Advancing Food Safety Surveillance: Rapid and Sensitive Biosensing Technologies for Foodborne Pathogenic Bacteria
Abstract
1. Introduction
2. Biorecognition Elements for Monitoring Foodborne Pathogenic Bacteria
2.1. Antibodies
2.2. Aptamers
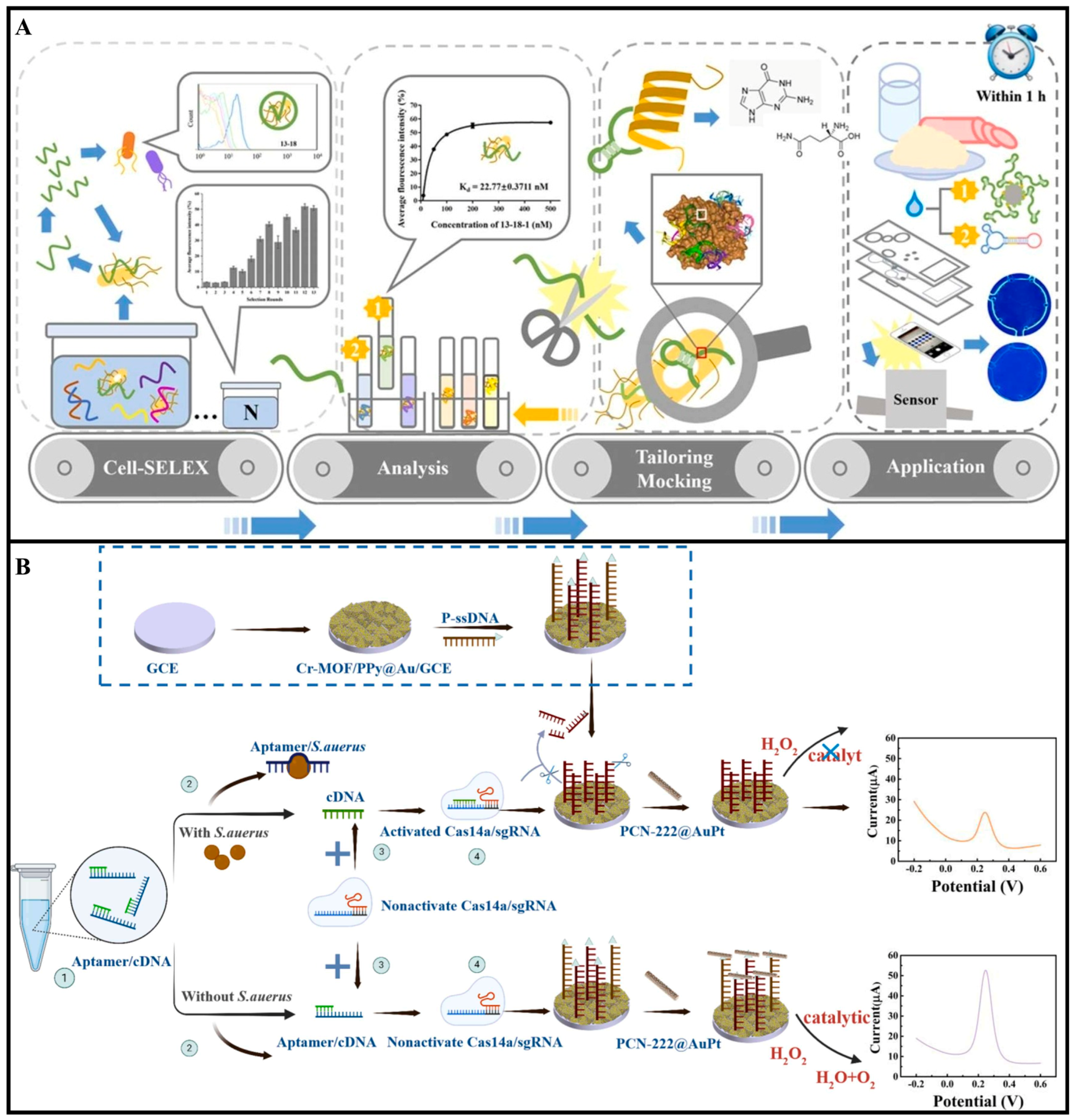
2.3. Enzymes
2.4. Cell Receptors
2.5. Molecularly Imprinted Polymers
2.6. Bacteriophages
3. Transducers for Detecting Foodborne Pathogenic Bacteria
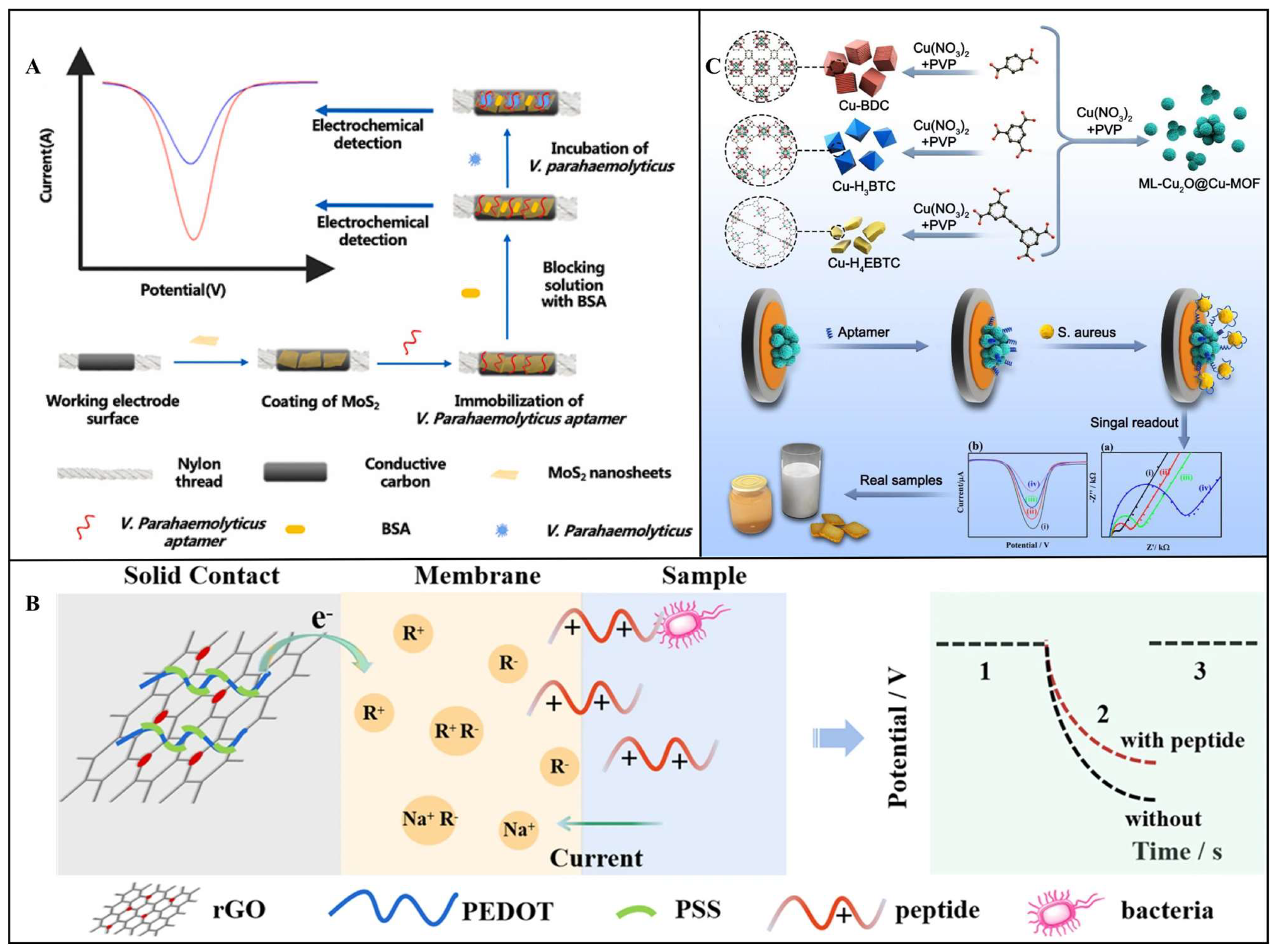
3.1. Electrochemical Transduction
3.1.1. Voltammetric Transducer
3.1.2. Potentiometric Transducer
3.1.3. Impedimetric Transducer
| Electrochemical Methods | Targets | Range of Detection | LOD | Reference |
|---|---|---|---|---|
| DPV | S. typhimurium | 101 to 107 CFU/mL | 3 CFU/mL | [107] |
| CV | S. typhimurium | 101–105 CFU/mL | 10 CFU/mL | [108] |
| CV/DPV | S. typhimurium | 1 to 1 × 105 CFU/mL | 23 CFU/mL | [109] |
| CV | S. typhimurium | 6.7 × 101 to 6.7 × 105 CFU/mL | 55 CFU/mL | [110] |
| EIS/CV | S. aureus | 101–105 CFU/mL | 0.28 CFU/mL | [111] |
| EIS/CV | S. aureus | 0.01 fM–10 nM | 10−17 M | [112] |
| EIS | S. aureus | 102 to 107 CFU/mL | 17 CFU/mL | [113] |
| DPV | S. aureus | 5.0 × 100–5.0 × 108 CFU/mL | 0.97 CFU/mL | [114] |
| EIS | S. aureus | 10 to 107 CFU/mL | 7 CFU/mL | [115] |
| EIS/CV | S. aureus | 12 to 6250 CFU/mL | 3 CFU/mL | [116] |
| EIS/CV | S. aureus | 102 to 108 CFU/mL | 10 CFU/mL | [117] |
| CV | S. aureus | - | 39 CFU | [118] |
| DPV/EIS | L. monocytogenes | 1.9 × 101 to 1.9 × 106 CFU/mL | 1.9 × 101 CFU/mL | [119] |
| EIS/CV | E. coli | 102–109 CFU/mL | 10 CFU/mL | [120] |
| EIS | E. coli O157:H7 | 1.5 × 101 to 1.5 × 105 CFU/mL | 4.0 CFU/mL | [121] |
| CV | E. coli | - | 104 CFU/mL | [122] |
| DPV/EIS | S. aureus | 60 to 6 × 107 CFU/mL | 9 CFU/mL | [123] |
| EIS/CV | V. parahaemolyticus | 101 to 106 CFU/mL | 32 CFU/mL | [124] |
3.2. Optical Transduction
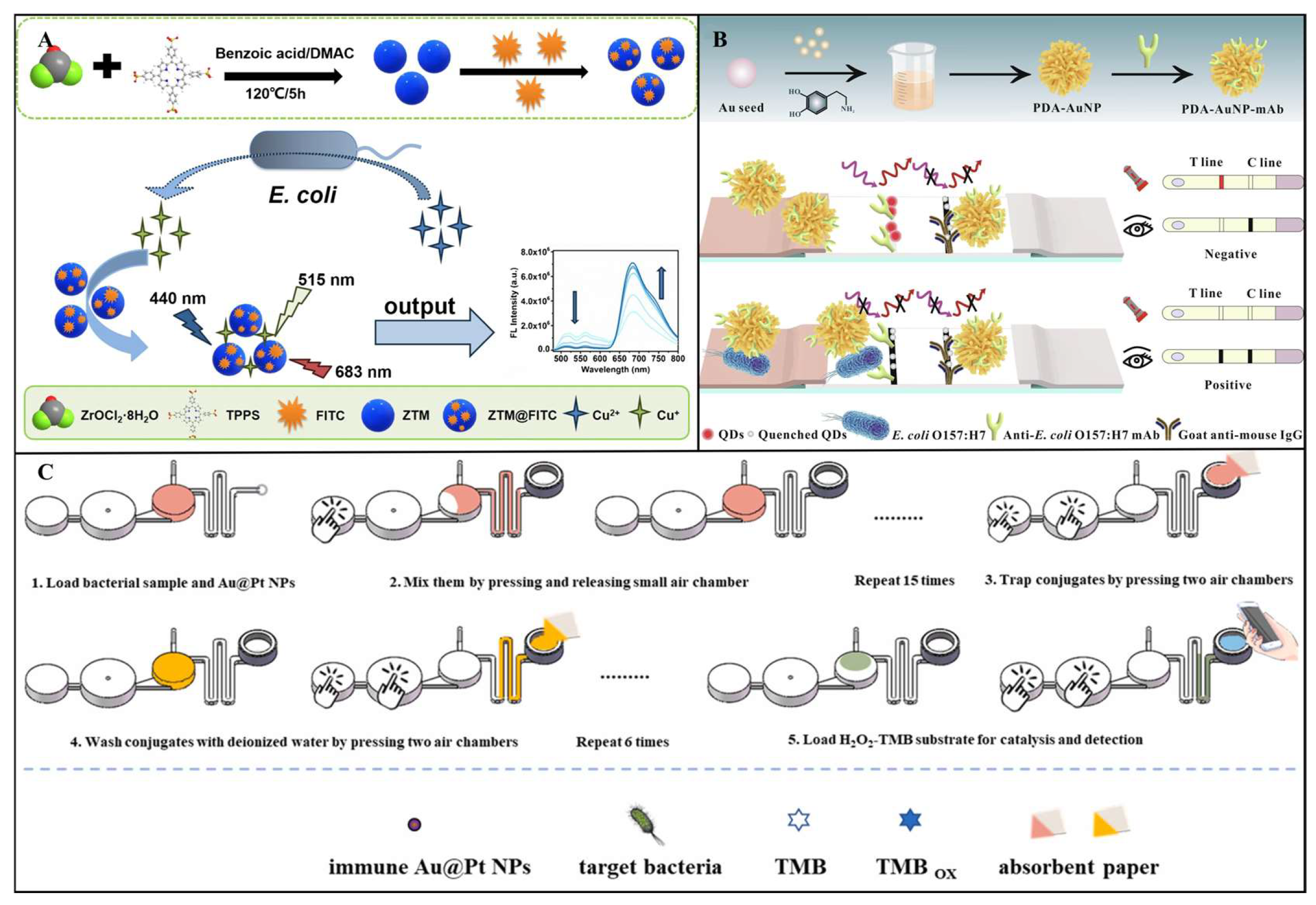
3.2.1. Fluorescent Transducer
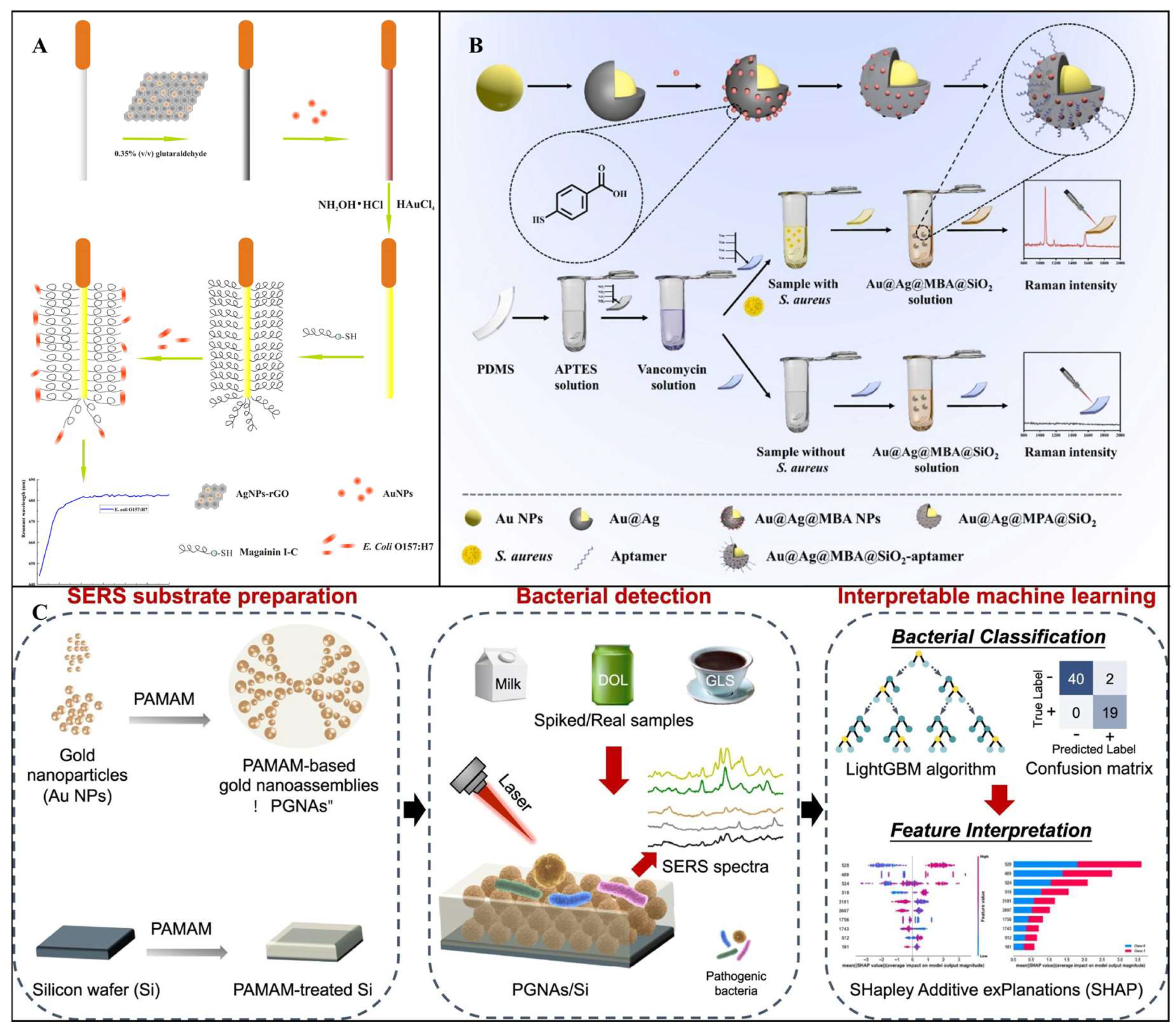
3.2.2. Colorimetric Transducer
3.2.3. SPR Transducer
3.2.4. SERS Transducer
| Optical Methods | Targets | Range of Detection | LOD | Reference |
|---|---|---|---|---|
| Colorimetric biosensor | S. typhimurium | - | 8.59 pM | [174] |
| Colorimetric biosensor | Salmonella | 1.8 × 101 to 1.8 × 105 CFU/mL | 18 CFU/mL | [175] |
| Colorimetric biosensor | Salmonella | 102 to 105 CFU/mL | 41 CFU/mL | [176] |
| SERS biosensor | S. typhimurium | 3.3 × 102–3.3 × 106 CFU/mL | 110 CFU/mL | [177] |
| Colorimetric biosensor | S. aureus | 10 to 1 × 106 CFU/mL | 2 CFU/mL | [178] |
| Fluorescent genosensor | S. aureus | 1 × 10−17 to 1 × 10−11 mol /L | 0.98 × 10−17 mol /L | [179] |
| Fluorescence biosensor | S. aureus | 10 to 106 CFU/mL | 6.9 CFU/mL | [180] |
| Ratiometric fluorescence biosensor | S. aureus | 7.9 × 100 to 7.9 × 108 CFU/mL | 3 CFU/mL | [181] |
| Fluorescence biosensor | S. aureus | 63–6.3 × 106 CFU/mL | 25 CFU/mL | [182] |
| Colorimetric biosensor | L. monocytogenes | 3.1 × 101 to 3.1 × 105 CFU/mL | 3.1 × 101 CFU/mL | [183] |
| Colorimetric biosensor | L. monocytogenes | 3.1 × 100 to 3.1 × 106 CFU/mL | 3.1 × 101 CFU/mL | [184] |
| Fluorescence aptasensor | L. monocytogenes | 68 to 68 × 106 CFU/mL | 8 CFU/mL | [185] |
| Colorimetric biosensor | S. typhimurium | 1.6 × 102–1.6 × 105 CFU/m3 | 100 CFU/m3 | [186] |
| Fluorescence biosensor | Salmonella enterica (S. enterica) | 6 × 101–6 × 107 CFU/mL | 1 CFU/mL | [187] |
| Fluorescence biosensor | S. typhimurium | 10–107 CFU/mL | 4 CFU/mL | [188] |
| Colorimetric biosensor | Salmonella | 5 × 101–5 × 105 CFU/mL | 41 CFU/mL | [189] |
| Ratiometric SERS biosensor | S. aureus | 10–108 CFU/mL | 10 CFU/mL | [190] |
| Colorimetric biosensor | S. aureus | 10−2 × 108 CFU/mL | 2.35 CFU/mL | [191] |
| Colorimetric-SERS dual-mode aptasensor | S. aureus | 101 to 107 CFU/mL | 0.926 CFU/mL (colorimetric) and 1.561 CFU/mL (SERS) | [192] |
| SERS biosensor | S. aureus | 2.15 to 2.15 × 105 CFU/mL | 1.0 CFU/mL | [193] |
| Colorimetric biosensor | S. aureus | 1 × 102 to 1 × 108 CFU/mL | 2 × 101 CFU/mL | [194] |
| SERS biosensor | S. aureus | 8.0 to 8.0 × 106 CFU/mL | 1.5 CFU/mL | [195] |
| Fluorescence-enhanced lateral flow biosensor | S. aureus | - | 5.4 × 102 CFU/mL | [196] |
| Microfluidic colorimetric biosensor | E. coli O157:H7 | 5 × 101∼5 × 106 CFU/mL | 17 CFU/mL | [197] |
| Colorimetric biosensor | E. coli O157:H7 | 0 to 107 CFU/mL | 2 CFU/mL | [198] |
| SERS biosensor | E. coli O157:H7 | 10 to 107 CFU/mL | 2 CFU/mL | [199] |
| FRET immunosensor | E. coli O157:H7 | 0 to 106 CFU/mL | 7 CFU/mL | [200] |
| Fluorescence biosensor | E. coli O157:H7 | 10 to 108 CFU/mL | 17.4 CFU/mL | [201] |
| Fluorescence biosensor | E. coli O157:H7 | 2.4 × 102 to 2.4 × 107 CFU/mL | 2.4 × 102 CFU/mL | [202] |
| Fluorescence biosensor | E. coli O157:H7 | 500–106 CFU/mL | 487 CFU/mL | [203] |
| Fluorescence biosensor | V. parahaemolyticus | 102–105 CFU/mL | 102 CFU/mL | [204] |
| Colorimetric-SERS dual-mode | V. parahaemolyticus | 101–105 CFU/mL | 9 CFU/mL (Colorimetric) and 7 CFU/mL (SERS) | [205] |
3.3. Piezoelectric Transduction
3.3.1. QCM Transducer
3.3.2. SAW Transducer
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riley, L.W. Extraintestinal Foodborne Pathogens. Annu. Rev. Food Sci. Technol. 2020, 11, 275–294. [Google Scholar] [CrossRef]
- Deng, R.; Bai, J.; Yang, H.; Ren, Y.; He, Q.; Lu, Y. Nanotechnology-Leveraged Nucleic Acid Amplification for Foodborne Pathogen Detection. Coord. Chem. Rev. 2024, 506, 215745. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Ali, S.S.; Moawad, M.S.; Hussein, M.A.; Azab, M.; Abdelkarim, E.A.; Badr, A.; Sun, J.; Khalil, M. Efficacy of Metal Oxide Nanoparticles as Novel Antimicrobial Agents against Multi-Drug and Multi-Virulent Staphylococcus aureus Isolates from Retail Raw Chicken Meat and Giblets. Int. J. Food Microbiol. 2021, 344, 109116. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Sun, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Synergistic Antibiofilm Effects of Ultrasound and Phenyllactic Acid against Staphylococcus aureus and Salmonella enteritidis. Foods 2021, 10, 2171. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the Anti-Bacterial Mechanism of Litsea Cubeba Essential Oil against E. coli O157:H7 and Its Application in Vegetable Juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Shi, C.; Hu, W.; Cui, H.; Lin, L. Characterization of Controlled-Release Eucalyptus Citriodora Oil/Zinc Ions Nanoparticles with Enhanced Antibacterial Properties against E. Coli O157:H7 in Fruit Juice. Food Res. Int. 2022, 162, 112138. [Google Scholar] [CrossRef]
- Cui, H.; Chen, Y.; Aziz, T.; Al-Asmari, F.; Alwethaynani, M.S.; Shi, C.; Lin, L. Antibacterial Mechanisms of Diacetyl on Listeria Monocytogenes and Its Application in Inner Mongolian Cheese Preservation via Gelatin-Based Edible Films. Food Control 2025, 168, 110920. [Google Scholar] [CrossRef]
- Hoffmann, S.; White, A.E.; McQueen, R.B.; Ahn, J.-W.; Gunn-Sandell, L.B.; Walter, E.J.S. Economic Burden of Foodborne Illnesses Acquired in the United States. Foodborne Pathog. Dis. 2024, 22, 4–14. [Google Scholar] [CrossRef]
- Liu, R.; Ali, S.; Huang, D.; Zhang, Y.; Lü, P.; Chen, Q. A Sensitive Nucleic Acid Detection Platform for Foodborne Pathogens Based on CRISPR-Cas13a System Combined with Polymerase Chain Reaction. Food Anal. Methods 2023, 16, 356–366. [Google Scholar] [CrossRef]
- Guo, Q.; Han, J.-J.; Shan, S.; Liu, D.-F.; Wu, S.-S.; Xiong, Y.-H.; Lai, W.-H. DNA-Based Hybridization Chain Reaction and Biotin–Streptavidin Signal Amplification for Sensitive Detection of Escherichia coli O157:H7 through ELISA. Biosens. Bioelectron. 2016, 86, 990–995. [Google Scholar] [CrossRef]
- Ravan, H.; Amandadi, M.; Sanadgol, N. A Highly Specific and Sensitive Loop-Mediated Isothermal Amplification Method for the Detection of Escherichia coli O157:H7. Microb. Pathog. 2016, 91, 161–165. [Google Scholar] [CrossRef]
- Wang, D.-B.; Cui, M.-M.; Li, M.; Zhang, X.-E. Biosensors for the Detection of Bacillus anthracis. Acc. Chem. Res. 2021, 54, 4451–4461. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Hazra, S.; Patra, S.; Gogoi, M. Biosensors for Cancer Detection: A Review. TrAC Trends Anal. Chem. 2024, 180, 117978. [Google Scholar] [CrossRef]
- Akbari Nakhjavani, S.; Tokyay, B.K.; Soylemez, C.; Sarabi, M.R.; Yetisen, A.K.; Tasoglu, S. Biosensors for Prostate Cancer Detection. Trends Biotechnol. 2023, 41, 1248–1267. [Google Scholar] [CrossRef]
- Chen, J.; Andler, S.M.; Goddard, J.M.; Nugen, S.R.; Rotello, V.M. Integrating Recognition Elements with Nanomaterials for Bacteria Sensing. Chem. Soc. Rev. 2017, 46, 1272–1283. [Google Scholar] [CrossRef]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for Breast Cancer Diagnosis: A Review of Bioreceptors, Biotransducers and Signal Amplification Strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-N.; Luo, L.; Wang, B.-Z.; Lei, H.-T.; Guan, T.; Shen, Y.-D.; Wang, H.; Xu, Z.-L. Biosensors for Detection of Paralytic Shellfish Toxins: Recognition Elements and Transduction Technologies. Trends Food Sci. Technol. 2023, 133, 205–218. [Google Scholar] [CrossRef]
- Van Dorst, B.; Mehta, J.; Bekaert, K.; Rouah-Martin, E.; De Coen, W.; Dubruel, P.; Blust, R.; Robbens, J. Recent Advances in Recognition Elements of Food and Environmental Biosensors: A Review. Biosens. Bioelectron. 2010, 26, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xianyu, Y. Array-Based Biosensors for Bacteria Detection: From the Perspective of Recognition. Small 2021, 17, 2006230. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Luo, L.; Duan, C.; Shen, J.; Wang, Z. Application of Germline Antibody Features to Vaccine Development, Antibody Discovery, Antibody Optimization and Disease Diagnosis. Biotechnol. Adv. 2023, 65, 108143. [Google Scholar] [CrossRef] [PubMed]
- Alanine, D.G.W.; Quinkert, D.; Kumarasingha, R.; Mehmood, S.; Donnellan, F.R.; Minkah, N.K.; Dadonaite, B.; Diouf, A.; Galaway, F.; Silk, S.E.; et al. Human Antibodies That Slow Erythrocyte Invasion Potentiate Malaria-Neutralizing Antibodies. Cell 2019, 178, 216–228.e21. [Google Scholar] [CrossRef] [PubMed]
- Crivianu-Gaita, V.; Thompson, M. Aptamers, Antibody scFv, and Antibody Fab’ Fragments: An Overview and Comparison of Three of the Most Versatile Biosensor Biorecognition Elements. Biosens. Bioelectron. 2016, 85, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, L.; Bi, W.; Shen, H.; Li, D.; Yu, Z.; Gan, N. Ultrasensitive Microfluidic Immunosensor with Stir Bar Enrichment for Point-of-Care Test of Staphylococcus aureus in Foods Triggered by DNAzyme-Assisted Click Reaction. Food Chem. 2022, 378, 132093. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, D.; Wang, S.; Cai, G.; Xue, L.; Li, Y.; Liao, M.; Lin, J. A Microfluidic Biosensor for Rapid Detection of Salmonella typhimurium Based on Magnetic Separation, Enzymatic Catalysis and Electrochemical Impedance Analysis. Chin. Chem. Lett. 2022, 33, 3156–3160. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, X.; Meng, Q.; Zheng, P.; Zhang, J.; He, Z.; Jiang, H. Gold Interdigitated Micro-Immunosensor Based on Mn-MOF-74 for the Detection of Listeria Monocytogens. Biosens. Bioelectron. 2021, 183, 113186. [Google Scholar] [CrossRef]
- Asaadi, Y.; Jouneghani, F.F.; Janani, S.; Rahbarizadeh, F. A Comprehensive Comparison between Camelid Nanobodies and Single Chain Variable Fragments. Biomark. Res. 2021, 9, 87. [Google Scholar] [CrossRef]
- Gao, S.; Yang, W.; Zheng, X.; Wang, T.; Zhang, D.; Zou, X. Advances of Nanobody-Based Immunosensors for Detecting Food Contaminants. Trends Food Sci. Technol. 2025, 156, 104871. [Google Scholar] [CrossRef]
- Currie, S.; Cortes De La Torre, A.J.; Kumar, A.; Logsetty, S.; Liu, S. Next-Generation Wound Care: Aptamer-Conjugated Polydiacetylene/Polyurethane Nanofibrous Biosensors for Selective In Situ Colorimetric Detection of Pseudomonas. Adv. Funct. Mater. 2024, 34, 2403440. [Google Scholar] [CrossRef]
- Cao, X.; Liu, D.; Shi, R.; Li, T.; Fang, X.; Feng, X.; Li, P.; Zhang, Y.; Xiao, M.; Wang, L. Real-Time, Ultrasensitive, and Accurate Dual Detection of Escherichia coli O157 and Listeria Monocytogenes Using a Field-Effect Transistor Biosensor Functionalized with Aptamer Groups. Chem. Eng. J. 2025, 509, 161218. [Google Scholar] [CrossRef]
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Zhou, Z.; Lan, X.; Zhu, L.; Zhang, Y.; Chen, K.; Zhang, W.; Xu, W. Portable Dual-Aptamer Microfluidic Chip Biosensor for Bacillus Cereus Based on Aptamer Tailoring and Dumbbell-Shaped Probes. J. Hazard. Mater. 2023, 445, 130545. [Google Scholar] [CrossRef]
- Yan, L.; Tian, L.; Zhang, Y.; Guo, Q.; Sun, X.; Guo, Y.; Li, F.; Yang, Q.; Zhang, Y. Coreactant-Free Electrochemiluminescent Biosensor for Detection of Staphylococcus aureus Based on Host–Guest Structure of Arg/ATT-AuNCs and DNA Nanomachines. Chem. Eng. J. 2025, 506, 160268. [Google Scholar] [CrossRef]
- Van Dongen, J.E.; Berendsen, J.T.W.; Steenbergen, R.D.M.; Wolthuis, R.M.F.; Eijkel, J.C.T.; Segerink, L.I. Point-of-Care CRISPR/Cas Nucleic Acid Detection: Recent Advances, Challenges and Opportunities. Biosens. Bioelectron. 2020, 166, 112445. [Google Scholar] [CrossRef]
- Wachholz Junior, D.; Kubota, L.T. CRISPR-Based Electrochemical Biosensors: An Alternative for Point-of-Care Diagnostics? Talanta 2024, 278, 126467. [Google Scholar] [CrossRef]
- Aman, R.; Mahas, A.; Mahfouz, M. Nucleic Acid Detection Using CRISPR/Cas Biosensing Technologies. ACS Synth. Biol. 2020, 9, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Yudin Kharismasari, C.; Irkham; Zein, M.I.H.L.; Hardianto, A.; Nur Zakiyyah, S.; Umar Ibrahim, A.; Ozsoz, M.; Wahyuni Hartati, Y. CRISPR/Cas12-Based Electrochemical Biosensors for Clinical Diagnostic and Food Monitoring. Bioelectrochemistry 2024, 155, 108600. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Man, S.; Ye, S.; Liu, G.; Ma, L. CRISPR-Cas-based Detection for Food Safety Problems: Current Status, Challenges, and Opportunities. Comp. Rev. Food Sci. Food Safe 2022, 21, 3770–3798. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Chen, X.; Wei, L.; Yang, D.; Pu, M.; Mao, Y.; Wang, Z.; Wang, B. Rapid Detection of Staphylococcus aureus Using a CRISPR/Cas14a-Assisted Electrochemical Aptasensor and PCN-222@AuPt Nanozyme-Induced Amplification Strategy. Sens. Actuators B Chem. 2025, 437, 137751. [Google Scholar] [CrossRef]
- Dhara, D.; Hill, A.C.; Ramesh, A.; Wood, M.J.A.; El-Sagheer, A.H.; Brown, T. Synthesis, Biophysical and Biological Evaluation of Splice-Switching Oligonucleotides with Multiple LNA-Phosphothiotriester Backbones. J. Am. Chem. Soc. 2024, 146, 29773–29781. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Du, C.; Li, Y.; Ma, X.; Yang, C.; Xu, W.; Sun, C. Structure-Switching Aptamer Triggering Signal Amplification Strategy for Tobramycin Detection Based on Hybridization Chain Reaction and Fluorescence Synergism. Talanta 2022, 243, 123318. [Google Scholar] [CrossRef]
- An, Q.; Wang, Y.; Tian, Z.; Han, J.; Li, J.; Liao, F.; Yu, F.; Zhao, H.; Wen, Y.; Zhang, H.; et al. Molecular and Structural Basis of an ATPase-Nuclease Dual-Enzyme Anti-Phage Defense Complex. Cell Res. 2024, 34, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Béguin, P.; Chekli, Y.; Sezonov, G.; Forterre, P.; Krupovic, M. Sequence Motifs Recognized by the Casposon Integrase of Aciduliprofundum Boonei. Nucleic Acids Res. 2019, 47, 6386–6395. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Zhu, W.; Yuan, Y.; Du, X.; Xu, A.; Zhang, D.; Cao, S.; Chen, W.; Lin, Y.; Xie, J.; et al. Duality of Immune Recognition by Tomato and Virulence Activity of the Ralstonia solanacearum Exo-Polygalacturonase PehC. Plant Cell 2023, 35, 2552–2569. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Song, Z.; Zhou, C.; Ma, P.; Li, C.; Lu, Q.; Liao, Z.; Huang, Z.; Tang, Y.; Li, H.; et al. Development of Nanobody-Horseradish Peroxidase-Based Sandwich ELISA to Detect Salmonella enteritidis in Milk and in Vivo Colonization in Chicken. J. Nanobiotechnol. 2022, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, X.; Wang, Z.; Zhang, Y.; Huang, X.; Li, Z.; Daglia, M.; Xiao, J.; Shi, J.; Zou, X. Bioinspired Nanozyme Enabling Glucometer Readout for Portable Monitoring of Pesticide under Resource-Scarce Environments. Chem. Eng. J. 2022, 429, 132243. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Liu, R.; Wang, Y.; Li, A.; Tian, S.; Cheng, W.; Ding, S.; Li, W.; Zhao, M.; et al. A Novel CRISPR/Cas14a-Based Electrochemical Biosensor for Ultrasensitive Detection of Burkholderia Pseudomallei with PtPd@PCN-224 Nanoenzymes for Signal Amplification. Biosens. Bioelectron. 2023, 225, 115098. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, Z.; Wang, X.; Zhang, C.; Li, X.; Wei, Y.; Wang, J.; Fang, Y.; Wang, Y.; Zhang, D. Synergistic Optimization Strategies for the Development of Multienzymatic Cascade System-Based Electrochemical Biosensors with Enhanced Performance. Biosens. Bioelectron. 2025, 274, 117222. [Google Scholar] [CrossRef]
- Porębska, N.; Poźniak, M.; Matynia, A.; Żukowska, D.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Galectins as Modulators of Receptor Tyrosine Kinases Signaling in Health and Disease. Cytokine Growth Factor Rev. 2021, 60, 89–106. [Google Scholar] [CrossRef]
- Parker, J.E.; Hessler, G.; Cui, H. A New Biochemistry Connecting Pathogen Detection to Induced Defense in Plants. New Phytol. 2022, 234, 819–826. [Google Scholar] [CrossRef]
- Qiu, X.; Ding, J.; Wang, Y.; Fang, L.; Li, D.; Huo, Z. Identification and Function Analysis of Toll–like Receptor 4 (TLR4) from Manila Clam (Ruditapes Philippinarum). Int. J. Biol. Macromol. 2025, 290, 139000. [Google Scholar] [CrossRef]
- Tomasek, K.; Leithner, A.; Glatzova, I.; Lukesch, M.S.; Guet, C.C.; Sixt, M. Type 1 Piliated Uropathogenic Escherichia coli Hijack the Host Immune Response by Binding to CD14. eLife 2022, 11, e78995. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.J.; Patiño, E.B.; Orozco, J. IL-5Rα-Based Electrochemical Biosensor: Towards Building Biosensors with Natural Receptors. Chem. Eng. J. 2025, 505, 159789. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles–Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, H.; Han, Y.; Yu, F.; Shi, X. Development of a Biomimetic Enzyme-Linked Immunosorbent Assay Based on Molecularly Imprinted Polymers on Paper for the Detection of Carbaryl. Food Chem. 2018, 240, 893–897. [Google Scholar] [CrossRef]
- Cieplak, M.; Kutner, W. Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef]
- Dar, K.K.; Shao, S.; Tan, T.; Lv, Y. Molecularly Imprinted Polymers for the Selective Recognition of Microorganisms. Biotechnol. Adv. 2020, 45, 107640. [Google Scholar] [CrossRef] [PubMed]
- Agar, M.; Laabei, M.; Leese, H.S.; Estrela, P. Aptamer-Molecularly Imprinted Polymer Sensors for the Detection of Bacteria in Water. Biosens. Bioelectron. 2025, 272, 117136. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Ma, Z.; Gu, H.; Luo, X.; Yin, X.; Yi, H.; Chen, Y. Hybrid Recognition-Enabled Ratiometric Electrochemical Sensing of Staphylococcus aureus via in-Situ Growth of MOF/Ti3C2Tx-MXene and a Self-Reporting Bacterial Imprinted Polymer. Food Chem. 2025, 463, 141496. [Google Scholar] [CrossRef]
- Wang, X.; Zang, X.; Deng, L.; Tan, F.; Liu, X.; Zhang, Z.; Cui, B.; Fang, Y. Molecularly Imprinted Photoelectrochemical Sensor for Escherichia coli Based on Cu:ZIF-8/KZ3TTz Heterojunction. Food Chem. 2024, 458, 140495. [Google Scholar] [CrossRef]
- Narula, K.; Rajpal, S.; Bhakta, S.; Kulanthaivel, S.; Mishra, P. Rationally Designed Protein A Surface Molecularly Imprinted Magnetic Nanoparticles for the Capture and Detection of Staphylococcus aureus. J. Mater. Chem. B 2024, 12, 5699–5710. [Google Scholar] [CrossRef] [PubMed]
- Skurnik, M.; Alkalay-Oren, S.; Boon, M.; Clokie, M.; Sicheritz-Pontén, T.; Dąbrowska, K.; Hatfull, G.F.; Hazan, R.; Jalasvuori, M.; Kiljunen, S.; et al. Phage Therapy. Nat. Rev. Methods Primers 2025, 5, 9. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Lindback, M.M.; Bastien, G.E.; Solonenko, N.; Zayed, A.A.; Jang, H.; Andreopoulos, B.; Brewer, H.M.; Glavina Del Rio, T.; Adkins, J.N.; et al. Phage-Specific Metabolic Reprogramming of Virocells. ISME J. 2020, 14, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.V.; Wenner, N.; Dulberger, C.L.; Rodwell, E.V.; Bowers-Barnard, A.; Quinones-Olvera, N.; Rigden, D.J.; Rubin, E.J.; Garner, E.C.; Baym, M.; et al. Prophages Encode Phage-Defense Systems with Cognate Self-Immunity. Cell Host Microbe 2021, 29, 1620–1633.e8. [Google Scholar] [CrossRef]
- Lin, S.; Xie, G.; He, J.; Meng, L.; Pang, Y.; Liu, J. Enhancing Phage Therapy by Coating Single Bacteriophage-Infected Bacteria with Polymer to Preserve Phage Vitality. Nat. Biomed. Eng. 2025, 9, 1155–1171. [Google Scholar] [CrossRef]
- Wu, S.; Sheng, L.; Lu, X.; Ye, Y.; Sun, J.; Ji, J.; Shao, J.; Zhang, Y.; Sun, X. Screening of Bio-Recognition Elements by Phage Display and Their Application in the Detection of Foodborne Pathogens. TrAC Trends Anal. Chem. 2024, 171, 117481. [Google Scholar] [CrossRef]
- Zhao, J.; Han, M.; Ma, A.; Jiang, F.; Chen, R.; Dong, Y.; Wang, X.; Ruan, S.; Chen, Y. A Machine Vision-Assisted Argonaute-Mediated Fluorescence Biosensor for the Detection of Viable Salmonella in Food without Convoluted DNA Extraction and Amplification Procedures. J. Hazard. Mater. 2024, 466, 133648. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, R.; Ma, A.; Dong, Y.; Han, M.; Yu, X.; Chen, Y. CuO2@SiO2 Nanoparticle Assisted Click Reaction-Mediated Magnetic Relaxation Biosensor for Rapid Detection of Salmonella in Food. Biosens. Bioelectron. 2025, 273, 117188. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepulveda, D.R.; Rios-Velasco, C.; Acosta-Muñiz, C.H. Incorporation of A511 Bacteriophage in a Whey Protein Isolate-Based Edible Coating for the Control of Listeria Monocytogenes in Cheese. Food Packag. Shelf Life 2023, 37, 101095. [Google Scholar] [CrossRef]
- Kamali, S.; Yavarmanesh, M.; Habibi Najafi, M.B.; Koocheki, A. Development of Whey Protein Concentrate/Pullulan Composite Films Containing Bacteriophage A511: Functional Properties and Anti-Listerial Effects during Storage. Food Packag. Shelf Life 2022, 33, 100902. [Google Scholar] [CrossRef]
- Tabib-Salazar, A.; Liu, B.; Shadrin, A.; Burchell, L.; Wang, Z.; Wang, Z.; Goren, M.G.; Yosef, I.; Qimron, U.; Severinov, K.; et al. Full Shut-off of Escherichia coli RNA-Polymerase by T7 Phage Requires a Small Phage-Encoded DNA-Binding Protein. Nucleic Acids Res. 2017, 45, 7697–7707. [Google Scholar] [CrossRef]
- Cao, Y.; Khanal, D.; Kim, J.; Chang, R.Y.K.; Byun, A.S.; Morales, S.; Banaszak Holl, M.M.; Chan, H.-K. Stability of Bacteriophages in Organic Solvents for Formulations. Int. J. Pharm. 2023, 646, 123505. [Google Scholar] [CrossRef]
- Brady, A.; Felipe-Ruiz, A.; Gallego Del Sol, F.; Marina, A.; Quiles-Puchalt, N.; Penadés, J.R. Molecular Basis of Lysis–Lysogeny Decisions in Gram-Positive Phages. Annu. Rev. Microbiol. 2021, 75, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sindhu, A.; Dilbaghi, N.; Chaudhury, A. Biosensors as Innovative Tools for the Detection of Food Borne Pathogens. Biosens. Bioelectron. 2011, 28, 1–12. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Nanomaterial-based Biosensors for Sensing Key Foodborne Pathogens: Advances from Recent Decades. Comp. Rev. Food Sci. Food Safe 2020, 19, 1465–1487. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Wang, J. Multi-Mode/Signal Biosensors: Electrochemical Integrated Sensing Techniques. Adv. Funct. Mater. 2024, 34, 2403122. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, X.; Ye, Q.; Zou, J.; Wan, Q.; Jiang, F.; Cai, Z.; Zhang, J.; Qu, X.; Huang, J.; et al. Isothermal Nucleic Acid Amplification-Based Biosensors: The next Generation Analytical Toolkit for Point-of-Care Assay of Foodborne Pathogens. Trends Food Sci. Technol. 2025, 157, 104882. [Google Scholar] [CrossRef]
- Lu, W.; Dai, X.; Yang, R.; Liu, Z.; Chen, H.; Zhang, Y.; Zhang, X. Fenton-like Catalytic MOFs Driving Electrochemical Aptasensing toward Tracking Lead Pollution in Pomegranate Fruit. Food Control 2025, 169, 111006. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Wang, J.; Huang, X.; El-Mesery, H.S.; Shi, Y.; Zou, Y.; Li, Z.; Li, Y.; Shi, J.; et al. Simple-Easy Electrochemical Sensing Mode Assisted with Integrative Carbon-Based Gel Electrolyte for in-Situ Monitoring of Plant Hormone Indole Acetic Acid. Food Chem. 2025, 467, 142342. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Zhang, W.; Li, Y.; Zhang, X.; Huang, X.; Shi, Y.; Zou, Y.; Li, Z.; Shi, J.; et al. Highly Catalytic Ce-Based MOF for Powering Electrochemical Aptasensing toward Evaluating Dissolution Rate of Microelement Copper from Tea-Leaves. J. Food Compos. Anal. 2025, 140, 107266. [Google Scholar] [CrossRef]
- Huang, X.; Huang, C.; Zhou, L.; Hou, G.; Sun, J.; Zhang, X.; Zou, X. Allosteric Switch for Electrochemical Aptasensor toward Heavy Metals Pollution of Lentinus Edodes Sensitized with Porphyrinic Metal-Organic Frameworks. Anal. Chim. Acta 2023, 1278, 341752. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Huang, A.; He, L.; Cai, C.; You, T. Recent Advances in Foodborne Pathogen Detection Using Photoelectrochemical Biosensors: From Photoactive Material to Sensing Strategy. Front. Sustain. Food Syst. 2024, 8, 1432555. [Google Scholar] [CrossRef]
- Lin, X.; Liu, P.P.; Yan, J.; Luan, D.; Sun, T.; Bian, X. Dual Synthetic Receptor-Based Sandwich Electrochemical Sensor for Highly Selective and Ultrasensitive Detection of Pathogenic Bacteria at the Single-Cell Level. Anal. Chem. 2023, 95, 5561–5567. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Chen, T.; You, T. Label-Free Ratiometric Homogeneous Electrochemical Aptasensor Based on Hybridization Chain Reaction for Facile and Rapid Detection of Aflatoxin B1 in Cereal Crops. Food Chem. 2022, 373, 131443. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in Nanomaterial-Based Electrochemical Biosensors for the Detection of Microbial Toxins, Pathogenic Bacteria in Food Matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef]
- Yang, L.; Ding, Y.; Ma, Y.; Wen, J.; Wang, J.; Dai, G.; Mo, F. An Electrochemical Sensor Based on 2D Zn-MOFs and 2D C-Ti3C2Tx Composite Materials for Rapid and Direct Detection of Various Foodborne Pathogens. Food Chem. 2025, 462, 140922. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, S.; Shalini Devi, K.S.; Pandikumar, A. A Comprehensive Review on Graphitic Carbon Nitride Based Electrochemical and Biosensors for Environmental and Healthcare Applications. TrAC Trends Anal. Chem. 2021, 140, 116274. [Google Scholar] [CrossRef]
- Li, F.; Ye, Q.; Chen, M.; Zhou, B.; Zhang, J.; Pang, R.; Xue, L.; Wang, J.; Zeng, H.; Wu, S.; et al. An Ultrasensitive CRISPR/Cas12a Based Electrochemical Biosensor for Listeria Monocytogenes Detection. Biosens. Bioelectron. 2021, 179, 113073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, Z.; Guo, Q.; Weng, X. Microfluidic Thread-Based Electrochemical Aptasensor for Rapid Detection of Vibrio Parahaemolyticus. Biosens. Bioelectron. 2021, 182, 113191. [Google Scholar] [CrossRef]
- Yoon, Y.; Baek, C.; Yoo, D.; Seo, Y.; Lee, S.; Won Shin, S.; Min, J.; Lee, T. Construction of On-Site DNA Pre-Treatment Device and Rapid Electrochemical Biosensor Set for Escherichia coli Detection in Milk. Chem. Eng. J. 2024, 499, 155898. [Google Scholar] [CrossRef]
- Wang, C.; Wu, R.; Ling, H.; Zhao, Z.; Han, W.; Shi, X.; Payne, G.F.; Wang, X. Toward Scalable Fabrication of Electrochemical Paper Sensor without Surface Functionalization. npj Flex. Electron. 2022, 6, 12. [Google Scholar] [CrossRef]
- Silvestri, A.; Vázquez-Díaz, S.; Misia, G.; Poletti, F.; López-Domene, R.; Pavlov, V.; Zanardi, C.; Cortajarena, A.L.; Prato, M. An Electroactive and Self-Assembling Bio-Ink, Based on Protein-Stabilized Nanoclusters and Graphene, for the Manufacture of Fully Inkjet-Printed Paper-Based Analytical Devices. Small 2023, 19, e2300163. [Google Scholar] [CrossRef]
- Wonsawat, W.; Limvongjaroen, S.; Supromma, S.; Panphut, W.; Ruecha, N.; Ratnarathorn, N.; Dungchai, W. A Paper-Based Conductive Immunosensor for the Determination of Salmonella typhimurium. Analyst 2020, 145, 4637–4645. [Google Scholar] [CrossRef] [PubMed]
- Zdrachek, E.; Bakker, E. Potentiometric Sensor Array with Multi-Nernstian Slope. Anal. Chem. 2020, 92, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.; Zou, X. Rapid Determination of Cadmium in Rice Using an All-Solid RGO-Enhanced Light Addressable Potentiometric Sensor. Food Chem. 2018, 261, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Y.; Tahir, H.E.; Zou, X.; Wang, P. Rapid and Wide-Range Determination of Cd(II), Pb(II), Cu(II) and Hg(II) in Fish Tissues Using Light Addressable Potentiometric Sensor. Food Chem. 2017, 221, 541–547. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, J.; Luan, F.; Qin, W. Chronopotentiometric Sensors for Antimicrobial Peptide-Based Biosensing of Staphylococcus aureus. Microchim. Acta 2024, 191, 356. [Google Scholar] [CrossRef]
- Malvano, F.; Pilloton, R.; Albanese, D. A Novel Impedimetric Biosensor Based on the Antimicrobial Activity of the Peptide Nisin for the Detection of Salmonella spp. Food Chem. 2020, 325, 126868. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Shi, J.; Li, Z.; Huang, X.; Zou, X.; Tan, W.; Zhang, X.; Hu, X.; Wang, X.; et al. Impedimetric Aptasensor Based on Highly Porous Gold for Sensitive Detection of Acetamiprid in Fruits and Vegetables. Food Chem. 2020, 322, 126762. [Google Scholar] [CrossRef]
- Huang, F.; Xue, L.; Qi, W.; Cai, G.; Liu, Y.; Lin, J. An Ultrasensitive Impedance Biosensor for Salmonella Detection Based on Rotating High Gradient Magnetic Separation and Cascade Reaction Signal Amplification. Biosens. Bioelectron. 2021, 176, 112921. [Google Scholar] [CrossRef]
- Balser, S.; Röhrl, M.; Spormann, C.; Lindhorst, T.K.; Terfort, A. Selective Quantification of Bacteria in Mixtures by Using Glycosylated Polypyrrole/Hydrogel Nanolayers. ACS Appl. Mater. Interfaces 2024, 16, 14243–14251. [Google Scholar] [CrossRef]
- Tian, J.-Y.; Liu, X.; Zhang, S.; Chen, K.; Zhu, L.; Song, Y.; Wang, M.; Zhang, Z.; Du, M. Novel Aptasensing Strategy for Efficiently Quantitative Analyzing Staphylococcus aureus Based on Defective Copper-Based Metal–Organic Framework. Food Chem. 2023, 402, 134357. [Google Scholar] [CrossRef]
- Wachholz Junior, D.; Pontes, R.G.; Hryniewicz, B.M.; Kubota, L.T. Exploring a CRISPR/Cas12a-Powered Impedimetric Biosensor for Amplification-Free Detection of a Pathogenic Bacterial DNA. Biosens. Bioelectron. 2025, 285, 117607. [Google Scholar] [CrossRef] [PubMed]
- Ertuğrul Uygun, H.D.; Odaci, D. Impedimetric Single Carbon Fiber Electrode for Ultrasensitive Detection of Staphylococcus aureus Pathogen DNAs in Breast Milk by CRISPR Technology. ACS Omega 2024, 9, 25172–25180. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Liang, J.; Zhang, Y.; Zhang, M.; Ao, H.; Yang, T. An Antifouling Electrochemical Biosensor Using Self-Signal for Salmonella typhimurium Direct Detection in Food Sample. Food Chem. 2024, 452, 139536. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Wang, S.; Ma, J.; Hu, D.; Liang, H.; Ma, C.; Jin, Y.; Chen, X.; Xu, G.; et al. Rapid Detection of Salmonella typhimurium in Food Samples Using Electrochemical Sensor. LWT 2024, 206, 116567. [Google Scholar] [CrossRef]
- Mahari, S.; Roberts, A.; Gandhi, S. Probe-Free Nanosensor for the Detection of Salmonella Using Gold Nanorods as an Electroactive Modulator. Food Chem. 2022, 390, 133219. [Google Scholar] [CrossRef]
- He, Y.; Jia, F.; Sun, Y.; Fang, W.; Li, Y.; Chen, J.; Fu, Y. An Electrochemical Sensing Method Based on CRISPR/Cas12a System and Hairpin DNA Probe for Rapid and Sensitive Detection of Salmonella typhimurium. Sens. Actuators B Chem. 2022, 369, 132301. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Yuan, R.; Qi, Y. Construction of an Electrochemical Aptasensor Based on a Covalent Organic Framework for Rapid and Ultrasensitive Detection of Staphylococcus aureus. Sens. Actuators B Chem. 2025, 428, 137224. [Google Scholar] [CrossRef]
- Zheng, J.; Li, J.; Lin, T.; Ren, Z.; Wang, F.; Shi, Z.; Yu, H.; Jiang, W.; Tang, W. Amplification-Free and Label-Free Rapid Detection of Staphylococcus aureus Using Solution-Gated Graphene Transistor-Based DNA Biosensor with Hybridization Enhancement by Interface Engineering. Chem. Eng. J. 2024, 495, 153329. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, Q.; Liu, W.; Xiao, Z.; Jin, L.; Liu, Y.; Wu, Y.; Yu, X. Multi-Functional Electrochemiluminescence Biosensor for Efficient Capture, Elimination, and Sensitive Monitoring of Staphylococcus aureus. Biosens. Bioelectron. 2025, 272, 117112. [Google Scholar] [CrossRef]
- Lin, X.; Liu, C.; Lei, Q.; Nan, X.; Zhu, Y.; Liao, J.; Du, Z.; Ye, C.; Xiong, Y.; Yang, M.; et al. A Novel Ratiometric Electrochemical Aptasensor Based on Graphene Quantum Dots/Cu-MOF Nanocomposite for the on-Site Determination of Staphylococcus aureus. J. Hazard. Mater. 2025, 485, 136845. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.; Zhang, S.; Yang, A.; Ma, Q.; Deng, Z.; Fang, J.; Cai, Q.; He, J. A Supersensitive Electrochemical Sensor Based on RCA Amplification-Assisted “Silver Chain”-Linked Gold Interdigital Electrodes and CRISPR/Cas9 for the Detection of Staphylococcus aureus in Food. Food Chem. 2024, 440, 138197. [Google Scholar] [CrossRef]
- Nguyen, T.T.-Q.; Gu, M.B. An Ultrasensitive Electrochemical Aptasensor Using Tyramide-Assisted Enzyme Multiplication for the Detection of Staphylococcus aureus. Biosens. Bioelectron. 2023, 228, 115199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, Y.; Li, J.; Huang, B.; Wen, H.; Ren, J. Cascade Signal Enhancement by Integrating DNA Walking and RCA Reaction-Assisted “Silver-Link” Crossing Electrode for Ultrasensitive Electrochemical Detection of Staphylococcus aureus. Biosens. Bioelectron. 2022, 217, 114716. [Google Scholar] [CrossRef]
- Nguyen, T.T.-Q.; Kim, E.R.; Gu, M.B. A New Cognate Aptamer Pair-Based Sandwich-Type Electrochemical Biosensor for Sensitive Detection of Staphylococcus aureus. Biosens. Bioelectron. 2022, 198, 113835. [Google Scholar] [CrossRef]
- Li, W.; Song, Y.; Zhao, L.; Ling, Z.; Xu, H. MXene-CuBTC Nanozyme with Oxygen Vacancies and Charge Transfer for Biosensing of Listeria Monocytogenes. Chem. Eng. J. 2025, 509, 161261. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Yang, L.; Huang, B.; Lu, K.; Wen, H.; Ren, J. Ultrasensitive Electrochemical Biosensor for Bacteria Detection Based on Fe3O4@COF-AuNPs and Trigging Isothermal Circular Amplification. Sens. Actuators B Chem. 2025, 422, 136609. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Qi, H.; Huang, X.; Shi, J.; Zou, X. A Novel Renewable Electrochemical Biosensor Based on Mussel-Inspired Adhesive Protein for the Detection of Escherichia coli O157:H7 in Food. Sens. Actuators B Chem. 2022, 372, 132601. [Google Scholar] [CrossRef]
- Ramanujam, A.; Neyhouse, B.; Keogh, R.A.; Muthuvel, M.; Carroll, R.K.; Botte, G.G. Rapid Electrochemical Detection of Escherichia coli Using Nickel Oxidation Reaction on a Rotating Disk Electrode. Chem. Eng. J. 2021, 411, 128453. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, S.; Chen, L.; Li, M.; Zhang, Y.; Zhou, N. Self-Assembled DNA Nanoflowers Triggered by a DNA Walker for Highly Sensitive Electrochemical Detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2021, 13, 4905–4914. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, Q.; Meng, X.; Yan, C.; Yao, B.; Chen, Z.; Wang, Z.; Chen, W. CRISPR/Cas12a-Mediated Cyclic Signal Amplification and Electrochemical Reporting Strategy for Rapid and Accurate Sensing of Vibrio Parahaemolyticus in Aquatic Foods. Biosens. Bioelectron. 2025, 277, 117284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, K.; Lin, J. Optical Biosensors for the Detection of Foodborne Pathogens: Recent Development and Future Prospects. TrAC Trends Anal. Chem. 2024, 177, 117785. [Google Scholar] [CrossRef]
- Dadmehr, M.; Sangachin, E.A.; Bazzi, F.; Li, J.; Hosseini, M. DNA Integrated Nanostructures for Optical-Based Detection of Foodborne Contaminants. TrAC Trends Anal. Chem. 2024, 178, 117836. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, M.; Peng, T.; Zhang, P.; Shen, R.; Jia, Y. Detection and Discrimination of Pathogenic Bacteria with Nanomaterials-Based Optical Biosensors: A Review. Food Chem. 2023, 426, 136578. [Google Scholar] [CrossRef]
- Qin, J.; Guo, N.; Yang, J.; Wei, J. Recent Advances in Metal Oxide Nanozyme-Based Optical Biosensors for Food Safety Assays. Food Chem. 2024, 447, 139019. [Google Scholar] [CrossRef]
- Sagar Shrikrishna, N.; Sharma, R.; Sahoo, J.; Kaushik, A.; Gandhi, S. Navigating the Landscape of Optical Biosensors. Chem. Eng. J. 2024, 490, 151661. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; He, J.; Zhang, Z.; Chai, Y.; Yuan, R.; Xu, W. Dynamic Switching Circuit Modulated by Intramolecular Conformation Transition of DNA Translator for Versatile Fluorescence Biosensors. Biosens. Bioelectron. 2025, 279, 117404. [Google Scholar] [CrossRef]
- Wang, L.; Ji, Y.; Chen, Y.; Zheng, S.; Wang, F.; Li, C. Recent Research Progress of Fluorescence Biosensors Based on Carbon Dots in Early Diagnosis of Diseases. TrAC Trends Anal. Chem. 2024, 180, 117962. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal Organic Framework Based Fluorescence Sensor for Detection of Antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Rong, Y.; Ali, S.; Ouyang, Q.; Wang, L.; Wang, B.; Chen, Q. A Turn-on Upconversion Fluorescence Sensor for Acrylamide in Potato Chips Based on Fluorescence Resonance Energy Transfer and Thiol-Ene Michael Addition. Food Chem. 2021, 351, 129215. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Liu, C.; Nan, X.; Zhu, Y.; Fu, L.; Lin, X.; Zhang, H.; Yang, M.; Fang, X.; Luo, Y.; et al. Carbon Dots-Based Electrochemical and Fluorescent Biosensors for the Detection of Foodborne Pathogens: Current Advance and Challenge. Coord. Chem. Rev. 2025, 529, 216457. [Google Scholar] [CrossRef]
- Li, H.; Ahmad, W.; Rong, Y.; Chen, Q.; Zuo, M.; Ouyang, Q.; Guo, Z. Designing an Aptamer Based Magnetic and Upconversion Nanoparticles Conjugated Fluorescence Sensor for Screening Escherichia coli in Food. Food Control 2020, 107, 106761. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Pan, W.; Chen, Q.; Ouyang, Q.; Zhao, J. Dual-Color Upconversion Nanoparticles (UCNPs)-Based Fluorescent Immunoassay Probes for Sensitive Sensing Foodborne Pathogens. Food Anal. Methods 2017, 10, 2036–2045. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A Highly Sensitive Detection of Carbendazim Pesticide in Food Based on the Upconversion-MnO2 Luminescent Resonance Energy Transfer Biosensor. Food Chem. 2021, 349, 129157. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, Q.; Liu, X.; Wang, Y.; Wang, J.; Wang, X. An Ultrasensitive Fluorescence Nano-Biosensor Based on RBP 41-Quantum Dot Microspheres for Rapid Detection of Salmonella in the Food Matrices. Food Chem. 2025, 468, 142504. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Liang, M.; Li, X.; Xiao, H.; Cao, D.; Zhao, X. Ratiometric Fluorescence Sensor for Escherichia coli Detection Using Fluorescein Isothiocyanate–Labeled Metal–Organic Frameworks. Microchim. Acta 2025, 192, 188. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Liu, L.; Jia, M.; Li, X.; Li, J. Nano-Biosensor Based on Manganese Dioxide Nanosheets and Carbon Dots for Dual-Mode Determination of Staphylococcus aureus. Food Chem. 2024, 432, 137144. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Z.; Xie, J.; Zhu, Z.; Feng, Y.; Yu, S.; Xue, L.; Wu, S.; Gu, Q.; Zhang, J.; et al. Dual-Mode Immunochromatographic Assay Based on Dendritic Gold Nanoparticles with Superior Fluorescence Quenching for Ultrasensitive Detection of E. coli O157:H7. Food Chem. 2023, 424, 136366. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, Y.; Xu, J.; Wu, H.; Li, T.; Yu, Z.; Huang, S.; Gan, N. A Multi-Channel Microfluidic Chip Based on Fluorescent Distance Readout-Mode for Rapid, Simultaneous and Visual Detection of Multiplex Pathogens Using Phage- AIEgen-Antimicrobial Peptide-Encoded Tags. Sens. Actuators B Chem. 2025, 424, 136867. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Huang, X.; Huang, Q.; Wen, Y.; Li, B.; Holmes, M.; Shi, J.; Zou, X. Uniform Stain Pattern of Robust MOF-Mediated Probe for Flexible Paper-Based Colorimetric Sensing toward Environmental Pesticide Exposure. Chem. Eng. J. 2023, 451, 138928. [Google Scholar] [CrossRef]
- Zhu, W.; Li, L.; Zhou, Z.; Yang, X.; Hao, N.; Guo, Y.; Wang, K. A Colorimetric Biosensor for Simultaneous Ochratoxin A and Aflatoxins B1 Detection in Agricultural Products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, H.; Lin, J.; Yao-Say Solomon Adade, S.; Chen, Q.; Xue, Z.; Chan, C. Non-Destructive Detection of Multi-Component Heavy Metals in Corn Oil Using Nano-Modified Colorimetric Sensor Combined with near-Infrared Spectroscopy. Food Control 2022, 133, 108640. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric Aptasensor for the Detection of Salmonella Enterica Serovar Typhimurium Using ZnFe2O4 -Reduced Graphene Oxide Nanostructures as an Effective Peroxidase Mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- Qi, W.; Zheng, L.; Hou, Y.; Duan, H.; Wang, L.; Wang, S.; Liu, Y.; Li, Y.; Liao, M.; Lin, J. A Finger-Actuated Microfluidic Biosensor for Colorimetric Detection of Foodborne Pathogens. Food Chem. 2022, 381, 131801. [Google Scholar] [CrossRef]
- Lin, H.; Wang, F.; Lin, J.; Yang, W.; Kang, W.; Jiang, H.; Adade, S.Y.-S.S.; Cai, J.; Xue, Z.; Chen, Q. Detection of Wheat Toxigenic Aspergillus Flavus Based on Nano-Composite Colorimetric Sensing Technology. Food Chem. 2023, 405, 134803. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, L.; Li, W.; Xu, X.; Xu, Q.; Xu, H. Sea Cucumber-Inspired Self-Assembly Nanozyme for Ultrasensitive and Tri-Modal Colorimetric Quantification of Pathogenic Bacteria. Sens. Actuators B Chem. 2025, 431, 137314. [Google Scholar] [CrossRef]
- Lin, H.; Man, Z.; Kang, W.; Guan, B.; Chen, Q.; Xue, Z. A Novel Colorimetric Sensor Array Based on Boron-Dipyrromethene Dyes for Monitoring the Storage Time of Rice. Food Chem. 2018, 268, 300–306. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Sun, H.; Wang, M.; Chen, Q.; Guo, Z.; Wu, J. Rapid Pseudomonas Species Identification from Chicken by Integrating Colorimetric Sensors with Near-Infrared Spectroscopy. Food Anal. Methods 2018, 11, 1199–1208. [Google Scholar] [CrossRef]
- Guan, B.; Zhao, J.; Jin, H.; Lin, H. Determination of Rice Storage Time with Colorimetric Sensor Array. Food Anal. Methods 2017, 10, 1054–1062. [Google Scholar] [CrossRef]
- Jin, N.; Xue, L.; Ding, Y.; Liu, Y.; Jiang, F.; Liao, M.; Li, Y.; Lin, J. A Microfluidic Biosensor Based on Finger-Driven Mixing and Nuclear Track Membrane Filtration for Fast and Sensitive Detection of Salmonella. Biosens. Bioelectron. 2023, 220, 114844. [Google Scholar] [CrossRef]
- Špringer, T.; Bocková, M.; Slabý, J.; Sohrabi, F.; Čapková, M.; Homola, J. Surface Plasmon Resonance Biosensors and Their Medical Applications. Biosens. Bioelectron. 2025, 278, 117308. [Google Scholar] [CrossRef]
- Islam, M.A.; Masson, J.-F. Plasmonic Biosensors for Health Monitoring: Inflammation Biomarker Detection. ACS Sens. 2025, 10, 577–601. [Google Scholar] [CrossRef]
- Balbinot, S.; Srivastav, A.M.; Vidic, J.; Abdulhalim, I.; Manzano, M. Plasmonic Biosensors for Food Control. Trends Food Sci. Technol. 2021, 111, 128–140. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Li, M.; Sun, C.; Ren, D.; Li, Y. Fiber Optic Surface Plasmon Resonance Sensor for Detection of E. coli O157:H7 Based on Antimicrobial Peptides and AgNPs-rGO. Biosens. Bioelectron. 2018, 117, 347–353. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Liu, D.; An, J.; Zhang, M.; Hua Li, J.; Jiang, C. Plasmonic AgNPs Reinforced Flexible Hydrogel Surface-Enhanced Raman Scattering (SERS) Sensor for in-Situ Detection of Curved Samples. Chem. Eng. J. 2024, 494, 153082. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, R.; Chen, H.; Zhang, X. Recent Advances in Food Safety: Nanostructure-Sensitized Surface-Enhanced Raman Sensing. Foods 2025, 14, 1115. [Google Scholar] [CrossRef] [PubMed]
- Kutsanedzie, F.Y.H.; Agyekum, A.A.; Annavaram, V.; Chen, Q. Signal-Enhanced SERS-Sensors of CAR-PLS and GA-PLS Coupled AgNPs for Ochratoxin A and Aflatoxin B1 Detection. Food Chem. 2020, 315, 126231. [Google Scholar] [CrossRef]
- Su, T.; Chang, Y.; Lu, M.; Lin, X.; Ning, Z.; Wu, S.; Wang, Z.; Duan, N. Bimetallic Loaded ZIF-8 with Peroxidase-like and Photothermal Activities for Sensitive Detection and Efficient Elimination of Listeria Monocytogenes. Chem. Eng. J. 2024, 497, 154918. [Google Scholar] [CrossRef]
- Hassan, M.M.; Ahmad, W.; Zareef, M.; Rong, Y.; Xu, Y.; Jiao, T.; He, P.; Li, H.; Chen, Q. Rapid Detection of Mercury in Food via Rhodamine 6G Signal Using Surface-Enhanced Raman Scattering Coupled Multivariate Calibration. Food Chem. 2021, 358, 129844. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Ouyang, Q.; Chen, Q. Advances in Surface-enhanced Raman Spectroscopy Technology for Detection of Foodborne Pathogens. Comp. Rev. Food Sci. Food Safe 2023, 22, 1466–1494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ahmad, W.; Chen, M.; Wang, J.; Jiao, T.; Wei, J.; Chen, Q.; Li, D.; Chen, X.; Chen, Q. Active Capture-Directed Bimetallic Nanosubstrate for Enhanced SERS Detection of Staphylococcus aureus by Combining Strand Exchange Amplification and Wavelength-Selective Machine Learning. Biosens. Bioelectron. 2025, 278, 117363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, Y.; Shi, J.; Zhang, W.; Zhang, X.; Huang, X.; Zou, X.; Li, Z.; Wei, R. Facile Synthesis of Au@Ag Core–Shell Nanorod with Bimetallic Synergistic Effect for SERS Detection of Thiabendazole in Fruit Juice. Food Chem. 2022, 370, 131276. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Zheng, Z.; Haruna, S.A.; Chen, Q. Flexible SERS Sensor Using AuNTs-Assembled PDMS Film Coupled Chemometric Algorithms for Rapid Detection of Chloramphenicol in Food. Food Chem. 2023, 418, 135998. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. Salmonella typhimurium Detection Using a Surface-Enhanced Raman Scattering-Based Aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef]
- Wei, W.; Haruna, S.A.; Zhao, Y.; Li, H.; Chen, Q. Surface-Enhanced Raman Scattering Biosensor-Based Sandwich-Type for Facile and Sensitive Detection of Staphylococcus aureus. Sens. Actuators B Chem. 2022, 364, 131929. [Google Scholar] [CrossRef]
- Draz, M.S.; Lu, X. Development of a Loop Mediated Isothermal Amplification (LAMP)—Surface Enhanced Raman Spectroscopy (SERS) Assay for the Detection of Salmonella Enterica Serotype Enteritidis. Theranostics 2016, 6, 522–532. [Google Scholar] [CrossRef]
- Qiu, J.; Zhong, Y.; Shao, Y.; Zhang, G.; Yang, J.; Li, Z.; Cheng, Y. A Dendrimer-Based Platform Integrating Surface-Enhanced Raman Scattering and Class-Incremental Learning for Rapidly Detecting Four Pathogenic Bacteria. Chem. Eng. J. 2024, 499, 155987. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, P.; Zhai, J.; Wu, B.; Chen, X.; Ding, F.; Chen, Q.; Sun, B. Efficient Detection of Foodborne Pathogens via SERS and Deep Learning: An ADMIN-Optimized NAS-Unet Approach. J. Hazard. Mater. 2025, 489, 137581. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, A.K.; Rani, S.; Kumar, P.; Malik, A.; Gupta, S. Simultaneous Detection and Differentiation of Common Foodborne Pathogens Using Tri-Metallic Magnetic Microspheres as an Aluminium Foil Based SERS Substrate. Anal. Methods 2025, 17, 5176–5185. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hassan, M.M.; Zhu, A.; Li, H.; Chen, Q. Dual-Mode of Magnetic Assisted Au@Ag SERS Tags and Cationic Conjugated UCNPs for Qualitative and Quantitative Analysis of Multiple Foodborne Pathogens. Sens. Actuators B Chem. 2021, 344, 130305. [Google Scholar] [CrossRef]
- Pascual-Garrigos, A.; Lozano-Torres, B.; Das, A.; Molloy, J.C. Colorimetric CRISPR Biosensor: A Case Study with Salmonella Typhi. ACS Sens. 2025, 10, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Yuan, J.; Wang, L.; Li, M.; Lin, J. A Pd/Pt Nanocluster Enhanced Colorimetric Biosensor for Large-Volume Salmonella Detection. LWT 2025, 215, 117182. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, Q.; Zhao, Q.; Liu, K.; Bo, Q.; Qin, X.; Yan, C.; Huang, L.; Chen, W.; Qin, P. Using dCas9 as an Intermediate Bridge of Loop-Mediated Isothermal Amplification-Based Lateral Flow Colorimetric Biosensor for Point-of-Care Salmonella Detection. Sens. Actuators B Chem. 2023, 396, 134581. [Google Scholar] [CrossRef]
- Jia, F.; Li, B.; He, Y.; Shen, Y.; Chen, J.; Li, X.; Li, Y. An Amplification-Free CRISPR-SERS Biosensor for Specific, Sensitive and Rapid Detection of Salmonella typhimurium in Poultry. LWT 2023, 189, 115476. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Z.; Niu, X.; Zhan, X.; Tao, H.; Wu, Y. Novel Nanozyme-Catalyzed and Magnetically Assisted Colorimetric Biosensor for Staphylococcus aureus Detection with a Low Matrix Effect from Complex Environments. Sens. Actuators B Chem. 2022, 373, 132752. [Google Scholar] [CrossRef]
- Liu, R.; Haruna, S.A.; Ali, S.; Xu, J.; Zhang, Y.; Lü, P.; Li, H.; Chen, Q. A Sensitive and Accurate Fluorescent Genosensor for Staphylococcus aureus Detection. Sens. Actuators B Chem. 2022, 355, 131311. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Y.; Liu, Y.; Feng, X.; Dong, Q.; Li, J.; Wang, J.; Zhao, C. A Concise Detection Strategy of Staphylococcus aureus Using N-Succinyl-Chitosan-Dopped Bacteria-Imprinted Composite Film and AIE Fluorescence Sensor. J. Hazard. Mater. 2022, 423, 126934. [Google Scholar] [CrossRef]
- Guo, W.; Guo, Y.; Xu, H.; Li, C.; Zhang, X.; Zou, X.; Sun, Z. Ultrasensitive “On–Off” Ratiometric Fluorescence Biosensor Based on RPA-CRISPR/Cas12a for Detection of Staphylococcus aureus. J. Agric. Food Chem. 2025, 73, 2167–2173. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhang, M.; Yang, Y.; Din, Z.; Chen, Q. Mesoporous Silica-Modified Upconversion Biosensor Coupled with Real-Time Ion Release Properties for Ultrasensitive Detection of Staphylococcus aureus in Meat. Food Control 2023, 145, 109444. [Google Scholar] [CrossRef]
- Xiao, F.; Li, W.; Wang, Z.; Xu, Q.; Song, Y.; Huang, J.; Bai, X.; Xu, H. Smartphone-Assisted Biosensor Based on Broom-like Bacteria-Specific Magnetic Enrichment Platform for Colorimetric Detection of Listeria Monocytogenes. J. Hazard. Mater. 2023, 459, 132250. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Z.; Li, W.; Qi, W.; Bai, X.; Xu, H. Cefepime-Modified Magnetic Nanoparticles and Enzymatic Colorimetry for the Detection of Listeria Monocytogenes in Lettuces. Food Chem. 2023, 409, 135296. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Ali, S.; Haruna, S.A.; He, P.; Li, H.; Ouyang, Q.; Chen, Q. Development of a Fluorescence Aptasensor for Rapid and Sensitive Detection of Listeria Monocytogenes in Food. Food Control 2021, 122, 107808. [Google Scholar] [CrossRef]
- Li, M.; Yang, F.; Jia, K.; Zhang, Q.; Zheng, W.; Chen, H.; Liao, M.; Lin, J.; Wang, L. Bacterial Bioaerosol-Specific Capture and In Situ Detection Using an Immune ZIF-8-Melamine Foam-Functionalized Colorimetric Biosensor. ACS Appl. Mater. Interfaces 2025, 17, 9669–9679. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Tian, Y.; Wang, H.; Yang, J.; Qu, B.; Jiang, Y.; Zhao, Q.; Zhang, X.; Man, C. CRISPR/Cas System Meets CLICK-17 DNAzyme: A Click Chemistry-Based Fluorescence Biosensing Platform Designed for Highly Sensitive Detection of Salmonella. Anal. Chem. 2025, 97, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, F.; Zhang, L.; Zhao, C.; Li, J.; Wang, J. A Portable Smartphone-Assisted Highly Emissive Magnetic Covalent Organic Framework-Based Fluorescence Sensor for the Detection of Salmonella typhimurium. Sens. Actuators B Chem. 2023, 392, 134076. [Google Scholar] [CrossRef]
- Ding, Y.; Yuan, J.; Wang, L.; Jin, N.; Wang, S.; Li, Y.; Lin, J. Semi-Circle Magnetophoretic Separation under Rotated Magnetic Field for Colorimetric Biosensing of Salmonella. Biosens. Bioelectron. 2023, 229, 115230. [Google Scholar] [CrossRef]
- Kan, L.; Zhao, W.; Liu, M.; Pan, W.; Li, D.; Zhao, Y.; Jiang, L. Ratiometric SERS Sensor for Sensitive Quantification of Methicillin-Resistant Staphylococcus aureus Using Ti3C2@AuNP Films and Aptamer-Based Tags. Appl. Surf. Sci. 2025, 690, 162639. [Google Scholar] [CrossRef]
- Wu, S.; Sheng, L.; Kou, G.; Tian, R.; Ye, Y.; Wang, W.; Sun, J.; Ji, J.; Shao, J.; Zhang, Y.; et al. Double Phage Displayed Peptides Co-Targeting-Based Biosensor with Signal Enhancement Activity for Colorimetric Detection of Staphylococcus aureus. Biosens. Bioelectron. 2024, 249, 116005. [Google Scholar] [CrossRef]
- Dai, J.; Li, J.; Jiao, Y.; Yang, X.; Yang, D.; Zhong, Z.; Li, H.; Yang, Y. Colorimetric-SERS Dual-Mode Aptasensor for Staphylococcus aureus Based on MnO2@AuNPs Oxidase-like Activity. Food Chem. 2024, 456, 139955. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liu, Q.; Cheng, N. Sea Hedgehog-Inspired Surface-Enhanced Raman Scattering Biosensor Probe for Ultrasensitive Determination of Staphylococcus aureus in Food Supplements. Biosens. Bioelectron. 2024, 252, 116146. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, W.; Wu, Y.; Deng, A.; Yuan, L.; Gao, Y.; Li, H.; Wang, Z.; Wang, B.; Zhu, G.; et al. Characterisation of a Colourimetric Biosensor SapYZUM13@Mn3O4-NH2 Reveals the Mechanisms Underlying Its Rapid and Sensitive Detection of Viable Staphylococcus aureus in Food. Food Chem. 2024, 457, 140189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Wang, Z.; Peng, L.; Xu, Y.; Jiao, T.; Ouyang, Q.; Chen, Q. Sensitive SERS Detection of S. Aureus via HCR-Mediated G-Quadruplex DNAzyme Assembly. Sens. Actuators B Chem. 2025, 425, 136977. [Google Scholar] [CrossRef]
- Zhou, B.; Ye, Q.; Li, F.; Xiang, X.; Shang, Y.; Wang, C.; Shao, Y.; Xue, L.; Zhang, J.; Wang, J.; et al. CRISPR/Cas12a Based Fluorescence-Enhanced Lateral Flow Biosensor for Detection of Staphylococcus aureus. Sens. Actuators B Chem. 2022, 351, 130906. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, B.; Gao, T.; Xu, X.; Du, Z.; Han, J.; Hu, Y.; Bai, Y.; Wang, L.; Wang, C.; et al. A Novel Microfluidic Colorimetric Biosensor for Rapid and Automatic Detection Escherichia coli O157:H7 in Aquaponics Water. Comput. Electron. Agric. 2025, 229, 109941. [Google Scholar] [CrossRef]
- Pan, B.; El-Moghazy, A.Y.; Norwood, M.; Nitin, N.; Sun, G. Rapid and Ultrasensitive Colorimetric Biosensors for Onsite Detection of Escherichia coli O157:H7 in Fluids. ACS Sens. 2024, 9, 912–922. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Guo, Z.; Yang, T.; Zhang, X.; Huang, X.; Shi, J.; Gao, S.; Zou, X. Ultrasensitive Analysis of Escherichia coli O157:H7 Based on Immunomagnetic Separation and Labeled Surface-Enhanced Raman Scattering with Minimized False Positive Identifications. J. Agric. Food Chem. 2024, 72, 22349–22359. [Google Scholar] [CrossRef]
- Wang, S.; Liang, N.; Hu, X.; Li, W.; Guo, Z.; Zhang, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J. Carbon Dots and Covalent Organic Frameworks Based FRET Immunosensor for Sensitive Detection of Escherichia coli O157:H7. Food Chem. 2024, 447, 138663. [Google Scholar] [CrossRef]
- Song, D.; Han, X.; Xu, W.; Liu, J.; Zhuo, Y.; Zhu, A.; Long, F. Target Nucleic Acid Amplification-Free Detection of Escherichia coli O157:H7 by CRISPR/Cas12a and Hybridization Chain Reaction Based on an Evanescent Wave Fluorescence Biosensor. Sens. Actuators B Chem. 2023, 376, 133005. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, P.; Yan, Z.; Zhou, Y.; Du, Y.; Qu, C.; Song, Y.; Zhou, D.; Qu, S.; et al. Cell-Based Fluorescent Microsphere Incorporated with Carbon Dots as a Sensitive Immunosensor for the Rapid Detection of Escherichia coli O157 in Milk. Biosens. Bioelectron. 2021, 179, 113057. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Xu, Y.; Gan, Z.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Li, Y. Green One-Step Synthesis of Carbon Quantum Dots from Orange Peel for Fluorescent Detection of Escherichia coli in Milk. Food Chem. 2021, 339, 127775. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, L.; Gong, L.; Ouyang, Y.; Yang, T.; Sun, T.; Zeng, H. Rapid and Accurate Sensing Platform Based on Lanthanide Metal-Organic Framework and Aptamer for Vibrio Parahaemolyticus Diagnosis. Sens. Actuators B Chem. 2023, 389, 133881. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Wu, J.; Ying, D.; Duan, N.; Wang, Z.; Wu, S. Multifunctional Magnetic Composite Nanomaterial for Colorimetric-SERS Dual-Mode Detection and Photothermal Sterilization of Vibrio Parahaemolyticus. Chem. Eng. J. 2023, 477, 147113. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Li, G.; Du, J.; Xie, C.; Li, H.; Jin, Y.; He, Y. Hierarchically Structured Hollow PVDF Nanofibers for Flexible Piezoelectric Sensor. Chem. Eng. J. 2024, 498, 155661. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, F.; Zhang, C.; Sheng, X.; Zhong, Y.; Chong, H.; Yang, Z.; Wang, C. Detection of Avian Influenza Virus H9N2 Based on Self-Driving and Self-Sensing Microcantilever Piezoelectric Sensor. Chin. Chem. Lett. 2023, 34, 107700. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, J.; He, F. A New Aptamer/Polyadenylated DNA Interdigitated Gold Electrode Piezoelectric Sensor for Rapid Detection of Pseudomonas Aeruginosa. Biosens. Bioelectron. 2019, 132, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zheng, C.; Zhu, L.; Wang, J. A Review on Rapid Detection of Modified Quartz Crystal Microbalance Sensors for Food: Contamination, Flavour and Adulteration. TrAC Trends Anal. Chem. 2022, 157, 116805. [Google Scholar] [CrossRef]
- Wachiralurpan, S.; Chansiri, K.; Lieberzeit, P.A. Direct Detection of Listeria Monocytogenes DNA Amplification Products with Quartz Crystal Microbalances at Elevated Temperatures. Sens. Actuators B Chem. 2020, 308, 127678. [Google Scholar] [CrossRef]
- Beyazit, F.; Arica, M.Y.; Acikgoz-Erkaya, I.; Ozalp, C.; Bayramoglu, G. Quartz Crystal Microbalance–Based Aptasensor Integrated with Magnetic Pre-Concentration System for Detection of Listeria Monocytogenes in Food Samples. Microchim. Acta 2024, 191, 235. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Fu, W.; Lv, H.; Zu, X.; Guo, Y.; Gibson, D.; Fu, Y.-Q. Advances in Sensing Mechanisms and Micro/Nanostructured Sensing Layers for Surface Acoustic Wave-Based Gas Sensors. J. Mater. Chem. A 2023, 11, 9216–9238. [Google Scholar] [CrossRef]
- Sun, X.; Chen, T.; Liang, Y.; Zhang, C.; Zhai, S.; Sun, J.; Wang, W. Enhanced Sensitivity of SAW Based Ammonia Sensor Employing GO-SnO2 Nanocomposites. Sens. Actuators B Chem. 2023, 375, 132884. [Google Scholar] [CrossRef]
- Lamanna, L.; Rizzi, F.; Bhethanabotla, V.R.; De Vittorio, M. Conformable Surface Acoustic Wave Biosensor for E-coli Fabricated on PEN Plastic Film. Biosens. Bioelectron. 2020, 163, 112164. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, J.; Zhang, J.; Guo, Y.; Ji, Z.; Chen, H.; Fu, Y. Monitoring Gene Sequences of Staphylococcus aureus Using a Love-Mode Surface Acoustic Wave Biosensor Coated with Cellulose Acetate/Polyethylenimine Nanofibers and Au Nanoparticles. ACS Sens. 2024, 9, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.; Jirapanjawat, T.; Tay, K.P.; Ashtiani, D.; Greening, C.; Tuck, K.L.; Neild, A.; Cadarso, V.J. Rapid Concentration and Detection of Bacteria in Milk Using a Microfluidic Surface Acoustic Wave Activated Nanosieve. ACS Sens. 2024, 9, 3105–3114. [Google Scholar] [CrossRef] [PubMed]

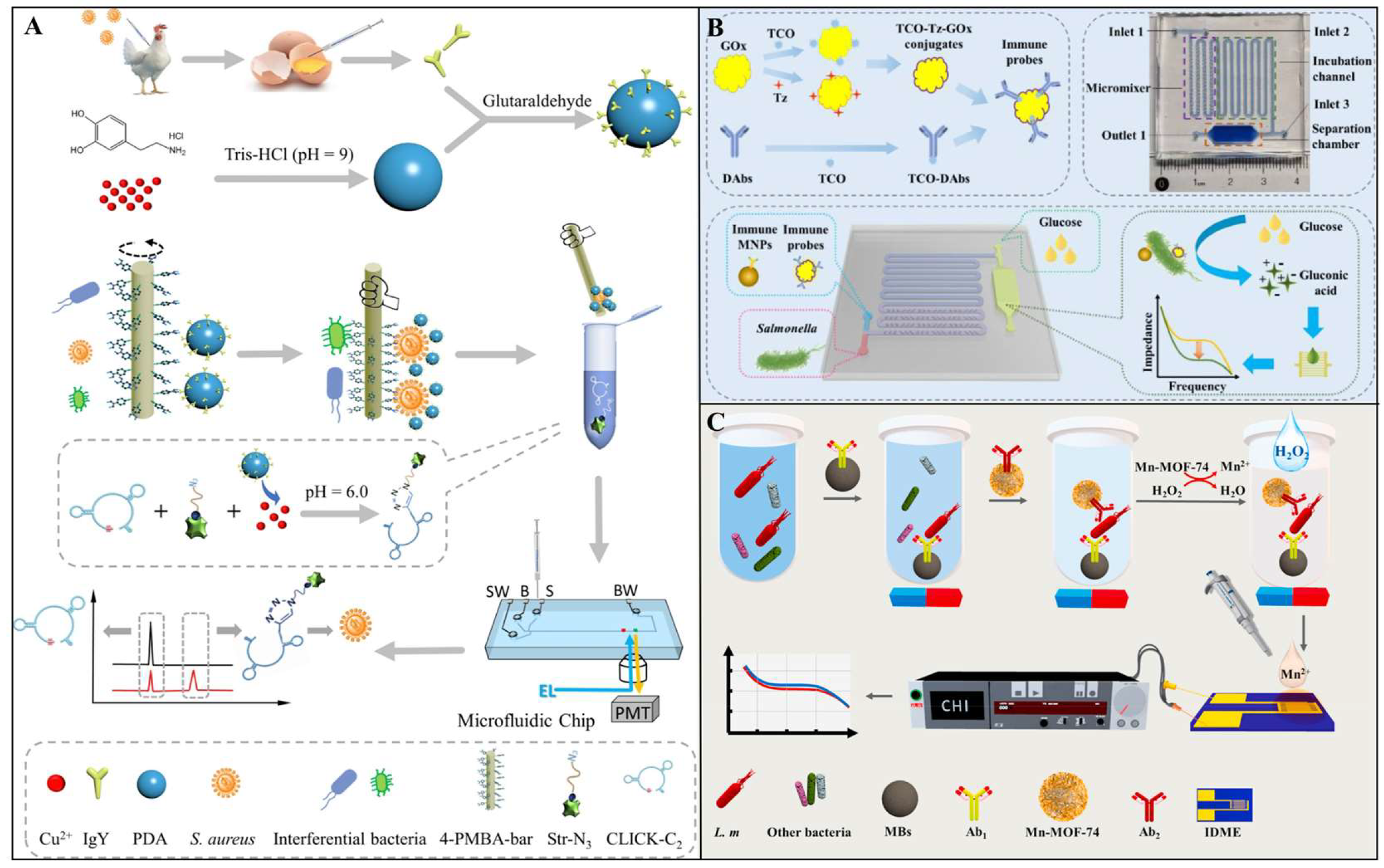
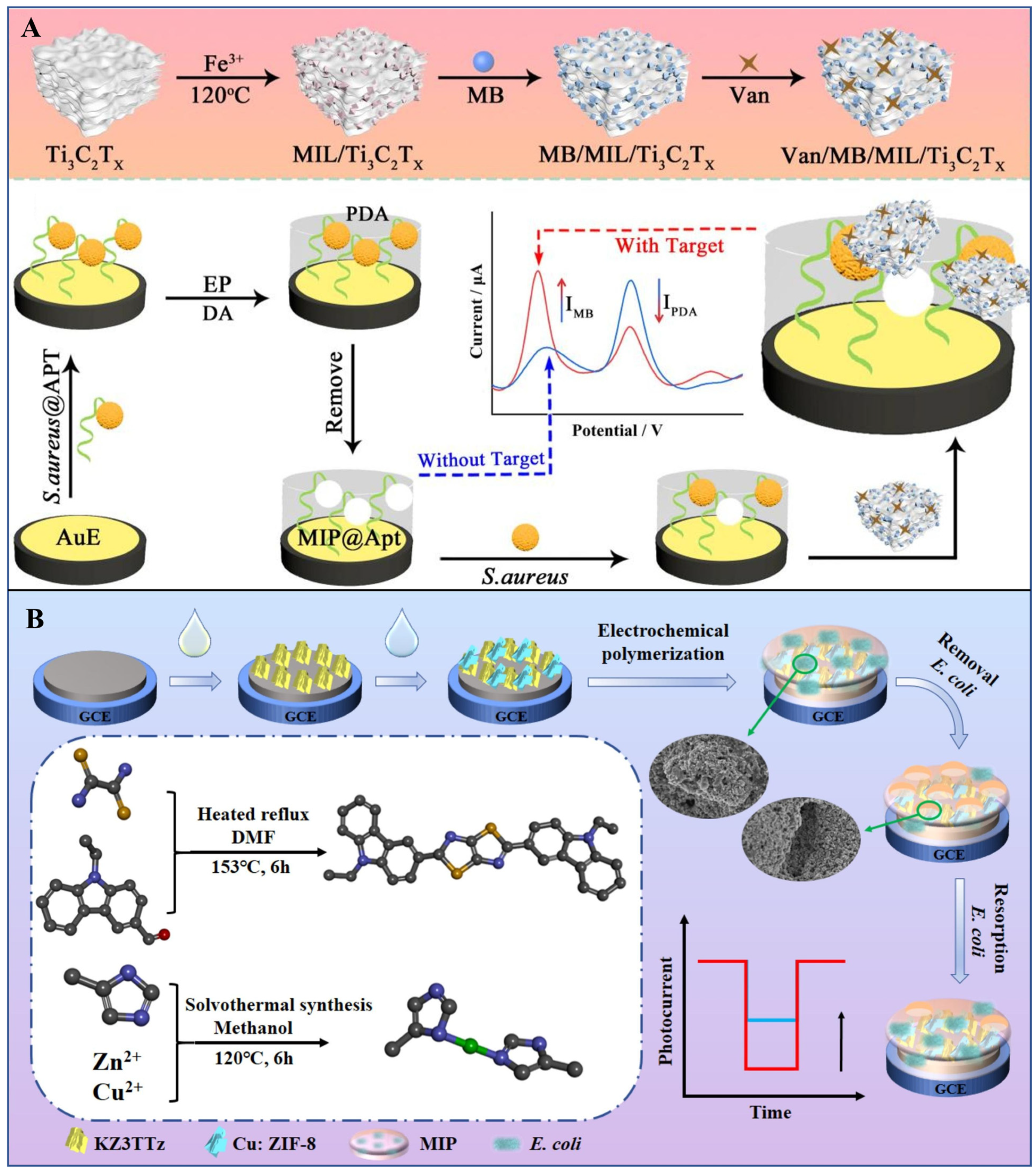
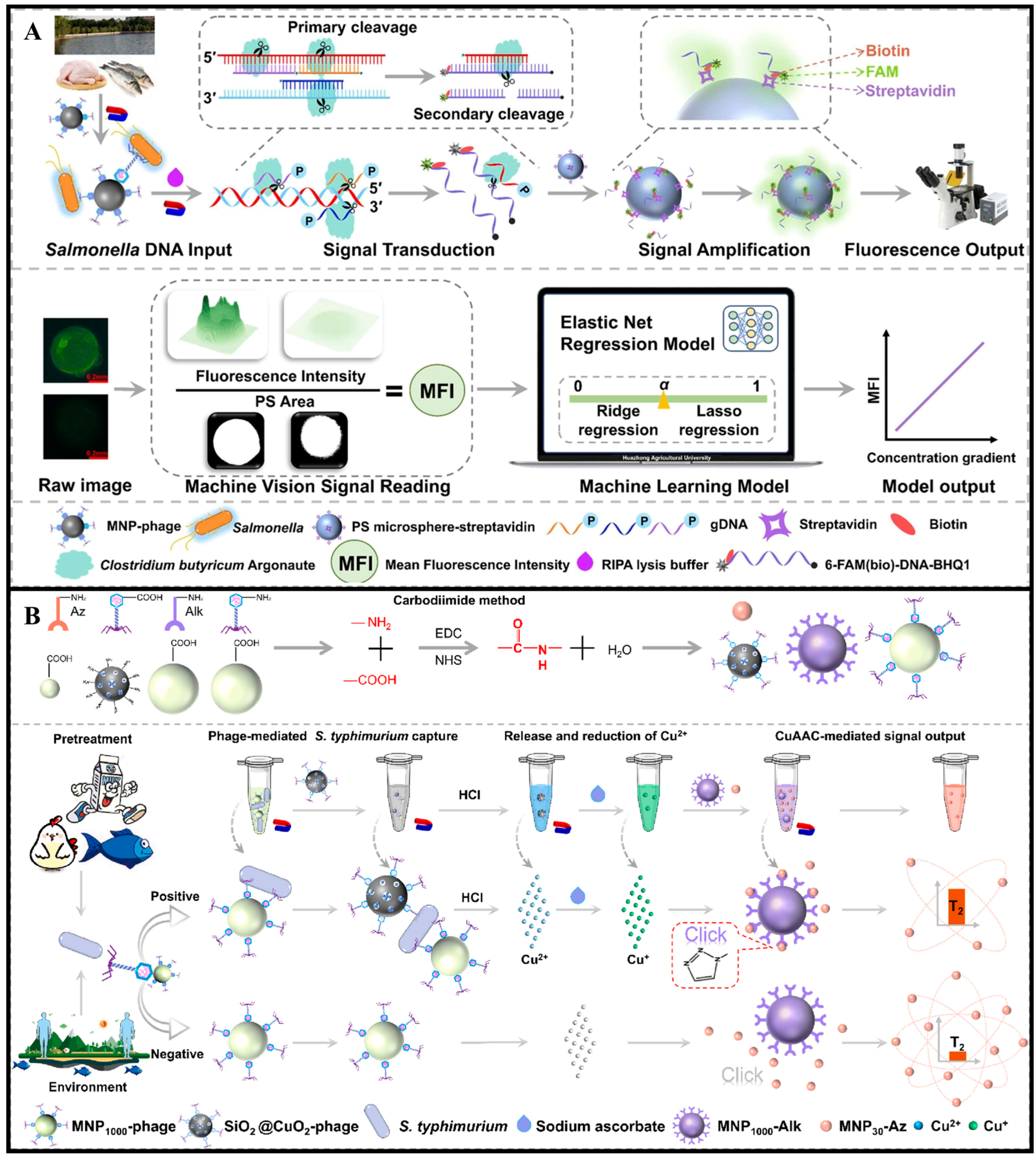
| Biorecognition Element | Recognition Mechanism | Key Features | Advantages | Limitations |
|---|---|---|---|---|
| Antibodies | Specific antigen–antibody binding | Y-shaped proteins with high affinity | High specificity; widely used; commercial availability | Poor stability to pH/temperature; high production cost |
| Aptamers | Target-induced conformational binding via nucleic acid sequences | Synthetic single-stranded DNA/RNA | Chemically stable; easily modified; cost-effective | Structural instability; off-target binding; SELEX selection is time-consuming |
| Enzymes | Catalytic reaction with target substrate | Biological catalysts | Signal amplification; well-characterized reactions | Sensitive to environmental conditions; short shelf life |
| Cell Receptors | Natural ligand–receptor interactions (e.g., host–pathogen mimicry) | Membrane or cytosolic proteins/glycoproteins | High biological relevance; specificity to pathogens | Complex structure; low availability; difficult immobilization |
| MIPs | Template-based molecular imprinting | Synthetic polymeric materials | High stability; low cost; suitable for harsh conditions | Lower selectivity; batch variability; complex preparation |
| Bacteriophages | Host-specific binding to bacterial surface structures | Viruses that infect specific bacteria | High specificity; self-replicating; can lyse target bacteria | Narrow host range; stability issues; limited commercial availability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Shi, J.; Liu, J.; Yuan, Z.; Gao, S. Advancing Food Safety Surveillance: Rapid and Sensitive Biosensing Technologies for Foodborne Pathogenic Bacteria. Foods 2025, 14, 2654. https://doi.org/10.3390/foods14152654
Feng Y, Shi J, Liu J, Yuan Z, Gao S. Advancing Food Safety Surveillance: Rapid and Sensitive Biosensing Technologies for Foodborne Pathogenic Bacteria. Foods. 2025; 14(15):2654. https://doi.org/10.3390/foods14152654
Chicago/Turabian StyleFeng, Yuerong, Jiyong Shi, Jiaqian Liu, Zhecong Yuan, and Shujie Gao. 2025. "Advancing Food Safety Surveillance: Rapid and Sensitive Biosensing Technologies for Foodborne Pathogenic Bacteria" Foods 14, no. 15: 2654. https://doi.org/10.3390/foods14152654
APA StyleFeng, Y., Shi, J., Liu, J., Yuan, Z., & Gao, S. (2025). Advancing Food Safety Surveillance: Rapid and Sensitive Biosensing Technologies for Foodborne Pathogenic Bacteria. Foods, 14(15), 2654. https://doi.org/10.3390/foods14152654







