Advances in the Application of Surface-Enhanced Raman Spectroscopy for Quality Control of Cereal Foods
Abstract
1. Introduction
2. SERS Technology
2.1. Enhancement Mechanism of SERS
2.2. SERS Substrates
3. Research Progress in the Application of SERS Technology in the Quality Control of Cereal Foods
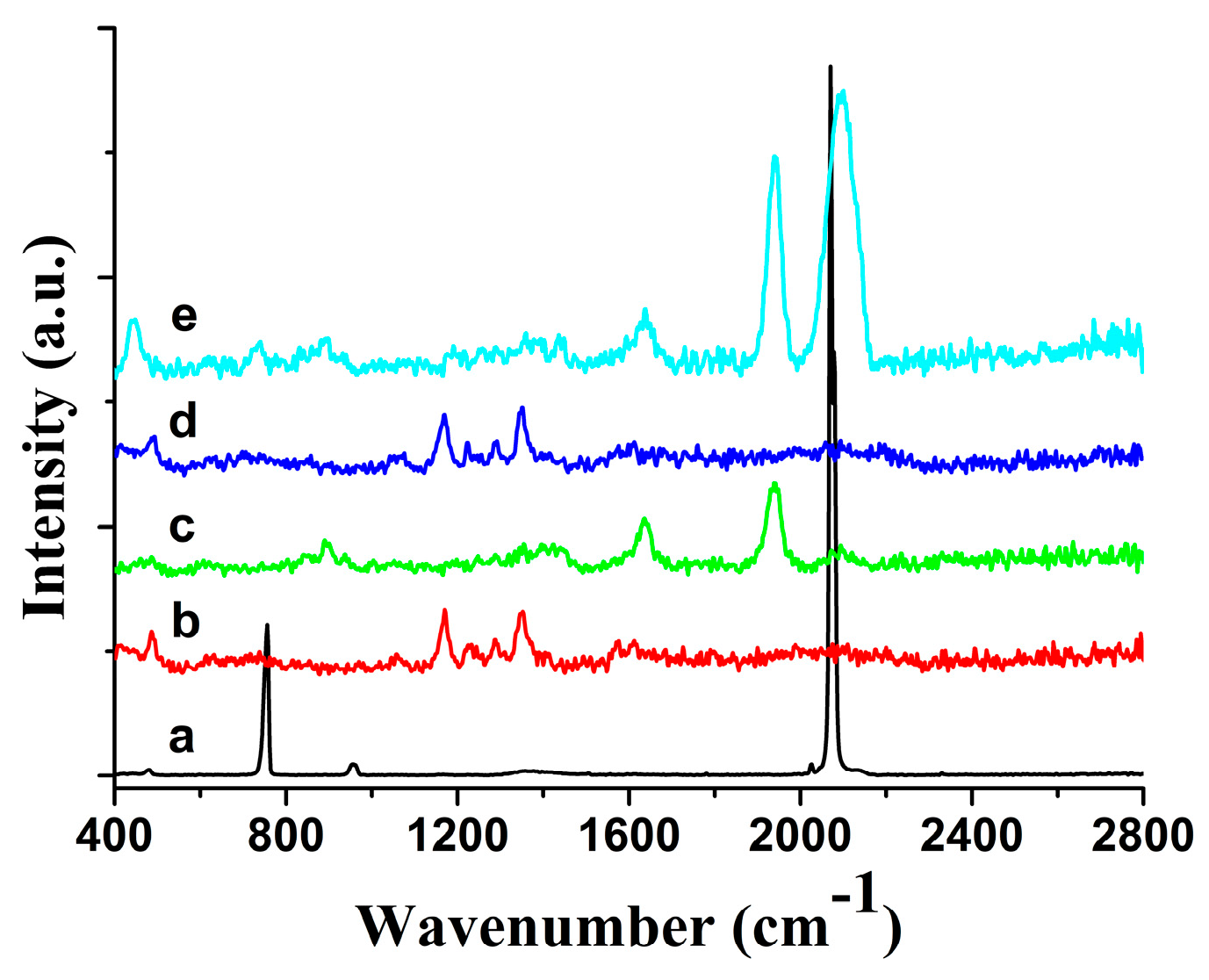
3.1. Detection of Microbial Contamination
3.2. Detection of Pesticide Residues
3.3. Applications in Other Fields
4. Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, Y.; Hewavithana, T.; Sharpe, A.G.; Jin, L. Understanding grain development in the Poaceae family by comparing conserved and distinctive pathways through omics studies in wheat and maize. Front. Plant Sci. 2024, 15, 1393140. [Google Scholar] [CrossRef]
- Yamini, V.; Singh, K.; Antar, M.; Sabagh, A.E. Sustainable cereal production through integrated crop management: A global review of current practices and future prospects. Front. Sustain. Food Syst. 2025, 9, 1428687. [Google Scholar] [CrossRef]
- Winarti, C.; Widaningrum; Widayanti, S.M.; Setyawan, N.; Qanytah; Juniawati; Suryana, E.A.; Widowati, S. Nutrient Composition of Indonesian Specialty Cereals: Rice, Corn, and Sorghum as Alternatives to Combat Malnutrition. Prev. Nutr. Food Sci. 2023, 28, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Gazza, L.; Nocente, F. The Contribution of Minor Cereals to Sustainable Diets and Agro-Food Biodiversity. Foods 2023, 12, 3500. [Google Scholar] [CrossRef] [PubMed]
- Yessembek, M.; Tarabayev, B.; Kakimov, M.; Gajdzik, B.; Wolniak, R.; Bembenek, M. Utilization of Secondary Raw Materials from Rice and Buckwheat Processing for the Production of Enriched Bread: Optimization of Formulation, Physicochemical and Organoleptic Properties, Structural and Mechanical Properties, and Microbiological Safety. Foods 2024, 13, 2678. [Google Scholar] [CrossRef]
- Fu, M.; Zhou, X.; Tian, H.; Chen, Y.; Jin, Z. Ethanolic Extrusion of Indica Rice Flour for Rice Noodle Production. Foods 2025, 14, 1453. [Google Scholar] [CrossRef]
- Shen, C.; Yu, Y.; Zhang, X.; Zhang, H.; Chu, M.; Yuan, B.; Guo, Y.; Li, Y.; Zhou, J.; Mao, J.; et al. The dynamic of physicochemical properties, volatile compounds and microbial community during the fermentation of Chinese rice wine with diverse cereals. Food Res. Int. 2024, 198, 115319. [Google Scholar] [CrossRef]
- Stępień, A.; Wojtkowiak, K.; Kolankowska, E.; Pietrzak-Fiećko, R. Organochlorine Contaminants in Maize Fertilized with Meat and Bone Meal Derived from Animal By-Products. Appl. Sci. 2025, 15, 5620. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Jiang, P.; Wang, R.; Guo, J.; Xiao, H.; Wu, J.; Shaaban, M.; Li, Y.; Huang, M. Organic Fertilizer Substituting 20% Chemical N Increases Wheat Productivity and Soil Fertility but Reduces Soil Nitrate-N Residue in Drought-Prone Regions. Front. Plant Sci. 2024, 15, 1379485. [Google Scholar] [CrossRef]
- Wohlers, J.; Stolz, P.; Geier, U. Intensive processing reduces quality of grains: A triangulation of three assessment methods. Biol. Agric. Hortic. 2024, 40, 107–126. [Google Scholar] [CrossRef]
- Mesías, M.; García, A.; Morales, F.J. Two Decades of Monitoring Acrylamide in Breakfast Cereals: Impact of Market Reformulation and Compliance with EU Regulation. Food Chem. X 2025, 31, 103039. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Drul, D.; Gaba, D.; Huang, M.; Richter, A.; Roscoe, M.; Serda, M.; Timofeiev, V.; et al. Contaminants and residues have varied distributions in large volumes of wheat. Food Addit. Contam. Part A 2024, 42, 92–102. [Google Scholar] [CrossRef]
- Khan, T.; Shahid, A.A.; Khan, H.A.A. Could biorational insecticides be used in the management of aflatoxigenic Aspergillus parasiticus and its insect vectors in stored wheat? PeerJ 2016, 4, e1665. [Google Scholar] [CrossRef]
- Choi, D.; Fan, X.; Yu, J.-H. Comprehensive Review of Dietary Probiotics in Reducing Aflatoxin B1 Toxicity. Toxins 2025, 17, 482. [Google Scholar] [CrossRef]
- Almashhadany, D.A.; Rashid, R.F.; Altaif, K.I.; Mohammed, S.H.; Mohammed, H.I.; Al-Bader, S.M. Heavy metal(loid) bioaccumulation in fish and its implications for human health. Ital. J. Food Saf. 2024, 14, 12782. [Google Scholar] [CrossRef]
- Bartholomew, S.K.; Winslow, W.; Sharma, R.; Pathak, K.V.; Tallino, S.; Judd, J.M.; Leon, H.; Turk, J.; Pirrotte, P.; Velazquez, R. Glyphosate exposure exacerbates neuroinflammation and Alzheimer’s disease-like pathology despite a 6-month recovery period in mice. J. Neuroinflammation 2024, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- DeWaal, C.S.; Okoruwa, A.; Yalch, T.; McClafferty, B. Regional Codex Guidelines and Their Potential to Impact Food Safety in Traditional Food Markets. J. Food Prot. 2022, 85, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Parker, C.; Johnson, H.; Haines, F.; Boatwright, M.; Northcott, T.; Baker, P. An ecological reorientation of the Codex Alimentarius Commission could help transform food systems. Nat. Food 2024, 5, 557–562. [Google Scholar] [CrossRef] [PubMed]
- CXS 198-2019; Standard for Rice. Codex Alimentarius Commission: Rome, Italy, 2019.
- CXS 153-2019; Standard for Maize (Corn). Codex Alimentarius Commission: Rome, Italy, 2019.
- CXS 152-2023; Standard for Wheat Flour. Codex Alimentarius Commission: Rome, Italy, 2023.
- CXS 201-2019; Standard for Oats. Codex Alimentarius Commission: Rome, Italy, 2019.
- Vavrouš, A.; Ševčík, V.; Dvořáková, M.; Čabala, R.; Moulisová, A.; Vrbík, K. Easy and inexpensive method for multiclass analysis of 41 food contact related contaminants in fatty food by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2019, 67, 10968–10976. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhuang, Y.; Guo, S.; Sohan, A.S.M.M.F.; Yin, B. Advances in Microfluidics Techniques for Rapid Detection of Pesticide Residues in Food. Foods 2023, 12, 2868. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Wang, H. Microchip-Based Surface Enhanced Raman Spectroscopy for the Determination of Sodium Thiocyanate in Milk. Anal. Lett. 2015, 48, 1930–1940. [Google Scholar] [CrossRef]
- Dong, J.; Qu, S.; Zhang, Z.; Liu, M.; Liu, G.; Yan, X.; Zheng, H. Surface enhanced fluorescence on three dimensional silver nanostructure substrate. J. Appl. Phys. 2012, 111, 93–101. [Google Scholar] [CrossRef]
- Jayan, H.; Sun, D.W.; Pu, H.; Wei, Q. Mesoporous silica coated core-shell nanoparticles substrate for size-selective SERS detection of chloramphenicol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 284, 121817. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Liang, P.; Wu, Y.; Dong, Q.; Li, J.; Bai, Y.; Xu, B.; Yu, Z.; Ni, D. Rapid qualitative and quantitative determination of food colorants by both Raman spectra and Surface-enhanced Raman scattering (SERS). Food Chem. 2018, 241, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Dai, S.; Dou, M.; Yang, J.; Wang, X.; Liu, X.; Wei, C.; Li, Q.; Li, J. Simultaneous, Label-Free and High-throughput SERS Detection of Multiple Pesticides on Ag@Three-Dimensional Silica Photonic Microsphere Array. J. Agric. Food Chem. 2023, 71, 3050–3059. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Ouyang, Q.; Chen, Q. Advances in surface-enhanced Raman spectroscopy technology for detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1466–1494. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, H.; Sun, D.W. Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy: Principles and recent applications. Trends Food Sci. Technol. 2021, 110, 393–404. [Google Scholar] [CrossRef]
- Tan, A.; Zhao, Y.; Sivashanmugan, K.; Squire, K.; Wang, A.X. Quantitative TLC-SERS detection of histamine in seafood with support vector machine analysis. Food Control 2019, 103, 111–118. [Google Scholar] [CrossRef]
- Dey, T. Microplastic pollutant detection by surface enhanced Raman spectroscopy (SERS): A mini-review. Nanotechnol. Environ. Eng. 2023, 8, 41–48. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Wei, L.; Huang, Y.; Wen, X.; Wang, D.; Cheng, G.; Zhao, R.; Lin, Y.; Yang, H.; et al. Rapid and Precise Differentiation and Authentication of Agricultural Products via Deep Learning-Assisted Multiplex SERS Fingerprinting. Anal. Chem. 2024, 96, 4682–4692. [Google Scholar] [CrossRef]
- Shen, W.; Wang, C.; Zheng, S.; Jiang, B.; Li, J.; Pang, Y.; Wang, C.; Hao, R.; Xiao, R. Ultrasensitive multichannel immunochromatographic assay for rapid detection of foodborne bacteria based on two-dimensional film-like SERS labels. J. Hazard. Mater. 2022, 437, 129347. [Google Scholar] [CrossRef]
- Rostron, P.; Gaber, S.; Gaber, D. Raman spectroscopy, review. Laser 2016, 21, 24. [Google Scholar]
- Liu, S.; Li, H.; Hassan, M.M.; Zhu, J.; Wang, A.; Ouyang, Q.; Zareef, M.; Chen, Q. Amplification of Raman spectra by gold nanorods combined with chemometrics for rapid classification of four Pseudomonas. Int. J. Food Microbiol. 2019, 304, 58–67. [Google Scholar] [CrossRef]
- Gala, U.; Chauhan, H. Principles and applications of Raman spectroscopy in pharmaceutical drug discovery and development. Expert Opin. Drug Discov. 2015, 10, 187–206. [Google Scholar] [CrossRef]
- Ma, H.; Pan, S.; Wang, W.; Yue, X.; Xi, X.; Yan, S.; Wu, D.; Wang, X.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Current understanding, challenges, and opportunities. ACS Nano 2024, 18, 14000–14019. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Wu, D.Y.; Li, J.F.; Ren, B.; Tian, Z.Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 2008, 37, 1025–1041. [Google Scholar] [CrossRef]
- Hoang, V.T.; Tufa, L.T.; Lee, J.; Quan, D.M.; Ha, A.N.; Tan, T.V.; Tuan, L.A. Tunable SERS activity of Ag@Fe3O4 core-shell nanoparticles: Effect of shell thickness on the sensing performance. J. Alloys Compd. 2022, 933, 167649. [Google Scholar] [CrossRef]
- Schatz, G.C.; Van Duyne, R.P. Electromagnetic mechanism of surface-enhanced spectroscopy. In Handbook of Vibrational Spectroscopy; John Wiley & Sons Ltd.: Chichester, UK, 2002; Volume 1, pp. 759–774. [Google Scholar]
- Willets, K.A.; Van-Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Jensen, L.; Aikens, C.M.; Schatz, G.C. Electronic structure methods for studying surface-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 1061–1073. [Google Scholar] [CrossRef]
- Long, Y.; Wang, W.; Xiong, W.; Li, H. Hybrid structure design, preparation of Ag-GO SERS optical fiber probe and its chemical, electromagnetic enhancement mechanism. J. Alloys Compd. 2022, 901, 163660. [Google Scholar] [CrossRef]
- Yaseen, T.; Pu, H.; Sun, D.W. Functionalization techniques for improving SERS substrates and their applications in food safety evaluation: A review of recent research trends. Trends Food Sci. Technol. 2018, 72, 162–174. [Google Scholar] [CrossRef]
- Nilghaz, A.; Mahdi-Mousavi, S.; Amiri, A.; Tian, J.; Cao, R.; Wang, X. Surface-enhanced Raman spectroscopy substrates for food safety and quality analysis. J. Agric. Food Chem. 2022, 70, 5463–5476. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, L.; Liu, J. Three-dimensional SERS hot spots for chemical sensing: Towards developing a practical analyze. Trends Anal. Chem. 2016, 80, 364–372. [Google Scholar] [CrossRef]
- Nilghaz, A.; Mousavi, S.M.; Tian, J.; Cao, R.; Guijt, R.M.; Wang, X. Noble-metal nanoparticle-based colorimetric diagnostic assays for point-of-need applications. ACS Appl. Nano Mater. 2021, 4, 12808–12824. [Google Scholar] [CrossRef]
- Adewuyi, A.; Lau, W.J. Chapter 3—Nanomaterial development and its applications for emerging pollutant removal in water. In Handbook of Nanotechnology Applications; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 67–97. [Google Scholar]
- Muntean, C.M.; Cuibus, D.; Boca, S.; Falamas, A.; Tosa, N.; Brezetean, I.A.; Bende, A.; Barbu-Tudoran, L.; Moldovan, R.; Bodoki, E.; et al. Gold vs. Silver Colloidal Nanoparticle Films for Optimized SERS Detection of Propranolol and Electrochemical-SERS Analyses. Biosensors 2023, 13, 530. [Google Scholar] [CrossRef]
- Davaraj, V.; Ruiz Alvarado, I.A.; Lee, J.-M.; Oh, J.-W.; Gerstmann, U.; Schmidt, W.G.; Zentgraf, T. Self-assembly of isolated plasmonic dimers with sub-5 nm gaps on a metallic mirror. Nanoscale Horiz. 2025, 10, 537–548. [Google Scholar] [CrossRef]
- Zhang, L. Self-assembly Ag nanoparticle monolayer film as SERS Substrate for pesticide detection. Appl. Surf. Sci. 2013, 270, 292–294. [Google Scholar] [CrossRef]
- Gong, X.; Tang, M.; Gong, Z.; Qiu, Z.; Wang, D.; Fan, M. Screening pesticide residues on fruit peels using portable Raman spectrometer combined with adhesive tape sampling. Food Chem. 2019, 295, 254–258. [Google Scholar] [CrossRef]
- Wu, H.; Kanike, C.; Marcati, A.; Zhang, X. Flexible Surface-Enhanced Raman Scattering Tape Based on Ag Nanostructured Substrate for On-Site Analyte Detection. Langmuir 2024, 40, 4218–4227. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and Adhesive Surface Enhance Raman Scattering Active Tape for Rapid Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef]
- Sabina, A.O.; Uzoamaka, G.-O.; Chinedu, O.S. Evaluation of the Thermal Stability Profile of Aflatoxins from Fungal Isolates from Dried Fishes. Int. J. Biochem. Biophys. 2023, 11, 17–26. [Google Scholar] [CrossRef]
- Kebede, H.; Liu, X.; Jin, J.; Xing, F. Current status of major mycotoxins contamination in food and feed in Africa. Food Control 2020, 110, 106975. [Google Scholar] [CrossRef]
- Massart, F.; Meucci, V.; Saggese, G.; Soldani, G. High growth rate of girls with precocious puberty exposed to estrogenic mycotoxins. J. Pediatr. 2008, 152, 690–695. [Google Scholar] [CrossRef]
- Belhassen, H.; Jiménez-Díaz, I.; Arrebola, J.P.; Olea, N.; Hedili, A. Zearalenone and its metabolites in urine and breast cancer risk: A case-control study in Tunisia. Chemosphere 2015, 128, 1–6. [Google Scholar] [CrossRef]
- Berg, T. How to establish international limits for mycotoxins in food and feed? Food Control 2003, 14, 219–224. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, J.; Xuan, Z.; Li, L.; Wang, H.; Wang, S.; Liu, H.; Wang, S. Development and validation of a rapid and efficient method for simultaneous determination of mycotoxins in coix seed using one-step extraction and UHPLC-HRMS. Food Addit. Contam. Part A 2021, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhu, L.; Chen, P.; Liu, H.; Loh, T.P.; Jia, Z.; Li, J.; Fu, F. Effective physical methods for aflatoxin B1 removal in food: A comprehensive review. Food Control 2025, 173, 111215. [Google Scholar] [CrossRef]
- Papp, D.A.; Kocsubé, S.; Farkas, Z.; Szekeres, A.; Vágvölgyi, C.; Hamari, Z.; Varga, M. Aflatoxin B1 Control by Various Pseudomonas Isolates. Toxins 2024, 16, 367. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Li, Y.; Ying, Y. A simple and rapid optical biosensor for detection of aflatoxin B1 based on competitive dispersion of gold nanorods. Biosens. Bioelectron. 2013, 47, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Liu, T.; Yao, Y.; Kong, D.; Liu, C.; Ye, H.; Zhang, Q.; Li, S.; Li, Y.; Shi, Q. A core-satellite self-assembled SERS aptasensor used for ultrasensitive detection of AFB1. Microchim. Acta 2025, 192, 190. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Ahmad, W.; Zhu, J.; Hassan, M.M.; Wang, J.; Rong, Y.; Guo, Z.; Li, H.; Ding, Z.; Lv, C.; et al. Aggregation triggered aflatoxin B1 determination in foodstuff employing 5-aminotetramethylrhodamine decorated gold-silver core-shell nanoparticles in surface enhanced Raman scattering. Sens. Actuators B Chem. 2021, 331, 129424. [Google Scholar] [CrossRef]
- Han, C.; Zhai, W.; Wang, Y.; Cao, J.; Wang, M. A SERS aptasensor for rapid detection of aflatoxin B1 in coix seed using satellite structured Fe3O4@Au nanocomposites. Food Control 2022, 142, 109228. [Google Scholar] [CrossRef]
- Miao, C.; Wu, Z.; Sun, Y.; Cao, Z. Deoxynivalenol Induces Intestinal Epithelial Barrier Damage Through RhoA/ROCK Pathway-Mediated Apoptosis and F-Actin-Associated Tight Junction Disruption. J. Agric. Food Chem. 2024, 72, 9411–9423. [Google Scholar] [CrossRef]
- Gabbitas, A.; Ahlborn, G.; Allen, K.; Pang, S. Advancing Mycotoxin Detection: Multivariate Rapid Analysis on Corn Using Surface Enhanced Raman Spectroscopy (SERS). Toxins 2023, 15, 610. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, H.; Huo, B.; Wang, Y.; Gao, Z. A novel bionic magnetic SERS aptasensor for the ultrasensitive detection of Deoxynivalenol based on “dual antennae” nano-silver. Biosens. Bioelectron. 2022, 211, 114383. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Zhao, H.; Ren, X.; Lin, T.; Zhang, P.; Zheng, D. Rapid detection and identification of fungi in grain crops using colloidal Au nanoparticles based on surface-enhanced Raman scattering and multivariate statistical analysis. World J. Microbiol. Biotechnol. 2022, 39, 26. [Google Scholar] [CrossRef]
- Wu, F.; Wang, F.; Tang, Z.; Yang, X.; Liu, Y.; Zhao, M.; Liu, S.; Han, S.; Chen, B. Zearalenone causes ovarian damage and abnormal estradiol secretion in meat rabbits by inducing oxidative stress and inflammatory responses. Front. Vet. Sci. 2025, 12, 1566284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, H.; Tan, X.; Lu, Z.; Han, H. Cauliflower-Inspired 3D SERS substrate for multiple mycotoxins detection. Anal. Chem. 2019, 91, 3885–3892. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, S.; Jin, Y.; Yang, C.; He, L.; Wang, J.; Chen, Y. Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J. Hazard. Mater. 2020, 393, 122348. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, J.; Wu, W.; Chen, H.; Li, S.; Zhang, Z.; Rong, S.; Wang, H. Dual colorimetric platforms for direct detection of glyphosate based on Os-Rh nanozyme with peroxidase-like activity. Anal. Chim. Acta 2024, 1326, 343150. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, Z.; Cui, B.; Xu, E. Aptamer and gold nanorod-based fumonisin B1 assay using both fluorometry and SERS. Microchim. Acta 2020, 187, 215. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Niazi, S.; Pasha, I.; Khan, M.K.I.; Yue, L.; Ye, H.; Mohsin, A.; Shoaib, M.; Zhang, Y.; Wang, Z.; et al. Novel Metal enhanced dual-mode fluorometric and SERS aptasensor implicating heterostructure nanoassembly for ultrasensitive T-2 Toxin detection. J. Mater. Chem. B 2022, 11, 441–451. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Demsie, A.F.; Yimer, G.T.; Sota, S.S. Pesticide Residues and Associated Public Health Risks in Vegetables from Irrigated Farms Adjacent to Rift Valley Lake Ziway, Ethiopia. J. Food Qual. 2024, 15, 5516159. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Assessment of fall-back MRLs for revoked CXLs previously implemented in the EU legislation. EFSA J. 2025, 23, 9299. [Google Scholar]
- Neng, J.; Liao, C.; Wang, Y.; Wang, Y.; Yang, K. Rapid and sensitive detection of pentachloronitrobenzene by surface-enhanced Raman spectroscopy combined with molecularly imprinted polymers. Biosensors 2022, 12, 52. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Ali, S.; Li, H.; Chen, Q. SERS-based rapid detection of 2,4-dichlorophenoxyacetic acid in food matrices using molecularly imprinted magnetic polymers. Microchim. Acta 2020, 187, 454. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, L.; Yang, Y.; Wu, D.; Zhang, Y.; Cheng, W.; Tang, X. Fabrication of a metal organic framework (MOF)-modified Au nanoparticle array for sensitive and stable SERS sensing of paraquat in cereals. J. Food Sci. 2023, 88, 1769–1780. [Google Scholar] [CrossRef]
- Weng, S.; Yu, S.; Dong, R.; Zhao, J.; Liang, D. Detection of pirimiphos-methyl in wheat using surface-enhanced Raman spectroscopy and chemometric methods. Molecules 2019, 24, 1691. [Google Scholar] [CrossRef] [PubMed]
- Droghetti, E.; Nicoletti, F.P.; Guandalini, L.; Bartolucci, G.; Smulevich, G. SERS detection of benzophenones on viologen functionalized Ag nanoparticles: Application to breakfast cereals. J. Raman Spectrosc. 2013, 10, 1428–1434. [Google Scholar] [CrossRef]
- Zhao, Y.; Yamaguchi, Y.; Liu, C.; Li, M.; Dou, X. Rapid and quantitative detection of trace Sudan black B in dyed black rice by surface-enhanced Raman spectroscopy (SERS). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Chen, Y.; Chen, Z.P.; Yu, R.Q. Quantification of Cadmium in Rice by Surface-enhanced Raman Spectroscopy Based on a Ratiometric Indicator and Conical Holed Enhancing Substrates. Analyst 2018, 34, 1405–1410. [Google Scholar] [CrossRef]
- Radu, A.I.; Kuellmer, M.; Giese, B.; Hubner, U.; Weber, K.; Cialla-May, D.; Popp, J. Surface-enhanced Raman spectroscopy (SERS) in food analytics: Detection of vitamins B2 and B12 in cereals. Talanta 2016, 160, 289–297. [Google Scholar] [CrossRef]
- Lin, D.; Yu, C.; Ku, C.; Chung, C. Design, Fabrication, and Applications of SERS Substrates for Food Safety Detection: Review. Micromachines 2023, 14, 1343. [Google Scholar] [CrossRef]
- Sun, L.-H.; Yu, F.; Wang, Y.-Y.; Lv, S.-W.; He, L.-Y. Effects of ultrasound extraction on the physicochemical and emulsifying properties of rice bran protein. Int. J. Food Eng. 2021, 17, 327–335. [Google Scholar] [CrossRef]
- Lin, Z.; He, L. Recent advance in SERS techniques for food safety and quality analysis: A brief review. Curr. Opin. Food Sci. 2019, 28, 82–87. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Han, X.; Zhao, B. Innovative Application of SERS in Food Quality and Safety: A Brief Review of Recent Trends. Foods 2022, 11, 2097. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Charron, G.; Corts, E.; Kleipp, J.; De La Chapelle, M.L.; Langer, J.; Procházka, M.; Tran, V.; Schlicker, S. Towards Reliable and Quantitative Surface-Enhanced Raman Scattering (SERS): From Key Parameters to Good Analytical Practice. Angew. Chem. Int. Ed. 2020, 59, 5454–5462. [Google Scholar] [CrossRef]
- Vignesh, S.; Lu, D.; Bian, A.C.; Yap, F.L. Fabrication of Large-Area Flexible SERS Substrates by Nanoimprint Lithography. ACS Appl. Nano Mater. 2018, 1, 886–893. [Google Scholar] [CrossRef]
- Cho, W.J.; Kim, Y.; Kim, J.K. Ultrahigh-density array of silver nanoclusters for SERS substrate with high sensitivity and excellent reproducibility. ACS Nano 2012, 6, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhou, J.; Xing, X.; Wang, J.; Huang, J.; Liu, H.; Chen, J.; Dong, M.; Xiang, Q.; Dong, H.; et al. DNA Assembly of Plasmonic Nanostructures Enables In Vivo SERS-Based MicroRNA Detection and Tumor Photoacoustic Imaging. Anal. Chem. 2023, 95, 11236–11242. [Google Scholar]
- Zhang, H.; Duan, S.; Radjenovic, P.M.; Tian, Z.Q.; Li, J.F. Core-Shell Nanostructure-Enhanced Raman Spectroscopy for Surface Catalysis. Acc. Chem. Res. 2020, 53, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; You, T.; Arslan, M.; El-Seedi, R.; Guo, Z.; Zou, X.; Cai, J. Dual-layers Raman reporter-tagged Au@Ag combined with core-satellite assemblies for SERS detection of Zearalenone. Food Chem. 2023, 429, 136834. [Google Scholar] [CrossRef]
- Wang, H.-P.; Lai, K.-Y.; Lin, Y.-R.; Lin, C.-A.; He, J.-H. Periodic Si Nanopillar Arrays Fabricated by Colloidal Lithography and Catalytic Etching for Broadband and Omnidirectional Elimination of Fresnel Reflection. Langmuir 2010, 26, 12855–12858. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, H.; Han, X.; Zhao, B. Metal-semiconductor heterostructures for surface-enhanced Raman scattering: Synergistic contribution of plasmons and charge transfer. Mater. Horiz. 2021, 8, 370–382. [Google Scholar] [CrossRef]
- Lin, L.L.; Alvarez-Puebla, R.; Liz-Marzán, L.M.; Trau, M.; Wang, J.; Fabris, L.; Wang, X.; Liu, G.; Xu, S.; Han, X.X.; et al. Surface-Enhanced Raman Spectroscopy for Biomedical Applications: Recent Advances and Future Challenges. ACS Appl. Mater. Interfaces 2025, 17, 16287–16379. [Google Scholar] [CrossRef]
- Oksenberg, E.; Shlesinger, I.; Tek, G.; Koenderink, A.F.; Garnett, E.C. Complementary Surface-Enhanced Raman Scattering (SERS) and IR Absorption Spectroscopy (SEIRAS) with Nanorods-on-a-Mirror. Adv. Funct. Mater. 2023, 33, 2211154. [Google Scholar] [CrossRef]
- Amirsalari, A.; Razi, S.; Gawinkowski, S. Multimodal microscope system for hyperspectral imaging of scattering directionality and Raman excitation spectroscopy. J. Raman Spectrosc. 2023, 54, 976–987. [Google Scholar] [CrossRef]
- Jurisch, M.; Fantini, C.; Augusti, R.; Almeida, M.R. Combining surface-enhanced Raman spectroscopy and paper spray mass spectrometry for the identification and confirmation of psychotropic substances in alcoholic beverages. J. Mass Spectrom. 2024, 59, 4997. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, L. Optimization of artificial intelligence in localized big data real-time query processing task scheduling algorithm. Front. Phys. 2024, 12, 1484115. [Google Scholar] [CrossRef]
- Liu, Q.; Vanmol, K.; Lycke, S.; Van Erps, J.; Vandenabeele, P.; Thienpont, H.; Ottevaere, H. SERS using two-photon polymerized nanostructures for mycotoxin detection. RSC Adv. 2020, 10, 14274–14282. [Google Scholar] [CrossRef]
- Bi, X.; Lin, L.; Chen, Z.; Ye, J. Artificial Intelligence for Surface-Enhanced Raman Spectroscopy. Small Methods 2023, 8, e2301243. [Google Scholar] [CrossRef]
- Lussier, F.; Thibault, V.; Charron, B.; Wallace, G.Q.; Masson, J.F. Deep learning and artificial intelligence methods for Raman and surface-enhanced Raman scattering. TrAC Trends Anal. Chem. 2020, 24, 115796. [Google Scholar] [CrossRef]
- Jabbar, H.; Zgair, I.A.; Heydaryan, K.; Kadhim, S.A.; Mehmandoust, S.; Eskandari, V.; Sahbafa, H. Applications of artificial intelligence and machine learning in combination with surface-enhanced Raman spectroscopy (SERS). Chemom. Intell. Lab. Syst. 2025, 263, 105445. [Google Scholar] [CrossRef]
- Dos Santos, D.P.; Sena, M.M.; Almeida, M.R.; Mazali, I.O.; Olivieri, A.C.; Villa, J.E. Unraveling surface-enhanced Raman spectroscopy results through chemometrics and machine learning: Principles, progress, and trends. Anal. Bioanal. Chem. 2023, 415, 3945–3966. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ren, Z.; Zhu, J.; Lee, C. Machine learning-augmented surface-enhanced spectroscopy toward next-generation molecular diagnostics. Nanoscale Adv. 2023, 5, 538–570. [Google Scholar] [CrossRef]
- Bahlol, H.S.; Li, J.; Deng, J.; Foda, M.F.; Han, H. Recent Progress in Nanomaterial-Based Surface-Enhanced Raman Spectroscopy for Food Safety Detection. Nanomaterials 2024, 14, 1750. [Google Scholar] [CrossRef] [PubMed]

| Cereal Categories | Analytes | SERS Substrates | Limit of Detection | Recoveries | Ref. |
|---|---|---|---|---|---|
| Wheat | Aflatoxin B1 | NH2-Rh-Au@Ag CSNPs | 0.03 ng/mL | — | [69] |
| T-2 toxin | rGO-AuNS | 1.2 × 10−11 mol/L | — | [80] | |
| Corn | Deoxynivalenol | β-CD@Ag NPs | 0.032 pg/mL | 88.7–110.58% | [71] |
| Toxin-producing fungi | Au NPs | — | — | [74] | |
| Zearalenone | Au NPs/PDMS@AAO | 24.8 ng/mL | — | [76] | |
| Zearalenone | 4-MBA/DTNB-Au@Ag CSNPs | 0.21 pg/mL | 78.9–106.2% | [77] | |
| Fumonisin B1 | Au NR@Pt | 10 pg/mL | 92–107% | [79] | |
| Coix seed | Aflatoxin B1 | Fe3O4@Au nanocomposite | 0.006 ng/mL | 98.2–101.2% | [70] |
| Cereal Categories | Analytes | SERS Substrates | Limit of Detection | Quantitative Linear Range | Recoveries | Ref. |
|---|---|---|---|---|---|---|
| Rice | Pentachloronitrobenzene | Oil-Soluble Ag NPs- Embedded MIPs | 5.0 ng/mL | 0.005–0.15 μg/mL | 94.4–103.3% | [84] |
| Wheat, Rice | 2,4-Dichlorophen- Oxyacetic acid | Mag@MIP/Au NPs | 0.00147 ng/mL | 10−1–105 ng/mL | 93.5–102.2% | [85] |
| Wheat, Corn, Rice | Paraquat | Au@MIL-101/PMMA/DT | 1.83 μg/kg | 10−8–10−2 mol/L | 91.57–102.32% | [86] |
| Wheat | Pirimiphos-methyl | mPEG-SH-coated GNRs | 0.25 mg/L | 0.25–23.93 mg/L | 94.12–106.63% (Predicted) | [87] |
| Cereal Categories | Analytes | SERS Substrates | Limit of Detection | Quantitative Linear Range | Recoveries | Ref. |
|---|---|---|---|---|---|---|
| Oat | Benzophenone, 4-Methylbenzophenone | Lucigenin-functionalized Ag NPs | 16 μmol/L | — | 84% | [88] |
| Black rice | Sudan Black B | Ag NPs | 0.1 mg/kg | — | — | [89] |
| Rice | Cd2+ | TMT-AuNPs | 8 μg/kg | 0.5–100 μg/L | 93.8–109.4% | [90] |
| Corn | Vitamin B2, Vitamin B12 | — | 10−7 mol/L | — | — | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, P.; Sha, M.; Zhang, Z. Advances in the Application of Surface-Enhanced Raman Spectroscopy for Quality Control of Cereal Foods. Foods 2025, 14, 3551. https://doi.org/10.3390/foods14203551
Meng P, Sha M, Zhang Z. Advances in the Application of Surface-Enhanced Raman Spectroscopy for Quality Control of Cereal Foods. Foods. 2025; 14(20):3551. https://doi.org/10.3390/foods14203551
Chicago/Turabian StyleMeng, Pan, Min Sha, and Zhengyong Zhang. 2025. "Advances in the Application of Surface-Enhanced Raman Spectroscopy for Quality Control of Cereal Foods" Foods 14, no. 20: 3551. https://doi.org/10.3390/foods14203551
APA StyleMeng, P., Sha, M., & Zhang, Z. (2025). Advances in the Application of Surface-Enhanced Raman Spectroscopy for Quality Control of Cereal Foods. Foods, 14(20), 3551. https://doi.org/10.3390/foods14203551





