Influence of Light Regimes on Production of Beneficial Pigments and Nutrients by Microalgae for Functional Plant-Based Foods
Abstract
1. Introduction
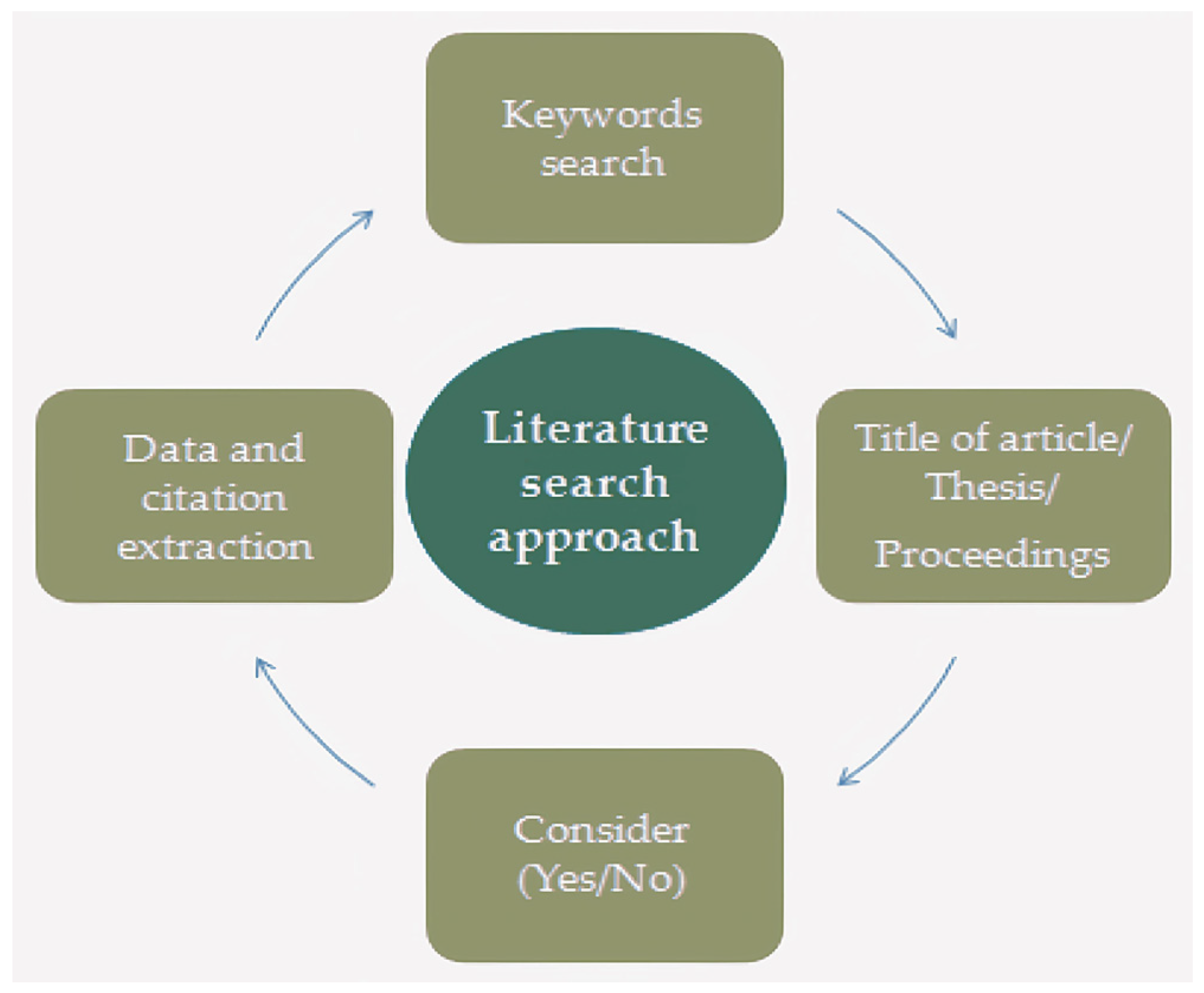
2. Methodology
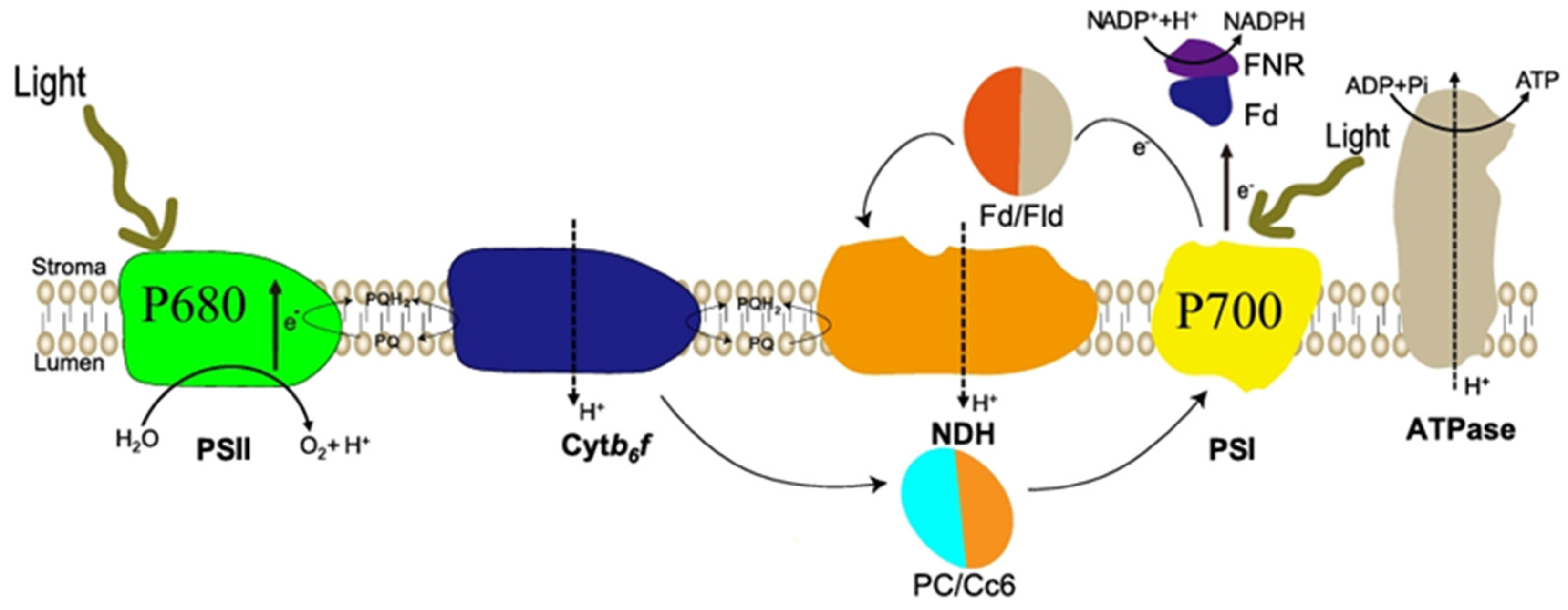
3. Effects of Light Changes on Photosynthesis and Growth of Microalgae
3.1. Microalgal Photoadaptation and Biomass Accumulation Under Variable Light Intensities
3.2. Microalgal Photoadaptation and Biomass Accumulation Under Variable Light Quality
4. Light Conditions for the High-Value Products of Microalgae Synthesis
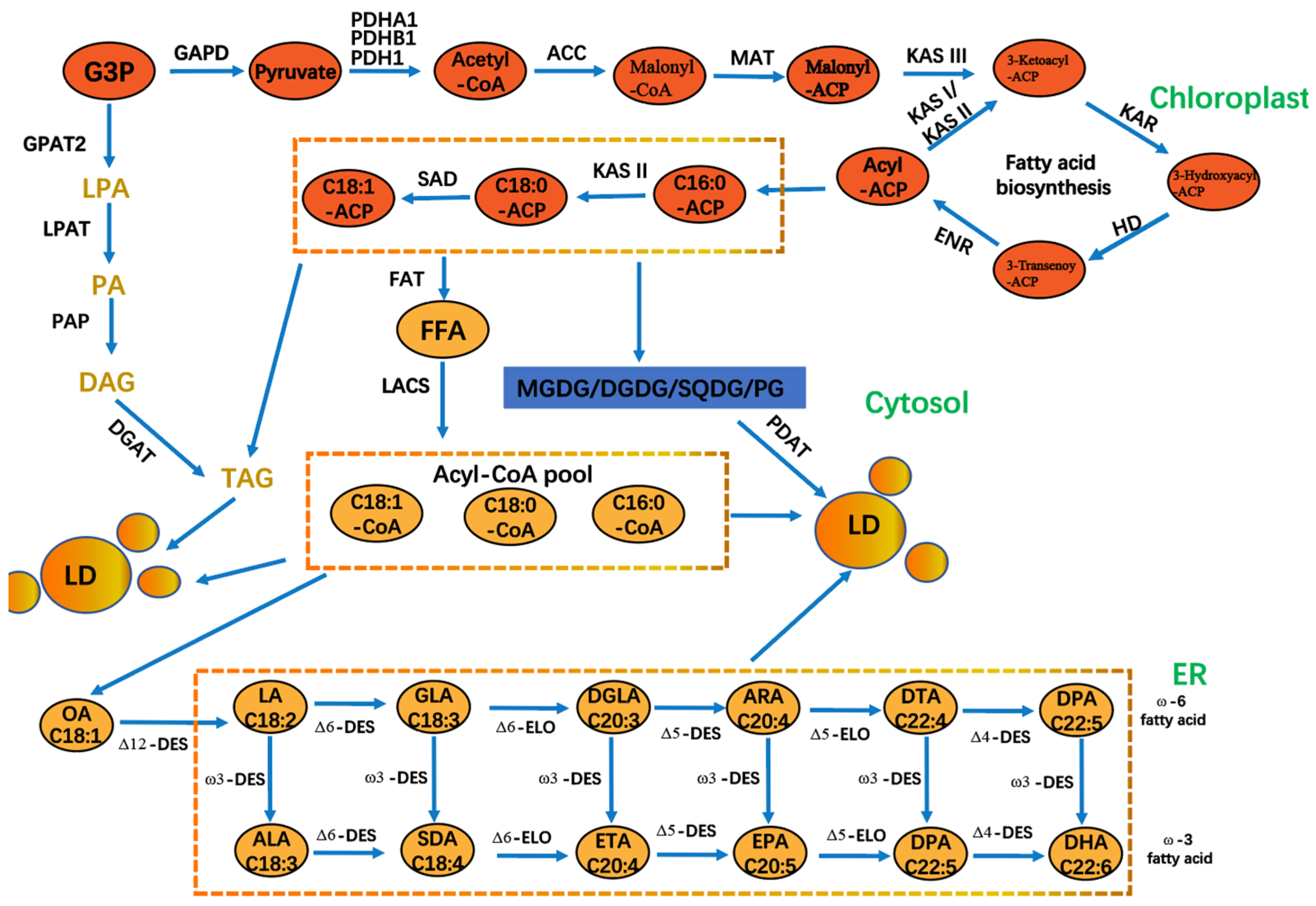
4.1. Long-Chain Unsaturated Fatty Acids
4.2. Fucoxanthin
4.3. Microalgal Protein
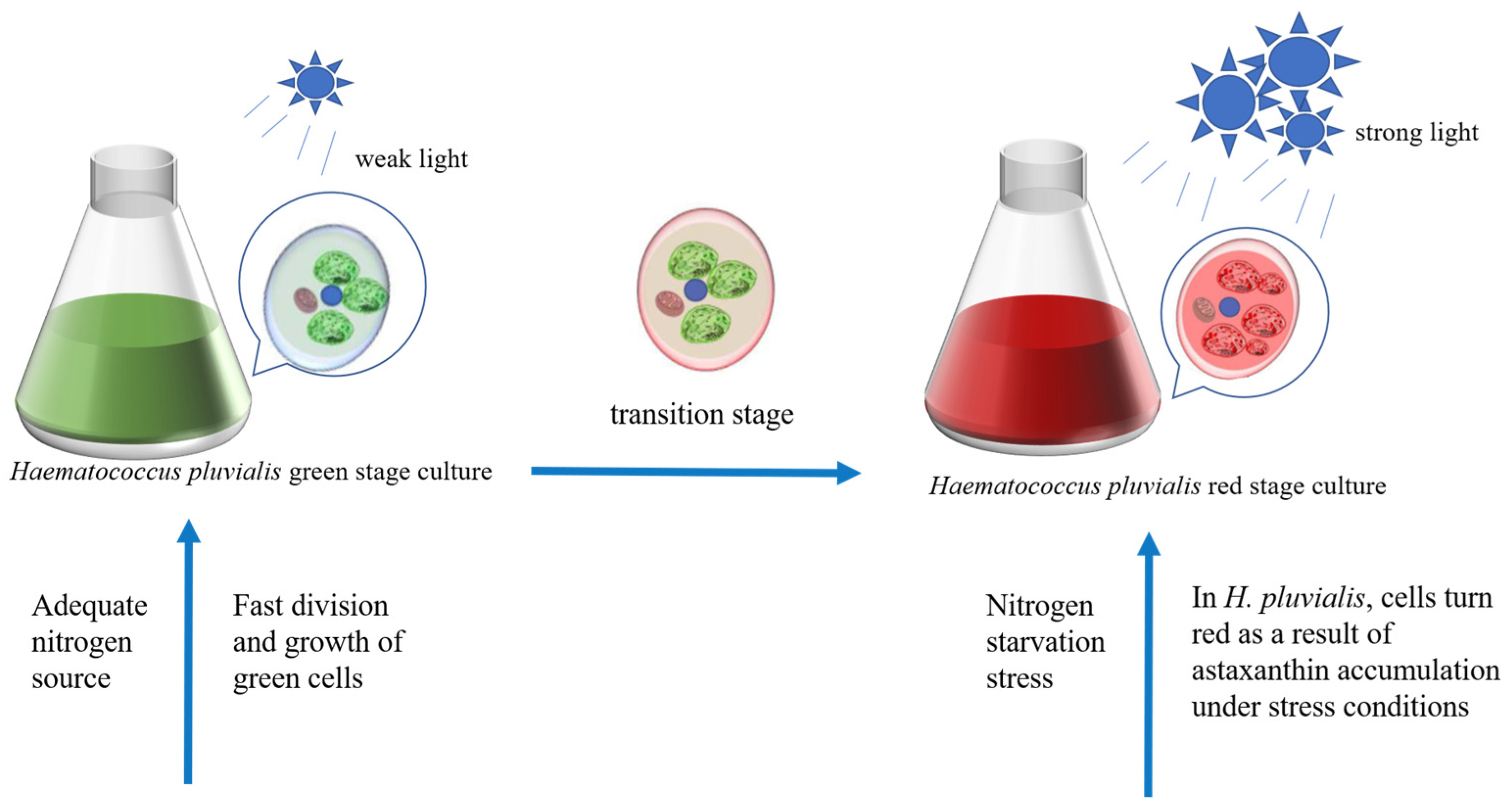
4.4. Astaxanthin
4.5. Microalgal Polysaccharides
5. Co-Regulatory Effects of Light and Other Environmental Factors
6. Safety of Microalgae in Food Applications
7. Prospects
7.1. Application of AI in Microalgae Cultivation
7.2. Limitations and Solutions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J.; Grossmann, L. Next-Generation Plant-Based Foods: Challenges and Opportunities. Annu. Rev. Food Sci. Technol. 2024, 15, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Tonsor, G.T.; Lusk, J.L. U.S. Perspective: Meat Demand Outdoes Meat Avoidance. Meat Sci. 2022, 190, 108843. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Grossmann, L. (Eds.) Meat and Fish Alternatives. In Next-Generation Plant-Based Foods: Design, Production, and Properties; Springer International Publishing: Cham, Switzerland, 2022; pp. 285–339. ISBN 978-3-030-96764-2. [Google Scholar]
- McClements, D.J.; Grossmann, L. (Eds.) Eggs and Egg Products. In Next-Generation Plant-Based Foods: Design, Production, and Properties; Springer International Publishing: Cham, Switzerland, 2022; pp. 341–388. ISBN 978-3-030-96764-2. [Google Scholar]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef]
- Shah, M.A.R.; Zhang, Y.; Cui, Y.; Hu, X.; Zhu, F.; Kumar, S.; Li, G.; Kubar, A.A.; Mehmood, S.; Huo, S. Ultrasonic-Assisted Green Extraction and Incorporation of Spirulina platensis Bioactive Components into Turmeric Essential Oil-in-Water Nanoemulsion for Enhanced Antioxidant and Antimicrobial Activities. Food Chem. 2024, 452, 139561. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Marine Algae as Efficacious Bioresources Housing Antimicrobial Compounds for Preserving Foods—A Review. Int. J. Food Microbiol. 2021, 358, 109416. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, N.; Zhu, Y.; Yang, M.; Shi, C.; Tang, Y.; Sun, W.; Sheng, K.; Liu, D.; Zhang, X. Blue Source-Based Food Alternative Proteins: Exploring Aquatic Plant-Based and Cell-Based Sources for Sustainable Nutrition. Trends Food Sci. Technol. 2024, 147, 104439. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, X.; Du, W.; Cai, Y.; Yang, Z.; Yin, Y.; Wakisaka, M.; Wang, J.; Zhou, Z.; Liu, D.; et al. Leveraging Microalgae as a Sustainable Ingredient for Meat Analogues. Food Chem. 2024, 450, 139360. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ucak, İ.; Afreen, M.; Sasidharan, A.; Yunusa, B.M.; Bhowmik, S.; Pandiselvam, R.; Ambartsumov, T.G.; Shah, M.A. Microalgae as a Potential Raw Material for Plant-Based Seafood Alternatives: A Comprehensive Review. Food Sci. Nutr. 2024, 12, 8559–8593. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Gururani, P.; Parveen, A.; Gautam, P.; Chandra Joshi, N.; Tomar, M.S.; Nanda, M.; Vlaskin, M.S.; Kumar, V. Algae: A Promising and Sustainable Protein-Rich Food Ingredient for Bakery and Dairy Products. Food Chem. 2024, 441, 138322. [Google Scholar] [CrossRef]
- Coleman, B.; Van Poucke, C.; Dewitte, B.; Ruttens, A.; Moerdijk-Poortvliet, T.; Latsos, C.; De Reu, K.; Blommaert, L.; Duquenne, B.; Timmermans, K.; et al. Potential of Microalgae as Flavoring Agents for Plant-Based Seafood Alternatives. Future Foods 2022, 5, 100139. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Cassani, L.; Garcia-Oliveira, P.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A.; Lucini, L. Algal Nutraceuticals: A Perspective on Metabolic Diversity, Current Food Applications, and Prospects in the Field of Metabolomics. Food Chem. 2023, 409, 135295. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of Kiwi Agricultural Waste and Industry By-Products by Recovering Bioactive Compounds and Applications as Food Additives: A Circular Economy Model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- Akyil, S.; İlter, I.; Koç, M.; Ertekin, F. Recent Trends in Extraction Techniques for High Value Compounds from Algae as Food Additives. Turk. J. Agric. Food Sci. Technol. 2018, 6, 1008–1014. [Google Scholar] [CrossRef]
- Shitanaka, T.; Fujioka, H.; Khan, M.; Kaur, M.; Du, Z.-Y.; Khanal, S.K. Recent Advances in Microalgal Production, Harvesting, Prediction, Optimization, and Control Strategies. Bioresour. Technol. 2024, 391, 129924. [Google Scholar] [CrossRef]
- Jing, H.; Nie, M.; Dai, Z.; Xiao, Y.; Song, J.; Zhang, Z.; Zhou, C.; Li, D. Identification of Carotenoids from Fruits and Vegetables with or without Saponification and Evaluation of Their Antioxidant Activities. J. Food Sci. 2023, 88, 2693–2703. [Google Scholar] [CrossRef]
- Ahmad, N.; Hussain, A.; Khan, S.; Korma, S.A.; Hussain, G.; Aadil, R.M.; Siddique, R.; Ali, A.; Shabbir, U.; Haq, A.U.; et al. Impact of Thermal Extrusion and Microwave Vacuum Drying on Fatty Acids Profile during Fish Powder Preparation. Food Sci. Nutr. 2021, 9, 2743–2753. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.-F.; Kong, F.; Ren, N.-Q.; Ren, H.-Y. Overview on Stress-Induced Strategies for Enhanced Microalgae Lipid Production: Application, Mechanisms and Challenges. Resour. Conserv. Recycl. 2022, 183, 106355. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Parveen, A.; Bhatnagar, P.; Gautam, P.; Bisht, B.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Enhancing the Bio-Prospective of Microalgae by Different Light Systems and Photoperiods. Photochem. Photobiol. Sci. 2023, 22, 2687–2698. [Google Scholar] [CrossRef]
- Chávez-Fuentes, P.; Ruiz-Marin, A.; Canedo-López, Y. Biodiesel Synthesis from Chlorella vulgaris under Effect of Nitrogen Limitation, Intensity and Quality Light: Estimation on the Based Fatty Acids Profiles. Mol. Biol. Rep. 2018, 45, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Baidya, A.; Akter, T.; Islam, M.R.; Shah, A.K.M.A.; Hossain, M.A.; Salam, M.A.; Paul, S.I. Effect of Different Wavelengths of LED Light on the Growth, Chlorophyll, β-Carotene Content and Proximate Composition of Chlorella Ellipsoidea. Heliyon 2021, 7, e08525. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light Emitting Diodes (LEDs) Applied to Microalgal Production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Sundui, B.; Ramirez Calderon, O.A.; Abdeldayem, O.M.; Lázaro-Gil, J.; Rene, E.R.; Sambuu, U. Applications of Machine Learning Algorithms for Biological Wastewater Treatment: Updates and Perspectives. Clean. Technol. Environ. Policy 2021, 23, 127–143. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Biji, A.P.; Lanuyanger, T.; Show, P.L.; Sriariyanun, M.; Upadhyayula, V.K.K.; Gadhamshetty, V.; Bhattacharyya, D. Artificial Intelligence and Machine Learning Tools for High-Performance Microalgal Wastewater Treatment and Algal Biorefinery: A Critical Review. Sci. Total Environ. 2023, 876, 162797. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Khoo, K.S.; Chew, K.W.; Ting, H.-Y.; Iwamoto, K.; Ruan, R.; Ma, Z.; Show, P.L. Artificial Intelligence-Driven Microalgae Autotrophic Batch Cultivation: A Comparative Study of Machine and Deep Learning-Based Image Classification Models. Algal Res. 2024, 79, 103400. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Tang, D.Y.Y.; Leong, H.Y.; Khoo, K.S.; Show, P.L.; Chew, K.W. Bridging Artificial Intelligence and Fucoxanthin for the Recovery and Quantification from Microalgae. Bioengineered 2023, 14, 2244232. [Google Scholar] [CrossRef]
- HÄDER, D.-P. Photosynthesis in Plants and Algae. Anticancer Res. 2022, 42, 5035–5041. [Google Scholar] [CrossRef]
- Lehmuskero, A.; Skogen Chauton, M.; Boström, T. Light and Photosynthetic Microalgae: A Review of Cellular- and Molecular-Scale Optical Processes. Prog. Oceanogr. 2018, 168, 43–56. [Google Scholar] [CrossRef]
- Rym, B.D. Photosynthetic Behavior of Microalgae in Response to Environmental Factors. In Applied Photosynthesis; IntechOpen: London, UK, 2012; ISBN 978-953-51-0061-4. [Google Scholar][Green Version]
- Esteves, A.F.; Salgado, E.M.; Vilar, V.J.P.; Gonçalves, A.L.; Pires, J.C.M. A Growth Phase Analysis on the Influence of Light Intensity on Microalgal Stress and Potential Biofuel Production. Energy Convers. Manag. 2024, 311, 118511. [Google Scholar] [CrossRef]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; van Grondelle, R.; Govindjee; Scholes, G.D. Light Absorption and Energy Transfer in the Antenna Complexes of Photosynthetic Organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef]
- Zhao, L.-S.; Wang, P.; Li, K.; Zhang, Q.-B.; He, F.-Y.; Li, C.-Y.; Su, H.-N.; Chen, X.-L.; Liu, L.-N.; Zhang, Y.-Z. Structural Basis and Evolution of the Photosystem I-Light-Harvesting Supercomplex of Cryptophyte Algae. Plant Cell 2023, 35, 2449–2463. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. Singlet Oxygen in Plants: Generation, Detection, and Signaling Roles. Int. J. Mol. Sci. 2020, 21, 3237. [Google Scholar] [CrossRef]
- Ramanna, L.; Rawat, I.; Bux, F. Light Enhancement Strategies Improve Microalgal Biomass Productivity. Renew. Sustain. Energy Rev. 2017, 80, 765–773. [Google Scholar] [CrossRef]
- Levin, G.; Yasmin, M.; Liveanu, V.; Burstein, C.; Hanna, R.; Kleifeld, O.; Simanowitz, M.C.; Meir, A.; Tadmor, Y.; Hirschberg, J.; et al. A Desert Chlorella sp. That Thrives at Extreme High-Light Intensities Using a Unique Photoinhibition Protection Mechanism. Plant J. 2023, 115, 510–528. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. The Influence of Light Intensity and Photoperiod on the Growth and Lipid Content of Microalgae Nannochloropsis sp. Bioresour. Technol. 2013, 129, 7–11. [Google Scholar] [CrossRef]
- Virtanen, O.; Khorobrykh, S.; Tyystjärvi, E. Acclimation of Chlamydomonas Reinhardtii to Extremely Strong Light. Photosynth. Res. 2021, 147, 91–106. [Google Scholar] [CrossRef]
- Remmers, I.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Dynamics of Triacylglycerol and EPA Production in Phaeodactylum tricornutum under Nitrogen Starvation at Different Light Intensities. PLoS ONE 2017, 12, e0175630. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Premaratne, R.G.M.M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Astaxanthin Accumulation in the Green Microalga Haematococcus pluvialis: Effect of Initial Phosphate Concentration and Stepwise/Continuous Light Stress. Biotechnol. Rep. 2020, 28, e00538. [Google Scholar] [CrossRef]
- Gudvilovich, I.N.; Lelekov, A.S.; Maltsev, E.I.; Kulikovskii, M.S.; Borovkov, A.B. Growth of Porphyridium Purpureum (Porphyridiales, Rhodophyta) and Production of B-Phycoerythrin under Varying Illumination. Russ. J. Plant Physiol. 2021, 68, 188–196. [Google Scholar] [CrossRef]
- Mishra, N.; Prasad, S.M.; Mishra, N. Influence of High Light Intensity and Nitrate Deprivation on Growth and Biochemical Composition of the Marine Microalgae Isochrysis Galbana. Braz. Arch. Biol. Technol. 2019, 62, e19180398. [Google Scholar] [CrossRef]
- Kebede, E.; Ahlgren, G. Optimum Growth Conditions and Light Utilization Efficiency of Spirulina Platensis (=Arthrospira Fusiformis) (Cyanophyta) from Lake Chitu, Ethiopia. Hydrobiologia 1996, 332, 99–109. [Google Scholar] [CrossRef]
- Oostlander, P.C.; van Houcke, J.; Wijffels, R.H.; Barbosa, M.J. Optimization of Rhodomonas sp. under Continuous Cultivation for Industrial Applications in Aquaculture. Algal Res. 2020, 47, 101889. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Selão, T.T.; Norling, B.; Nixon, P.J. Newly Discovered Synechococcus sp. PCC 11901 Is a Robust Cyanobacterial Strain for High Biomass Production. Commun. Biol. 2020, 3, 215. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of Light and Temperature on Fatty Acid Production in Nannochloropsis Salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Khozin-Goldberg, I.; Didi-Cohen, S.; Cohen, Z.; Merzlyak, M.N. Effects of Light Intensity and Nitrogen Starvation on Growth, Total Fatty Acids and Arachidonic Acid in the Green Microalga Parietochloris Incisa. J. Appl. Phycol. 2008, 20, 245–251. [Google Scholar] [CrossRef]
- Rise, M.; Cohen, E.; Vishkautsan, M.; Cojocaru, M.; Gottlieb, H.E.; Arad, S. Accumulation of Secondary Carotenoids in Chlorella zofingiensis. J. Plant Physiol. 1994, 144, 287–292. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of Light Intensity on Growth and Lipid Production in Microalgae Grown in Wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Pereira, S.; Otero, A. Haematococcus pluvialis Bioprocess Optimization: Effect of Light Quality, Temperature and Irradiance on Growth, Pigment Content and Photosynthetic Response. Algal Res. 2020, 51, 102027. [Google Scholar] [CrossRef]
- Erdoğan, A.; Karataş, A.; Demirel, Z.; Dalay, M. Induction of Lutein Production in Scenedesmus obliquus under Different Culture Conditions Prior to Its Semipreparative Isolation. Turk. J. Chem. 2022, 46, 796–804. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A Multidisciplinary Review of Tetradesmus obliquus: A Microalga Suitable for Large-Scale Biomass Production and Emerging Environmental Applications. Rev. Aquac. 2021, 13, 1594–1618. [Google Scholar] [CrossRef]
- Jallet, D.; Caballero, M.A.; Gallina, A.A.; Youngblood, M.; Peers, G. Photosynthetic Physiology and Biomass Partitioning in the Model Diatom Phaeodactylum tricornutum Grown in a Sinusoidal Light Regime. Algal Res. 2016, 18, 51–60. [Google Scholar] [CrossRef]
- Uwizeye, C.; Decelle, J.; Jouneau, P.-H.; Flori, S.; Gallet, B.; Keck, J.-B.; Bo, D.D.; Moriscot, C.; Seydoux, C.; Chevalier, F.; et al. Morphological Bases of Phytoplankton Energy Management and Physiological Responses Unveiled by 3D Subcellular Imaging. Nat. Commun. 2021, 12, 1049. [Google Scholar] [CrossRef]
- Leister, D. Enhancing the Light Reactions of Photosynthesis: Strategies, Controversies, and Perspectives. Mol. Plant 2023, 16, 4–22. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef]
- Niazkhani, A.; Mohammadi, A.; Mashhadi, H.; Mahmoudnia, F. An Investigation Amount of Cell Density, Biomass, Lipid and Biodiesel Production in Chlorella vulgaris Microalgae under Effect of Different Parameters. Period. Biol. 2022, 124, 1–10. [Google Scholar] [CrossRef]
- Pozzobon, V. Chlorella vulgaris Cultivation under Super High Light Intensity: An Application of the Flashing Light Effect. Algal Res. 2022, 68, 102874. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Fixler, D.; Dubinsky, Z.; Iluz, D. Flashing Light in Microalgae Biotechnology. Bioresour. Technol. 2016, 203, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Seyfabadi, J.; Ramezanpour, Z.; Khoeyi, Z.A. Protein, Fatty Acid, and Pigment Content of Chlorella vulgaris under Different Light Regimes. J. Appl. Phycol. 2010, 23, 721–726. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The Role of Photosynthesis Related Pigments in Light Harvesting, Photoprotection and Enhancement of Photosynthetic Yield in Planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Zarei, Z.; Zamani, H. Biorefinery Approach to Stimulate Astaxanthin and Biofuel Generation in Microalga Haematococcus pluvialis under Different Light Irradiance. Clean. Technol. Environ. Policy 2024, 26, 3333–3347. [Google Scholar] [CrossRef]
- Raqiba, H.; Sibi, G. Light Emitting Diode (LED) Illumination for Enhanced Growth and Cellular Composition in Three Microalgae. Adv. Microbiol. Res. 2019, 3, 7. [Google Scholar] [CrossRef]
- Jungandreas, A.; Schellenberger Costa, B.; Jakob, T.; von Bergen, M.; Baumann, S.; Wilhelm, C. The Acclimation of Phaeodactylum tricornutum to Blue and Red Light Does Not Influence the Photosynthetic Light Reaction but Strongly Disturbs the Carbon Allocation Pattern. PLoS ONE 2014, 9, e99727. [Google Scholar] [CrossRef]
- Ritchie, R.J.; Sma-Air, S. Microalgae Grown under Different Light Sources. J. Appl. Phycol. 2023, 35, 551–566. [Google Scholar] [CrossRef]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Evaluation of Growth Rate and Biomass Productivity of Scenedesmus Quadricauda and Chlorella vulgaris under Different LED Wavelengths and Photoperiods. Sustainability 2022, 14, 6108. [Google Scholar] [CrossRef]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.T.; Eltanahy, E.; Netzel, M.E.; Netzel, G.; Lu, Y.; Schenk, P.M. Blue Light Enhances Astaxanthin Biosynthesis Metabolism and Extraction Efficiency in Haematococcus pluvialis by Inducing Haematocyst Germination. Algal Res. 2018, 35, 215–222. [Google Scholar] [CrossRef]
- Cray, R.; Levine, I. Oxidative Stress Modulates Astaxanthin Synthesis in Haematococcus pluvialis. J. Appl. Phycol. 2022, 34, 2327–2338. [Google Scholar] [CrossRef]
- Hernández, H.; Nunes, M.C.; Prista, C.; Raymundo, A. Innovative and Healthier Dairy Products through the Addition of Microalgae: A Review. Foods 2022, 11, 755. [Google Scholar] [CrossRef]
- Araújo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe; Publications Office of the EU: Luxembourg, 2021. [Google Scholar] [CrossRef]
- Hlaing, S.A.A.; Sadiq, M.B.; Anal, A.K. Enhanced Yield of Scenedesmus obliquus Biomacromolecules through Medium Optimization and Development of Microalgae Based Functional Chocolate. J. Food Sci. Technol. 2020, 57, 1090–1099. [Google Scholar] [CrossRef]
- Kazir, M.; Livney, Y.D. Plant-Based Seafood Analogs. Molecules 2021, 26, 1559. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined Production of Fucoxanthin and EPA from Two Diatom Strains Phaeodactylum tricornutum and Cylindrotheca Fusiformis Cultures. Bioprocess. Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Y.; Yu, Q.; Liu, Q.; Zhou, X.; Jin, C. Regulation of Light Quality on Lipid Production, Biodiesel Quality, and Nutritional Quality of Phaeodactylum tricornutum. Aquacult. Int. 2023, 31, 1231–1251. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth Kinetics and Fucoxanthin Production of Phaeodactylum tricornutum and Isochrysis Galbana Cultures at Different Light and Agitation Conditions. J. Appl. Phycol. 2016, 28, 849–860. [Google Scholar] [CrossRef]
- Yi, Z.; Su, Y.; Cherek, P.; Nelson, D.R.; Lin, J.; Rolfsson, O.; Wu, H.; Salehi-Ashtiani, K.; Brynjolfsson, S.; Fu, W. Combined Artificial High-Silicate Medium and LED Illumination Promote Carotenoid Accumulation in the Marine Diatom Phaeodactylum tricornutum. Microb. Cell Fact. 2019, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhou, Z.; Zhu, Y.; Liu, Q.; Zhou, X. Photosynthetic Activity and Astaxanthin Production of Haematococcus pluvialis Regulated by Manipulated Light Quality. Aquacult. Int. 2024, 32, 3617–3635. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, X.; Zhang, Y.; Cheng, P.; Ma, R.; Cheng, W.; Chu, H. Enhancing Astaxanthin Accumulation in Haematococcus pluvialis by Coupled Light Intensity and Nitrogen Starvation in Column Photobioreactors. J. Microbiol. Biotechnol. 2018, 28, 2019–2028. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the Nutritional Value of Mysore Thorn Borer (Anoplophora chinensis) and Mealworm Larva (Tenebrio molitor): Amino Acid, Fatty Acid, and Element Profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Shabbir, U.; Gilani, S.M.; Sameen, A.; Ahmad, N.; Siddique, R.; Ahmed, Z.; Qayyum, A.; Rehman, A. Characterization of Bioactive Fatty Acids and Oxidative Stability of Microwave Vacuum Dried Fish Powder Supplemented Extruded Product. Food Sci. Technol. 2021, 42, e22720. [Google Scholar] [CrossRef]
- Munialo, C.D.; Vriesekoop, F. Plant-Based Foods as Meat and Fat Substitutes. Food Sci. Nutr. 2023, 11, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wen, S.; Ye, Q.; Lou, H.; Gao, Y.; Bajpai, V.K.; Carpena, M.; Prieto, M.-A.; Simal-Gandara, J.; Xiao, J.; et al. Advances on Delta 5-Unsaturated-Polymethylene-Interrupted Fatty Acids: Resources, Biosynthesis, and Benefits. Crit. Rev. Food Sci. Nutr. 2021, 63, 767–789. [Google Scholar] [CrossRef] [PubMed]
- Jesionowska, M.; Ovadia, J.; Hockemeyer, K.; Clews, A.C.; Xu, Y. EPA and DHA in Microalgae: Health Benefits, Biosynthesis, and Metabolic Engineering Advances. J. Am. Oil Chem. Soc. 2023, 100, 831–842. [Google Scholar] [CrossRef]
- Maréchal, E.; Lupette, J. Relationship between Acyl-Lipid and Sterol Metabolisms in Diatoms. Biochimie 2020, 169, 3–11. [Google Scholar] [CrossRef]
- Celi, C.; Fino, D.; Savorani, F. Phaeodactylum tricornutum as a Source of Value-Added Products: A Review on Recent Developments in Cultivation and Extraction Technologies. Bioresour. Technol. Rep. 2022, 19, 101122. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A Diatom Cell Factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef]
- Cui, Y.; Thomas-Hall, S.R.; Chua, E.T.; Schenk, P.M. Development of High-Level Omega-3 Eicosapentaenoic Acid (EPA) Production from Phaeodactylum tricornutum. J. Phycol. 2021, 57, 258–268. [Google Scholar] [CrossRef]
- Conceição, D.; Lopes, R.G.; Derner, R.B.; Cella, H.; do Carmo, A.P.B.; Montes D’Oca, M.G.; Petersen, R.; Passos, M.F.; Vargas, J.V.C.; Galli-Terasawa, L.V.; et al. The Effect of Light Intensity on the Production and Accumulation of Pigments and Fatty Acids in Phaeodactylum tricornutum. J. Appl. Phycol. 2020, 32, 1017–1025. [Google Scholar] [CrossRef]
- Sirisuk, P.; Ra, C.-H.; Jeong, G.-T.; Kim, S.-K. Effects of Wavelength Mixing Ratio and Photoperiod on Microalgal Biomass and Lipid Production in a Two-Phase Culture System Using LED Illumination. Bioresour. Technol. 2018, 253, 175–181. [Google Scholar] [CrossRef]
- Peng, M.; Lin, S.; Shen, Y.; Peng, R.; Li, S.; Jiang, X.; Jiang, M. Effects of Light Quality on the Growth, Productivity, Fucoxanthin Accumulation, and Fatty Acid Composition of Thalassiosira Pseudonana. J. Appl. Phycol. 2024, 36, 1667–1678. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Z.; Liu, W.; Hou, Z.; Zhang, D.; Huang, L.; Zhang, Y. Oleic Acid as a Protein Ligand Improving Intestinal Absorption and Ocular Benefit of Fucoxanthin in Water through Protein-Based Encapsulation. Food Funct. 2019, 10, 4381–4395. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Cassani, L.; Grosso, C.; Garcia-Oliveira, P.; Morais, S.L.; Echave, J.; Carpena, M.; Xiao, J.; Barroso, M.F.; Simal-Gandara, J.; et al. Recent Advances in Biological Properties of Brown Algae-Derived Compounds for Nutraceutical Applications. Crit. Rev. Food Sci. Nutr. 2022, 64, 1283–1311. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health Benefits of Fucoxanthin in the Prevention of Chronic Diseases. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, S.; Zhao, W.; Kong, Q.; Zhu, C.; Fu, X.; Zhang, F.; Liu, Z.; Zhan, Y.; Mou, H.; et al. Fucoxanthin from Marine Microalgae: A Promising Bioactive Compound for Industrial Production and Food Application. Crit. Rev. Food Sci. Nutr. 2023, 63, 7996–8012. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A.; El-Sayed, S.M.; Murad, S.A.; Abdallah, W.E.; El-Sayed, H.S. Evaluation of the Antioxidant and Antimicrobial Activities of Fucoxanthin from Dilophys Fasciola and as a Food Additive in Stirred Yoghurt. S. Afr. J. Sci. 2023, 119, 1–9. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, G.; Pan, K.; Wang, L.; Hu, Z. A Review on the Progress, Challenges and Prospects in Commercializing Microalgal Fucoxanthin. Biotechnol. Adv. 2021, 53, 107865. [Google Scholar] [CrossRef]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An Investigation into the Effect of Culture Conditions on Fucoxanthin Production Using the Marine Microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Truong, T.Q.; Park, Y.J.; Koo, S.Y.; Choi, J.-H.; Enkhbayar, A.; Song, D.-G.; Kim, S.M. Interdependence of Fucoxanthin Biosynthesis and Fucoxanthin-Chlorophyll a/c Binding Proteins in Phaeodactylum tricornutum under Different Light Intensities. J. Appl. Phycol. 2023, 35, 25–42. [Google Scholar] [CrossRef]
- Sharma, A.K.; Nymark, M.; Flo, S.; Sparstad, T.; Bones, A.M.; Winge, P. Simultaneous Knockout of Multiple LHCF Genes Using Single sgRNAs and Engineering of a High-Fidelity Cas9 for Precise Genome Editing in Marine Algae. Plant Biotechnol. J. 2021, 19, 1658–1669. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Gu, W.; Zheng, Z.; Chen, M.; Wang, G. LHCF15 Facilitates the Absorption of Longer Wavelength Light and Promotes Growth of Phaeodactylum tricornutum under Red Light. Algal Res. 2023, 75, 103249. [Google Scholar] [CrossRef]
- Song, Q.; Liu, C.; Xu, R.; Cai, L. Enhancement of Fucoxanthin Accumulation in Phaeodactylum tricornutum by Light Quality and Intensity Shift Strategy. Chem. Eng. J. 2025, 505, 159388. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, P.; Cai, Q.; Zhang, C.; Gao, B. Maximizing Fucoxanthin Production in Odontella Aurita by Optimizing the Ratio of Red and Blue Light-Emitting Diodes in an Auto-Controlled Internally Illuminated Photobioreactor. Bioresour. Technol. 2022, 344, 126260. [Google Scholar] [CrossRef]
- Böcker, L.; Bertsch, P.; Wenner, D.; Teixeira, S.; Bergfreund, J.; Eder, S.; Fischer, P.; Mathys, A. Effect of Arthrospira platensis microalgae protein purification on emulsification mechanism and efficiency. J. Colloid Interface Sci. 2021, 584, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.l.B.; Laurens, L.M.L. Microalgae as Biodiesel & Biomass Feedstocks: Review & Analysis of the Biochemistry, Energetics & Economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded Meat Analogues Based on Yellow, Heterotrophically Cultivated AuxenoChlorella protothecoides Microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The Potentials and Challenges of Using Microalgae as an Ingredient to Produce Meat Analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Shah, M.A.R.; Zhu, F.; Cui, Y.; Hu, X.; Chen, H.; Kayani, S.-I.; Huo, S. Mechanistic Insights into the Nutritional and Therapeutic Potential of Spirulina (Arthrospira) spp.: Challenges and Opportunities. Trends Food Sci. Technol. 2024, 151, 104648. [Google Scholar] [CrossRef]
- Shao, W.; Ebaid, R.; El-Sheekh, M.; Abomohra, A.; Eladel, H. Pharmaceutical Applications and Consequent Environmental Impacts of Spirulina (Arthrospira): An Overview. Grasas Aceites 2019, 70, e292. [Google Scholar] [CrossRef]
- Wang, F.; Yu, X.; Cui, Y.; Xu, L.; Huo, S.; Ding, Z.; Hu, Q.; Xie, W.; Xiao, H.; Zhang, D. Efficient Extraction of Phycobiliproteins from Dry Biomass of Spirulina platensis Using Sodium Chloride as Extraction Enhancer. Food Chem. 2023, 406, 135005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Li, Y.; Liu, S.; Chen, Y.; Jia, M.; Wang, X.; Zhang, L.; Gao, Q.; Zhang, L.; et al. Regulation of Different Light Conditions for Efficient Biomass Production and Protein Accumulation of Spirulina Platensis. J. Oceanol. Limnol. 2024, 42, 174–186. [Google Scholar] [CrossRef]
- Bhat, O.; Unpaprom, Y.; Ramaraj, R. Effect of Photoperiod and White LED on Biomass Growth and Protein Production by Spirulina. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Bhat, O.; Unpaprom, Y.; Ramaraj, R. Spirulina Cultivation Under Different Light-Emitting Diodes for Boosting Biomass and Protein Production. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Yan, B.; Chen, X.; Wang, Y.; Yuan, M.; Xian, J.; Lu, D.; Shao, Z.; Qiu, M.; Fu, T.; Zheng, X. Chlorella pyrenoidosa Ameliorates Ulcerative Colitis by Tuning Intestinal Microecology: Butyric Acid Is a Crucial Player. J. Funct. Foods 2024, 121, 106414. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Katsoulas, N.; Karapanagiotidis, I.T.; Papapolymerou, G. Effect of Nitrogen Concentration, Two-Stage and Prolonged Cultivation on Growth Rate, Lipid and Protein Content of Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2019, 94, 1466–1473. [Google Scholar] [CrossRef]
- Atta, M.; Idris, A.; Bukhari, A.; Wahidin, S. Intensity of Blue LED Light: A Potential Stimulus for Biomass and Lipid Content in Fresh Water Microalgae Chlorella vulgaris. Bioresour. Technol. 2013, 148, 373–378. [Google Scholar] [CrossRef]
- Sui, Y.; Harvey, P.J. Effect of Light Intensity and Wavelength on Biomass Growth and Protein and Amino Acid Composition of Dunaliella salina. Foods 2021, 10, 1018. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E. Recent Progress in Practical Applications of a Potential Carotenoid Astaxanthin in Aquaculture Industry: A Review. Fish Physiol. Biochem. 2024, 50, 97–126. [Google Scholar] [CrossRef]
- Abuzar; Sharif, H.R.; Sharif, M.K.; Arshad, R.; Rehman, A.; Ashraf, W.; Karim, A.; Awan, K.A.; Raza, H.; Khalid, W.; et al. Potential Industrial and Nutritional Applications of Shrimp By-Products: A Review. Int. J. Food Prop. 2023, 26, 3407–3432. [Google Scholar] [CrossRef]
- Xie, X.; Zhong, M.; Huang, X.; Yuan, X.; Mahna, N.; Mussagy, C.U.; Ren, M. Astaxanthin Biosynthesis for Functional Food Development and Space Missions. Crit. Rev. Biotechnol. 2024, 45, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Zhu, L.; Zhao, X. Effects of Haematococcus pluvialis Addition on the Sensory Properties of Plant-Based Meat Analogues. Foods 2023, 12, 3435. [Google Scholar] [CrossRef]
- Samhat, K.; Kazbar, A.; Takache, H.; Ismail, A.; Pruvost, J. Influence of Light Absorption Rate on the Astaxanthin Production by the Microalga Haematococcus pluvialis during Nitrogen Starvation. Bioresour. Bioprocess. 2023, 10, 78. [Google Scholar] [CrossRef]
- Morgado, D.; Fanesi, A.; Martin, T.; Tebbani, S.; Bernard, O.; Lopes, F. Exploring the Dynamics of Astaxanthin Production in Haematococcus pluvialis Biofilms Using a Rotating Biofilm-Based System. Biotechnol. Bioeng. 2023, 121, 991–1004. [Google Scholar] [CrossRef]
- Rizzo, A.; Ross, M.E.; Norici, A.; Jesus, B. A Two-Step Process for Improved Biomass Production and Non-Destructive Astaxanthin and Carotenoids Accumulation in Haematococcus pluvialis. Appl. Sci. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Ahirwar, A.; Meignen, G.; Jahir Khan, M.; Sirotiya, V.; Harish; Scarsini, M.; Roux, S.; Marchand, J.; Schoefs, B.; Vinayak, V. Light Modulates Transcriptomic Dynamics Upregulating Astaxanthin Accumulation in Haematococcus: A Review. Bioresour. Technol. 2021, 340, 125707. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, A.S.; González, M.A.; Vargas, S.; Hoeneisen, M.; González, N. Optimization of Biomass, Total Carotenoids and Astaxanthin Production in Haematococcus pluvialis Flotow Strain Steptoe (Nevada, USA) under Laboratory Conditions. Biol. Res. 2003, 36, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cui, D.; Sun, X.; Shi, J.; Xu, N. Primary Metabolism Is Associated with the Astaxanthin Biosynthesis in the Green Algae Haematococcus pluvialis under Light Stress. Algal Res. 2020, 46, 101768. [Google Scholar] [CrossRef]
- Boussiba, S. Carotenogenesis in the Green Alga Haematococcus pluvialis: Cellular Physiology and Stress Response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Huang, L.; Gao, B.; Wu, M.; Wang, F.; Zhang, C. Comparative Transcriptome Analysis of a Long-Time Span Two-Step Culture Process Reveals a Potential Mechanism for Astaxanthin and Biomass Hyper-Accumulation in Haematococcus pluvialis JNU35. Biotechnol. Biofuels 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hou, L.; Dong, M.; Shi, J.; Huang, X.; Ding, Y.; Cong, X.; Zhang, F.; Zhang, X.; Zang, X. Transcriptome Analysis in Haematococcus pluvialis: Astaxanthin Induction by High Light with Acetate and Fe2+. Int. J. Mol. Sci. 2018, 19, 175. [Google Scholar] [CrossRef]
- Gwak, Y.; Hwang, Y.; Wang, B.; Kim, M.; Jeong, J.; Lee, C.-G.; Hu, Q.; Han, D.; Jin, E. Comparative Analyses of Lipidomes and Transcriptomes Reveal a Concerted Action of Multiple Defensive Systems against Photooxidative Stress in Haematococcus pluvialis. J. Exp. Bot. 2014, 65, 4317–4334. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yong, H.I.; Kim, M.; Choi, Y.-S.; Jo, C. Status of Meat Alternatives and Their Potential Role in the Future Meat Market—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1533–1543. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Cha, J.Y.; Park, S.-Y.; Jung, S.; Choi, Y.-S. Drying-Induced Restructured Jerky Analog Developed Using a Combination of Edible Insect Protein and Textured Vegetable Protein. Food Chem. 2022, 373, 131519. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-H.; Keum, D.-H.; Hong, S.-J.; Kim, Y.-J.; Han, S.-G. Comparative Evaluation of Polysaccharide Binders on the Quality Characteristics of Plant-Based Patties. Foods 2023, 12, 3731. [Google Scholar] [CrossRef]
- Ouyang, Y.; Qiu, Y.; Liu, Y.; Zhu, R.; Chen, Y.; El-Seedi, H.R.; Chen, X.; Zhao, C. Cancer-Fighting Potentials of Algal Polysaccharides as Nutraceuticals. Food Res. Int. 2021, 147, 110522. [Google Scholar] [CrossRef]
- Wang, M.; Yin, Z.; Zeng, M. Microalgae as a Promising Structure Ingredient in Food: Obtained by Simple Thermal and High-Speed Shearing Homogenization. Food Hydrocoll. 2022, 131, 107743. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, S.; Li, S.; Feng, Y.; Wu, H.; Zeng, M. Microalgae Polysaccharides Ameliorates Obesity in Association with Modulation of Lipid Metabolism and Gut Microbiota in High-Fat-Diet Fed C57BL/6 Mice. Int. J. Biol. Macromol. 2021, 182, 1371–1383. [Google Scholar] [CrossRef]
- Frick, K.; Ebbing, T.; Yeh, Y.-C.; Schmid-Staiger, U.; Tovar, G.E.M. Influence of Light Conditions on the Production of Chrysolaminarin Using Phaeodactylum tricornutum in Artificially Illuminated Photobioreactors. MicrobiologyOpen 2023, 12, e1378. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Linking Microalgae and Cyanobacteria Culture Conditions and Key-Enzymes for Carbohydrate Accumulation. Biotechnol. Adv. 2012, 30, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C. Preliminary Characterization, Antioxidant Properties and Production of Chrysolaminarin from Marine Diatom Odontella Aurita. Mar. Drugs 2014, 12, 4883–4897. [Google Scholar] [CrossRef]
- Ran, X.; Shen, Y.; Jiang, D.; Wang, C.; Li, X.; Zhang, H.; Pan, Y.; Xie, C.; Xie, T.; Zhang, Y.; et al. Nutrient Deprivation Coupled with High Light Exposure for Bioactive Chrysolaminarin Production in the Marine Microalga Isochrysis Zhangjiangensis. Mar. Drugs 2022, 20, 351. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of Microalgae for Primary Metabolites Including β-Glucans and the Influence of Nitrate Starvation and Irradiance on β-Glucan Production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Ahmed, Z. Influence of Nitrogen Sources on Biomass Productivity of Microalgae Scenedesmus bijugatus. Bioresour. Technol. 2013, 131, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.E.; Damiani, M.C.; Leonardi, P.I.; Popovich, C.A. Temperature and Salinity Effect on Tolerance and Lipid Accumulation in Halamphora Coffeaeformis: An Approach for Outdoor Bioenergy Cultures. Bioenerg. Res. 2022, 15, 1545–1554. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, Photosynthesis, and Nutrient Uptake at Different Light Intensities and Temperatures in Lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef]
- Terada, R.; Shindo, A.; Moriyama, H.; Shimboku, N.; Nishihara, G.N. The Response of Photosynthesis to Temperature and Irradiance in a Green Alga, Ryuguphycus kuaweuweu (Ulvales) Reveals Adaptation to a Subtidal Environment in the Northern Ryukyu Islands. Algal Res. 2023, 74, 103189. [Google Scholar] [CrossRef]
- Ova Ozcan, D.; Ovez, B. Evaluation of the Interaction of Temperature and Light Intensity on the Growth of Phaeodactylum tricornutum: Kinetic Modeling and Optimization. Biochem. Eng. J. 2020, 154, 107456. [Google Scholar] [CrossRef]
- Cella, H.; Nader, C.; Bastolla, C.L.V.; Bonomi-Barufi, J.; Oliveira, C.Y.B.; Lopes, R.G.; Mattos, J.J.; Bauer, C.M.; Maraschin, M.; Rörig, L.R.; et al. PAR Regulation of Photoprotection in Phaeodactylum tricornutum (Bacillariophyceae): Roles of Doses and Irradiances. J. Appl. Phycol. 2023, 35, 2177–2191. [Google Scholar] [CrossRef]
- Şirin, P.A.; Serdar, S. Effects of Nitrogen Starvation on Growth and Biochemical Composition of Some Microalgae Species. Folia Microbiol. 2024, 69, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic Response to Nitrogen Starvation and High Light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- Li, F.; Cai, M.; Wu, Y.; Lian, Q.; Qian, Z.; Luo, J.; Zhang, Y.; Zhang, N.; Li, C.; Huang, X. Effects of Nitrogen and Light Intensity on the Astaxanthin Accumulation in Motile Cells of Haematococcus pluvialis. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Zhang, Y.; Gao, H.; Zhao, Y.; Yu, X. Chemical Inducers Regulate ROS Signalling to Stimulate Astaxanthin Production in Haematococcus pluvialis under Environmental Stresses: A Review. Trends Food Sci. Technol. 2023, 136, 181–193. [Google Scholar] [CrossRef]
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the Antioxidant Peptides from Edible Plant Sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, H.; Li, X.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Physiological and Metabolomics Analyses Reveal the Roles of Fulvic Acid in Enhancing the Production of Astaxanthin and Lipids in Haematococcus pluvialis under Abiotic Stress Conditions. J. Agric. Food Chem. 2019, 67, 12599–12609. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, A.; Wang, R.; Elsherbiny, S.M.; Bondzie-Quaye, P.; Huang, Q. Exogenous Arginine Promotes the Coproduction of Biomass and Astaxanthin under High-Light Conditions in Haematococcus pluvialis. Bioresour. Technol. 2024, 393, 130001. [Google Scholar] [CrossRef]
- Bo, Y.; Wang, S.; Ma, F.; Yurevich Manyakhin, A.; Zhang, G.; Li, X.; Zhou, C.; Ge, B.; Yan, X.; Ruan, R.; et al. The Influence of Spermidine on the Build-up of Fucoxanthin in Isochrysis sp. Acclimated to Varying Light Intensities. Bioresour. Technol. 2023, 387, 129688. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, A.P.; Bragotto, A.P.A. Microalgae-Based Products: Food and Public Health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001; Official Journal of the European Union L 327/1; European Parliament and Council of the European Union: Strasbourg, France, 2015. [Google Scholar]
- Martín-Girela, I.; Albero, B.; Tiwari, B.K.; Miguel, E.; Aznar, R. Screening of Contaminants of Emerging Concern in Microalgae Food Supplements. Separations 2020, 7, 28. [Google Scholar] [CrossRef]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T. Benefits, Problems and Challenges of Plant Factories with Artificial Lighting (PFALs): A Short Review. Acta Hortic. 2018, 25–30. [Google Scholar] [CrossRef]
- Teng, S.Y.; Yew, G.Y.; Sukačová, K.; Show, P.L.; Máša, V.; Chang, J.-S. Microalgae with Artificial Intelligence: A Digitalized Perspective on Genetics, Systems and Products. Biotechnol. Adv. 2020, 44, 107631. [Google Scholar] [CrossRef] [PubMed]
- Imamoglu, E. Artificial Intelligence and/or Machine Learning Algorithms in Microalgae Bioprocesses. Bioengineering 2024, 11, 1143. [Google Scholar] [CrossRef]
- Kushwaha, O.S.; Uthayakumar, H.; Kumaresan, K. Modeling of Carbon Dioxide Fixation by Microalgae Using Hybrid Artificial Intelligence (AI) and Fuzzy Logic (FL) Methods and Optimization by Genetic Algorithm (GA). Environ. Sci. Pollut. Res. 2023, 30, 24927–24948. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Ho, S.-H.; Show, P.L. Smart Microalgae Farming with Internet-of-Things for Sustainable Agriculture. Biotechnol. Adv. 2022, 57, 107931. [Google Scholar] [CrossRef]



| Company Name | Light Source Used | Species of Microalgae Cultured | Major Products |
|---|---|---|---|
| Arizona Algae Products LLC (USA) | Solar light | Spirulina sp., Chlorella vulgaris | Microalgal oil and powder |
| Vaxa Technologies(IS) | Solar light | Cyanobacteria | Phycocyanin biofuels and algal proteins |
| BioFields (MX) | Solar light | Spirulina sp., Chlorella vulgaris | Aquatic feed |
| Susewi (MOR) | Solar light | Brown algae, Green algae | Diatom material Algica, biofertilizer |
| Swedish Algae Factory (SE) | Solar light | Diatoms | Sustainable aviation fuels, microalgal proteins, cosmetics, and pharmaceuticals |
| Chitose Carbon Capture Central (MAS) | Solar light | Diatoms, Spirulina sp., Chlorella vulgaris | Natural pigments, cosmetics, and animal feed additives |
| Provectus Algae (AUS) | Artificial light | Chlorella vulgaris, Phaeodactylum tricornutum, Dunaliella salina | Natural astaxanthin, health products, cosmetics |
| Yunnan Xi Zao Biotechnology (CHN) | Artificial light | Haematococcus pluvialis | Biofuels, food additives |
| Festo (GER) | Artificial light | Cyanobacteria | Pharmaceutical, cosmetic, and bioplastic |
| Provectus Algae (USA) | Artificial light | Asparagopsis, Dunaliella salina, Diatoms | Natural pigments, health products, and cosmetics |
| Investigated Light Intensity (μmol Photons m−2 s−1) | Optimal Light Intensity (μmol Photons m−2 s−1) | Microalgae Species | Marine or Freshwater Algae | Reference |
|---|---|---|---|---|
| 60, 100, 250, 500, 750 | 60–112 | Phaeodactylum tricornutum | marine algae | [41] |
| 200, 500, 1000, 1500, 2000 | 1500 | Dunaliella salina | marine algae | [42] |
| 70, 140, 210 | 70 | Porphyridium purpureum | marine algae | [43] |
| 50, 125, 325 | 325 | Isochrysis galbana | marine algae | [44] |
| 20–500 | 300 | Arthrospira fusiformis | marine algae | [45] |
| 60, 195, 330, 465, 600 | 110–220 | Rhodomonas sp. | marine algae | [46] |
| 75, 100, 150, 500, 660, 750 | 660 | Synechococcus sp. | marine algae | [47] |
| 5, 25, 50, 100, 250, 850 | 26–55 | Microchloropsis salina (=Nannochloropsis salina) | marine algae | [48] |
| 35, 200, 400 | 400 | Lobosphaera incisa | freshwater algae | [49] |
| 150, 300 | 150 | Chromochloris zofingiensis (=Chlorella vulgariszofingiensis) | freshwater algae | [50] |
| 50, 150, 300, 500 | 150 | Chlorella vulgarisvulgaris | freshwater algae | [51] |
| 50~300 | 150 | Haematococcus pluvialis | freshwater algae | [52] |
| 50, 150, 300 | 150 | Scenedesmus obliquus | freshwater algae | [53] |
| 10, 50, 150, 200, 350, 1000 | 150 | Tetradesmus obliquus | freshwater algae | [54] |
| Microalgae Products for Plant-Based Foods | Descriptions | Microalgae Species | Reference |
|---|---|---|---|
| Microalgae protein | Plant-based meat and plant-based milk | Spirulina sp., Chlorella vulgaris | [71] |
| Nutrient enhancer | Pigments, Antioxidant, Omega-3 Phycobiliproteins, Polysaccharides | Haematococcus pluvialis, Scenedesmus obliquus | [72] |
| Antioxidants | Improved food stability | Tetraselmis sp., Dunaliella salina, Phaeodactylum tricornutumScenedesmus obliquus | [73] |
| Food Flavourings | Flavor components of plant-based seafood alternatives | Rhodomonas salina, Tetraselmis chui, Phaeodactylum tricornutum | [74] |
| Species and Strain | Optimal Light Intensity and Quality (μmol Photons m−2 s−1) | Nutrients | Productivity | Reference |
|---|---|---|---|---|
| Phaeodactylum tricornutum | 30 μmol photons m−2 s−1, blue light | EPA | Increase by 30% (17% of fatty acids) | [75,76] |
| 100 μmol photons m−2 s−1, red and blue (50:50) light | Fucoxanthin | Increase more than 100% (9~12 mg/g) | [77,78] | |
| Chlorella vulgaris | 100 μmol photons m−2 s−1, blue light | Protein | Increase by 35% (460 mg/g) | [58,62] |
| Haematococcus pluvialis | 400 μmol photons m−2 s−1, red and blue (40:60) light | Astaxanthin | Increase by more than 100% (15.28 mg/L) | [79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Wang, F.; Rehman, O.U.; Hu, X.; Zhu, F.; Wang, R.; Xu, L.; Cui, Y.; Huo, S. Influence of Light Regimes on Production of Beneficial Pigments and Nutrients by Microalgae for Functional Plant-Based Foods. Foods 2025, 14, 2500. https://doi.org/10.3390/foods14142500
Huang X, Wang F, Rehman OU, Hu X, Zhu F, Wang R, Xu L, Cui Y, Huo S. Influence of Light Regimes on Production of Beneficial Pigments and Nutrients by Microalgae for Functional Plant-Based Foods. Foods. 2025; 14(14):2500. https://doi.org/10.3390/foods14142500
Chicago/Turabian StyleHuang, Xiang, Feng Wang, Obaid Ur Rehman, Xinjuan Hu, Feifei Zhu, Renxia Wang, Ling Xu, Yi Cui, and Shuhao Huo. 2025. "Influence of Light Regimes on Production of Beneficial Pigments and Nutrients by Microalgae for Functional Plant-Based Foods" Foods 14, no. 14: 2500. https://doi.org/10.3390/foods14142500
APA StyleHuang, X., Wang, F., Rehman, O. U., Hu, X., Zhu, F., Wang, R., Xu, L., Cui, Y., & Huo, S. (2025). Influence of Light Regimes on Production of Beneficial Pigments and Nutrients by Microalgae for Functional Plant-Based Foods. Foods, 14(14), 2500. https://doi.org/10.3390/foods14142500








