Integrated Smart Packaging of Modified Silica/Anthocyanin/Nanocellulose for Preservation and Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanoparticles
2.3. Preparation of Nanocellulose-Based Films
2.4. Characterization of Nanoparticles and Films
2.5. Dissolution Rate and Swelling Rate
2.6. In Vitro Release Test
2.7. Antibacterial Experiments
2.8. Colorimetric Experiment
2.9. Statistical Analysis of Data
3. Results and Discussion
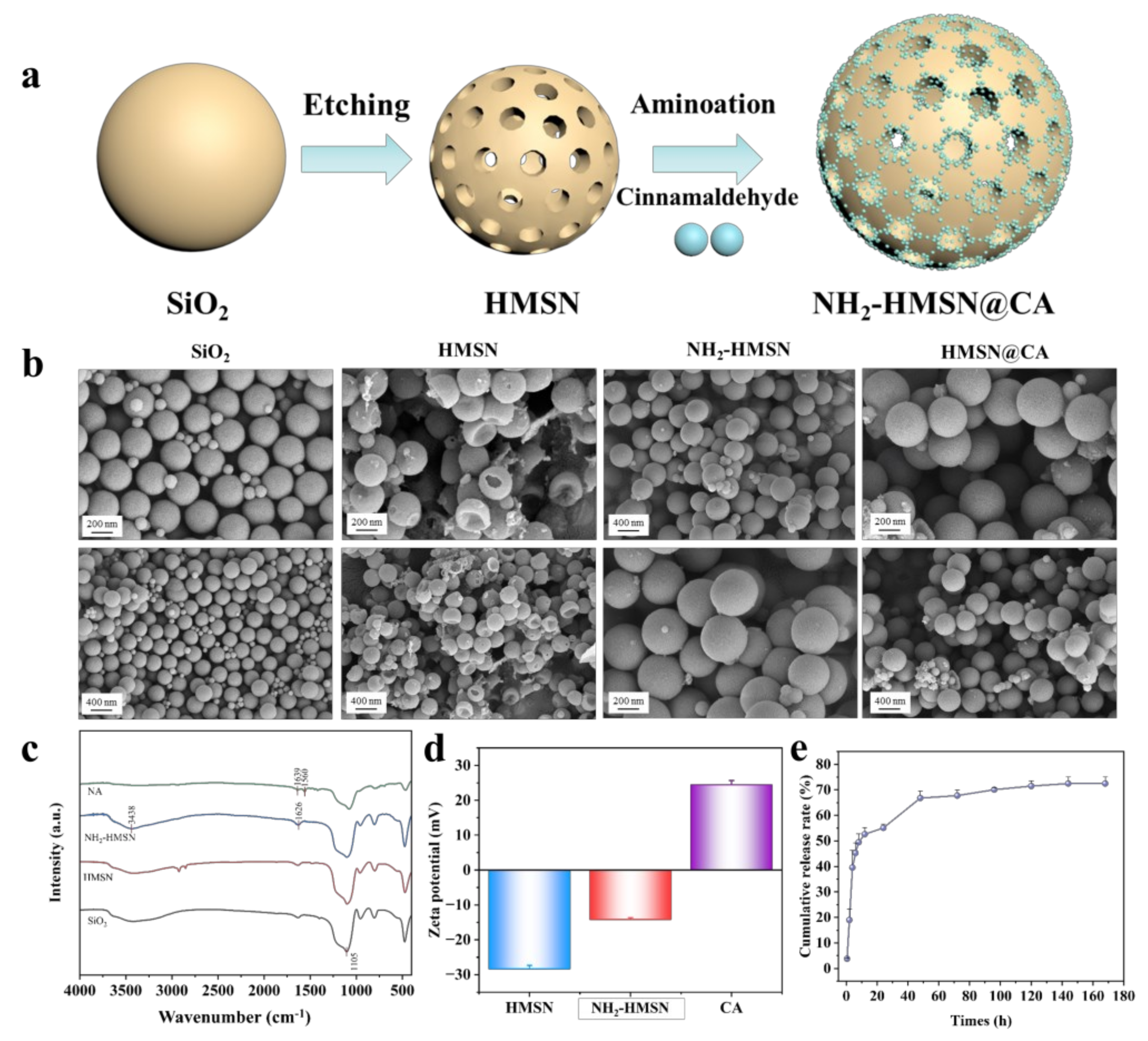
3.1. Characterization of Nanoparticles
3.2. Physicochemical Properties of the Films
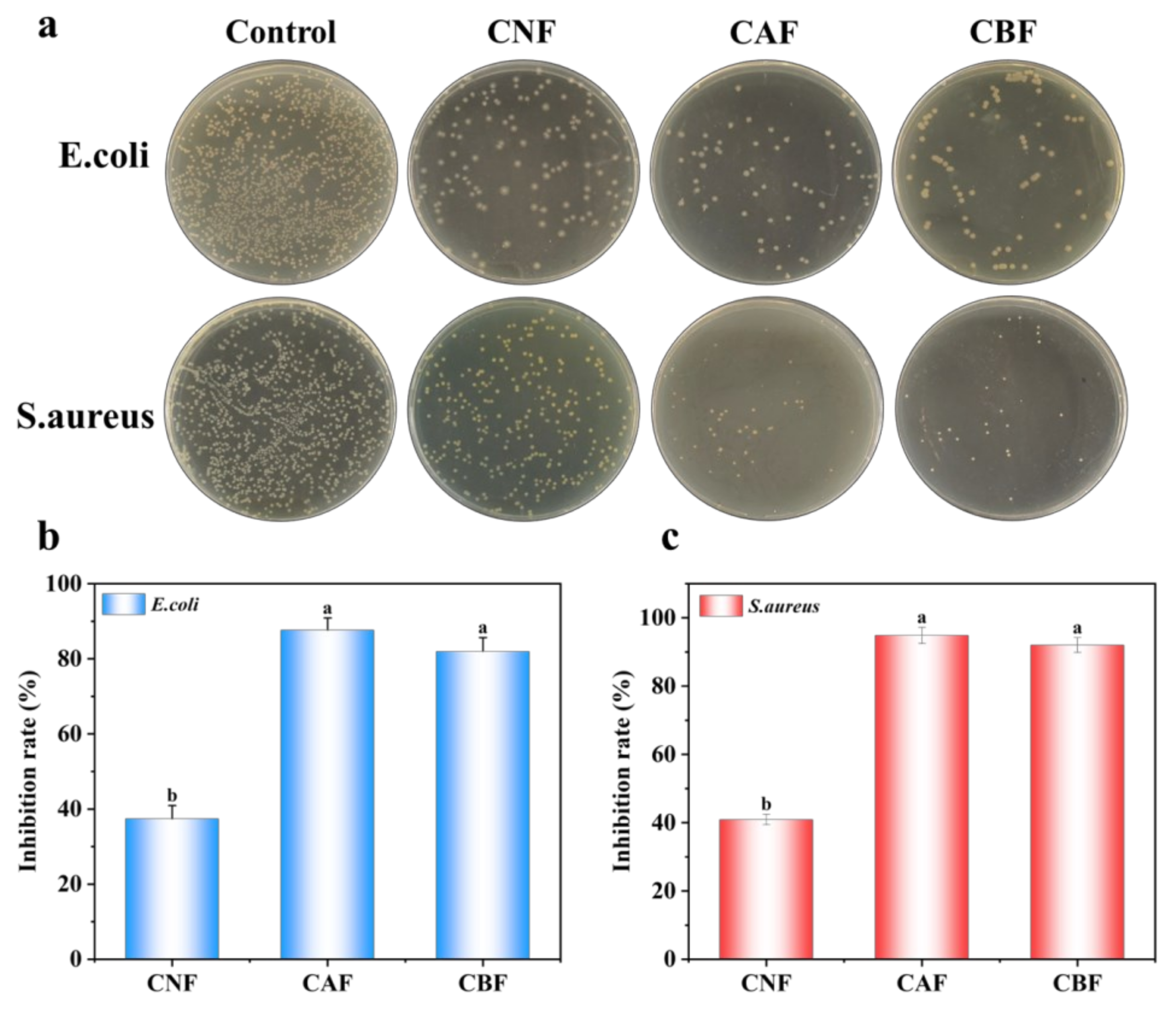
3.3. Antibactirial Properties
3.4. pH Sensitivity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Punia Bangar, S.; Thakur, N.; Trif, M. Recent Advancements in Smart Biogenic Packaging: Reshaping the Future of the Food Packaging Industry. Polymers 2022, 14, 829. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, T. Smart food packaging: Recent advancement and trends. Adv. Food Nutr. Res. 2024, 111, 1–33. [Google Scholar] [PubMed]
- Du, L.; Huang, X.; Li, Z.; Qin, Z.; Zhang, N.; Zhai, X.; Shi, J.; Zhang, J.; Shen, T.; Zhang, R.; et al. Application of Smart Packaging in Fruit and Vegetable Preservation: A Review. Foods 2025, 14, 447. [Google Scholar] [CrossRef]
- Gao, X.; Wu, W.; Chen, H.; Niu, B.; Han, Y.; Fang, X.; Chen, H.; Liu, R.; Gao, H. Nitric oxide treatment delays quality deterioration and enzymatic browning of Agaricus bisporus via reactive oxygen metabolism regulation. Food Front. 2023, 4, 447–458. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Riaz, S.; Khan, M.U.; Sultan, M.; Munekata, P.E.S.; Lorenzo, J.M. Biodegradable active, intelligent, and smart packaging materials for food applications. Food Packag. Shelf Life 2022, 33, 100903. [Google Scholar] [CrossRef]
- Mishra, B.; Panda, J.; Mishra, A.K.; Nath, P.C.; Nayak, P.K.; Mahapatra, U.; Sharma, M.; Chopra, H.; Mohanta, Y.K.; Sridhar, K. Recent advances in sustainable biopolymer-based nanocomposites for smart food packaging: A review. Int. J. Biol. Macromol. 2024, 279, 135583. [Google Scholar] [CrossRef]
- Upadhyay, A.; Agbesi, P.; Arafat, K.M.Y.; Urdaneta, F.; Dey, M.; Basak, M.; Hong, S.; Umeileka, C.; Argyropoulos, D. Bio-based smart packaging: Fundamentals and functions in sustainable food systems. Trends Food Sci. Technol. 2024, 145, 104369. [Google Scholar] [CrossRef]
- Gawel, J.P.F.; Aldridge, D.C.; Willer, D.F. Barriers and drivers to increasing sustainable bivalve seafood consumption in a mass market economy. Food Front. 2023, 4, 1257–1269. [Google Scholar] [CrossRef]
- Song, T.; Qian, S.; Lan, T.; Wu, Y.; Liu, J.; Zhang, H. Recent Advances in Bio-Based Smart Active Packaging Materials. Foods 2022, 11, 2228. [Google Scholar] [CrossRef]
- Yekta, R.; Abedi-Firoozjah, R.; Azimi Salim, S.; Khezerlou, A.; Abdolmaleki, K. Application of cellulose and cellulose derivatives in smart/intelligent bio-based food packaging. Cellulose 2023, 30, 9925–9953. [Google Scholar] [CrossRef]
- Xia, G.M.; Ma, Y.Y.; Ma, Q.C.; Yao, X.H.; Xu, Z.; Ji, X.X.; Zhang, F.S. Anti-ultraviolet, antioxidant and pH-responsive cellulose-based composite film incorporated with alizarin for intelligent packaging applications. Food Packag. Shelf Life 2024, 46, 101413. [Google Scholar] [CrossRef]
- Ge, H.; Li, T.; Yang, Q.; Tang, Y.; Liu, J.; Yu, Y.; Zhang, T. Egg white peptides administration in enhancing pathological immune response and regulating intestinal bacteria abundance: A new strategy for relieving young mice colitis. Food Front. 2023, 4, 782–794. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Development and characterization of plant oil-incorporated carboxymethyl cellulose/bacterial cellulose/glycerol-based antimicrobial edible films for food packaging applications. Adv. Compos. Hybrid Mater. 2022, 5, 973–990. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Yilmaz, B.; Mousavi Khaneghah, A.; Hano, C.; Shariati, M.A.; Punia Bangar, S.; Goksen, G.; Dhama, K.; Manuel Lorenzo, J. Cinnamon: An antimicrobial ingredient for active packaging. Food Packag. Shelf Life 2023, 35, 101026. [Google Scholar] [CrossRef]
- Alonso, P.; Fernandez-Pastor, S.; Guerrero, A. Application of Cinnamon Essential Oil in Active Food Packaging: A Review. Appl. Sci. 2024, 14, 6554. [Google Scholar] [CrossRef]
- Sun, J.; Leng, X.; Zang, J.; Zhao, G. Bio-based antibacterial food packaging films and coatings containing cinnamaldehyde: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 140–152. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; Castillo, R.; Jiménez, A.J.L.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. Polysaccharide film containing cinnamaldehyde-chitosan nanoparticles, a new eco-packaging material effective in meat preservation. Food Chem. 2024, 437, 137710. [Google Scholar] [CrossRef]
- Deng, S.; Cui, C.-X.; Liu, L.; Duan, L.; Wang, J.; Zhang, Y.; Qu, L. A facile and controllable one-pot synthesis approach to amino-functionalized hollow silica nanoparticles with accessible ordered mesoporous shells. Chin. Chem. Lett. 2021, 32, 1177–1180. [Google Scholar] [CrossRef]
- Cui, X.; Zheng, R.-R.; Wang, J.-Y.; Chen, S.-W.; Yu, Y.; Guo, L.-Y.; Wang, C.; Wang, L.-Y.; Ruan, X. Preparation and properties of mesoporous SiO2/polyimide composite films. Polym. Compos. 2024, 45, 2189–2201. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Yang, W. Synthesis of Mesoporous Silica Using the Sol-Gel Approach: Adjusting Architecture and Composition for Novel Applications. Nanomaterials 2024, 14, 903. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Lu, W.W.; Zhu, B.F.; Chen, H.Y.; Li, L.; Qin, Y.Y. Study on the property of mesoporous silica nanoparticles loaded with nisin for poly (lactic acid)-based packaging film. Polym. Compos. 2023, 44, 6676–6690. [Google Scholar] [CrossRef]
- Thongnoppakhun, N.; Amnuaypanich, S.; Prakobdee, J.; Rugmai, S.; Amnuaypanich, S. Sonochemical synthesis of hollow mesoporous silica spheres (HMSSs) and its effective utilization for one-step synthesis of curcumin-loaded HMSSs. Mater. Chem. Phys. 2024, 322, 129588. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, J.; Wang, J.; Xiang, C.; Wang, R. Synthesis of hollow mesoporous silica spheres functionalized with copper ferrocyanide and its application for Cs+ removal. Environ. Sci. Pollut. Res. 2022, 29, 53509–53521. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, D.; Yang, Y.; Huang, J.; Lin, B.; Zheng, Y.; Wang, Q. Characterization and in vitro assessment of probiotic potential of Lactiplantibacillus plantarum BXM2 from fermented honey passion fruit beverage. Food Front. 2023, 4, 1372–1381. [Google Scholar] [CrossRef]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Recent developments in the application of natural pigments as pH-sensitive food freshness indicators in biopolymer-based smart packaging: Challenges and opportunities. Int. J. Food Sci. Technol. 2024, 59, 2148–2161. [Google Scholar] [CrossRef]
- Neves, D.; Andrade, P.B.; Videira, R.A.; de Freitas, V.; Cruz, L. Berry anthocyanin-based films in smart food packaging: A mini-review. Food Hydrocoll. 2022, 133, 107885. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Wang, W.; Zhang, J. Effect of Anthocyanins on Colorimetric Indicator Film Properties. Coatings 2023, 13, 1682. [Google Scholar] [CrossRef]
- Gao, R.; Hu, H.; Shi, T.; Bao, Y.; Sun, Q.; Wang, L.; Ren, Y.; Jin, W.; Yuan, L. Incorporation of gelatin and Fe2+ increases the pH-sensitivity of zein-anthocyanin complex films used for milk spoilage detection. Curr. Res. Food Sci. 2022, 5, 677–686. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Liu, R.; Du, J.; Liu, Q.; Wang, T.; Wang, Y.; Wang, H. Intelligent double-layer pads containing blueberry anthocyanins/citric acid/tricolor lake for chilled pork real-time freshness monitoring. Food Chem. 2025, 476, 143372. [Google Scholar] [CrossRef]
- Xu, M.; Fang, D.; Shi, C.; Xia, S.; Wang, J.; Deng, B.; Kimatu, B.M.; Guo, Y.; Lyu, L.; Wu, Y.; et al. Anthocyanin-loaded polylactic acid/quaternized chitosan electrospun nanofiber as an intelligent and active packaging film in blueberry preservation. Food Hydrocoll. 2025, 158, 110586. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, Z.; Xie, F.; Tang, S.; Fang, J.; Wang, X. 3D printed nanocellulose-based label for fruit freshness keeping and visual monitoring. Carbohydr. Polym. 2021, 273, 118545. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Teng, H.; An, F.; Wang, Y.; Qiu, R.; Chen, L.; Song, H. Nanocelluloses review: Preparation, biological properties, safety, and applications in the food field. Food Front. 2023, 4, 85–99. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Li, Y.-C.; Xiao, H.; Wang, X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018, 199, 210–218. [Google Scholar]
- Pavaloiu, R.-D.; Sha’at, F.; Neagu, G.; Deaconu, M.; Bubueanu, C.; Albulescu, A.; Sha’at, M.; Hlevca, C. Encapsulation of Polyphenols from Lycium barbarum Leaves into Liposomes as a Strategy to Improve Their Delivery. Nanomaterials 2021, 11, 1938. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Jia, J.; Xing, H.; Yang, J.; He, T.; Liu, H.; Zhang, T.; Wu, W. pH-responsive antibacterial emulsion gel based on cinnamaldehyde and carboxymethyl chitosan for fruits preservation applications. Int. J. Biol. Macromol. 2025, 291, 139062. [Google Scholar]
- Yeldir, E.K.; Oral, A. Environmentally Friendly Preparation of Chitosan Microspheres for Cinnamaldehyde Encapsulation: A Sustainable Sustained-Release System. Maced. J. Chem. Chem. Eng. 2021, 40, 253–261. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, C.; Zhang, Y.; Tang, X. Structure and functionality of cinnamaldehyde/chitosan/gum Arabic complex particles. Food Hydrocoll. 2024, 146, 109220. [Google Scholar] [CrossRef]
- Muhoza, B.; Qi, B.; Harindintwali, J.D.; Koko, M.Y.F.; Zhang, S.; Li, Y. Encapsulation of cinnamaldehyde: An insight on delivery systems and food applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 2521–2543. [Google Scholar]
- Wei, L.; Ren, Y.; Hou, Y.; Jin, P.; Zheng, Y.; Wu, Z. MXene/nanocellulose/carbon sphere composite films with a multistage “egg-box” structure for electromagnetic-interference shielding and pressure sensors. J. Mater. Chem. A 2025, 13, 8876–8889. [Google Scholar] [CrossRef]
- Guo, J.; Ding, K.; Li, S.; Li, S.; Jin, P.; Zheng, Y.; Wu, Z. Polysaccharide-based high barrier food packaging film: Design and application. Crit. Rev. Food Sci. Nutr. 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ren, Y.; Hou, Y.; Zhan, Q.; Jin, P.; Zheng, Y.; Wu, Z. Polysaccharide-Based Composite Films: Promising Biodegradable Food Packaging Materials. Foods 2024, 13, 3674. [Google Scholar] [CrossRef]
- Xuan, S.; Shen, P.; Ren, Y.; Li, S.; Jin, P.; Zheng, Y.; Wu, Z. Modified SiO2@cinnamaldehyde/nanocellulose coating film for loquat preservation. Int. J. Biol. Macromol. 2024, 278, 134862. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Guo, J.; Li, M.; Qin, X.; Hou, Y.; Zhan, Q.; Jin, P.; Zheng, Y.; Wu, Z. Long-acting antibacterial nanochitin/nanocellulose bioplastic packaging film. Int. J. Biol. Macromol. 2025, 309, 142862. [Google Scholar] [CrossRef]
- Cox, H.J.; Li, J.; Saini, P.; Paterson, J.R.; Sharples, G.J.; Badyal, J.P.S. Bioinspired and eco-friendly high efficacy cinnamaldehyde antibacterial surfaces. J. Mater. Chem. B 2021, 9, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, P.; Luo, X.; Huang, A.; Wang, G. Study on the antibacterial activity and mechanism of Cinnamaldehyde against Methicillin-resistant Staphylococcus aureus. Eur. Food Res. Technol. 2024, 250, 1069–1081. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, T.; Hu, H.; Duan, X.; Wu, K.; Chai, X.; He, D. Trans-cinnamaldehyde fumigation inhibits Escherichia coli by affecting the mechanism of intracellular biological macromolecules. Nat. Prod. Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Luo, B.; Xuan, S.; Wang, X.; Ding, K.; Jin, P.; Zheng, Y.; Wu, Z. Liposome/chitosan coating film bioplastic packaging for Litchi fruit preservation. Food Chem. 2025, 464, 141850. [Google Scholar] [CrossRef]



| L | a | b | ∆E | Film Color | |
|---|---|---|---|---|---|
| CNF | 82.87 | −0.22 | −0.28 | 14.26 |  |
| CAF | 80.42 | −2.10 | 7.74 | 18.56 |  |
| CBF | 33.01 | 3.01 | −4.31 | 64.33 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Guo, J.; Zhong, Z.; Chen, J.; Jin, P.; Zheng, Y.; Wu, Z. Integrated Smart Packaging of Modified Silica/Anthocyanin/Nanocellulose for Preservation and Monitoring. Foods 2025, 14, 1888. https://doi.org/10.3390/foods14111888
Ren Y, Guo J, Zhong Z, Chen J, Jin P, Zheng Y, Wu Z. Integrated Smart Packaging of Modified Silica/Anthocyanin/Nanocellulose for Preservation and Monitoring. Foods. 2025; 14(11):1888. https://doi.org/10.3390/foods14111888
Chicago/Turabian StyleRen, Yu, Jing Guo, Zehao Zhong, Jinjin Chen, Peng Jin, Yonghua Zheng, and Zhengguo Wu. 2025. "Integrated Smart Packaging of Modified Silica/Anthocyanin/Nanocellulose for Preservation and Monitoring" Foods 14, no. 11: 1888. https://doi.org/10.3390/foods14111888
APA StyleRen, Y., Guo, J., Zhong, Z., Chen, J., Jin, P., Zheng, Y., & Wu, Z. (2025). Integrated Smart Packaging of Modified Silica/Anthocyanin/Nanocellulose for Preservation and Monitoring. Foods, 14(11), 1888. https://doi.org/10.3390/foods14111888







