---|---|
| Pea Protein Hydrolysate | Lvl. brevis SYLB 0016 | Substrate 5%, pH 5.0, 37 °C, 48 h | ~3.5 g/kg | [88] |
| Dairy Product | Lvl.brevis 877 G & Latilactobacillus sakei 795 co-fermentation | Skim milk, 29.57 mM MSG added | 22.51 mM | [89] |
| Germinated Brown Rice | Water soaking (endogenous GAD activation) | 40 °C, pH 4.0, with an 8-h immersion procedure. | Increase 8–10 fold (~300 mg/100 g) vs. non-germinated | [90] |
| Kimchi | Lbp. plantarum K255 | MRS broth, 2% MSG added | 163.6 mg/mL | [91] |
| Kimchi | Leuconostoc citreum S5 & L. plantarum KS2020 co-fermentation | Plant-based medium, 5% sucrose | Viable count reached 9.42 log CFU/mL | [92] |
| Active Component | Study Design | Basic Research Parameters | Dose/Intervention | Main Outcomes | Reference |
|---|---|---|---|---|---|
| GABA & Apocynum venetum Leaf Extract (AVLE) | EEG measurement | PSQI ≥ 6; Sample size: 16 subjects; Study duration: 2 weeks | GABA (100 mg) and/or AVLE (50 mg) | GABA: reduced sleep latency 5.3 min; AVLE: increased NREM sleep time 7.6%; Combination: synergistic effects | [93] |
| GABA & L-Theanine | Animal study | 8-week-old male mice; Study duration: 9 days | GABA and L-theanine mixture | Reduced sleep latency, improved NREM sleep. | [76] |
| Oral GABA | Randomized, single-blind, placebo-controlled, crossover | PSQI ≥ 6; Sample size: 10 subjects; Study duration: 2 weeks | Single oral GABA dose | Significantly shortened sleep latency, increased total NREM sleep time. | [94] |
| Probiotic Lp815 Lbp. plantarum | Randomized, double-blind, placebo-controlled | Self-reported individuals with sleep disorders; Sample size: 139 subjects; Study duration: 6 weeks | L. plantarum Lp815 (GABA-probiotic) | Improved sleep, reduced anxiety, and increased urinary GABA. | [5] |
| GABA & Asparagine Powder | Group trial | PSQI ≥ 6; Sample size: 54 subjects; Study duration: 2 weeks | Group A: GABA 120 mg + Asparagine 1500 mg; B: GABA 120 mg; C: Placebo | GABA and asparagine powder are beneficial for sleep (as assessed by the PSQI). | [95] |
| Poria Cocos, Ziziphus spinose, & GABA Combination | Randomized, double-blind, controlled trial | PSQI ≥ 7;Sample size: 70 subjects; Study duration: 4 weeks | Combination intervention for 4 weeks | Improved sleep quality, increased skin hydration, reduced skin roughness | [96] |
| Bifidobacterium animalis subsp. lactis BLa80 (GABA-producing in vitro) | Randomized, placebo-controlled | 6 ≤ PSQI ≤ 18; Sample size:106 subjects; Study duration: 8 weeks | B. lactis BLa80 | Improving sleep quality in healthy individuals and regulating gut microbiota. | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, Y.; Xue, C.; Zhang, Y.; Tong, T.; Ouyang, Z.; Liu, D.; Cai, J.; Sun, H. Progress in Research on the Mechanism of GABA in Improving Sleep. Foods 2025, 14, 3856. https://doi.org/10.3390/foods14223856
Li S, Li Y, Xue C, Zhang Y, Tong T, Ouyang Z, Liu D, Cai J, Sun H. Progress in Research on the Mechanism of GABA in Improving Sleep. Foods. 2025; 14(22):3856. https://doi.org/10.3390/foods14223856
Chicago/Turabian StyleLi, Shuyu, Yanhui Li, Chunxu Xue, Ying Zhang, Tong Tong, Zijun Ouyang, Dong Liu, Jun Cai, and Haiyan Sun. 2025. "Progress in Research on the Mechanism of GABA in Improving Sleep" Foods 14, no. 22: 3856. https://doi.org/10.3390/foods14223856
APA StyleLi, S., Li, Y., Xue, C., Zhang, Y., Tong, T., Ouyang, Z., Liu, D., Cai, J., & Sun, H. (2025). Progress in Research on the Mechanism of GABA in Improving Sleep. Foods, 14(22), 3856. https://doi.org/10.3390/foods14223856





