Microbial Assessment and Antibiotic Susceptibility of Isolated Pathogens in Retail Chicken
Abstract
:1. Introduction
2. Materials and Methods
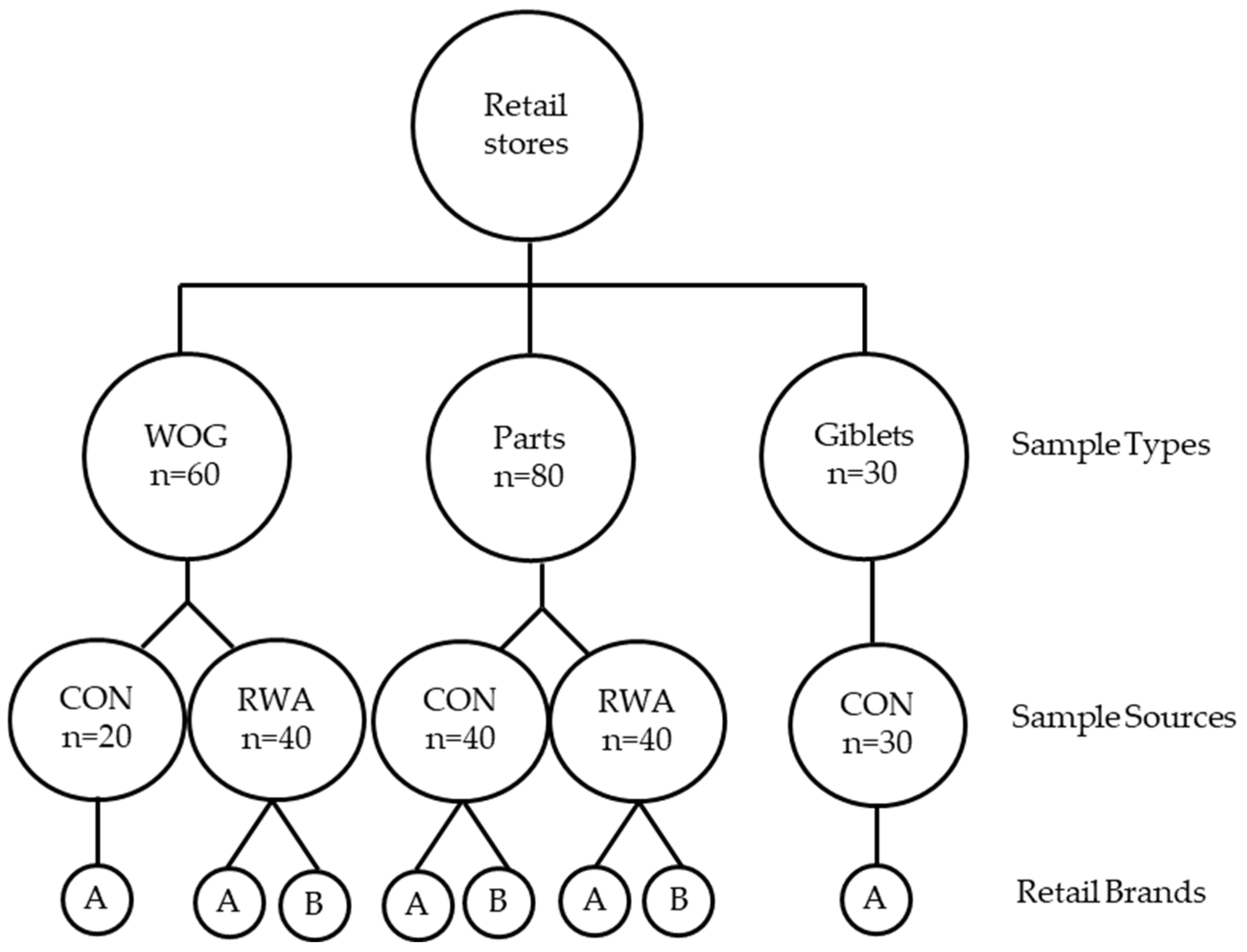
2.1. Experimental Design and Sample Collection
2.2. Sample Processing
2.3. Salmonella Isolation, Identification, and Quantification
2.4. Campylobacter Isolation, Quantification, and Identification
2.5. Detection of Indicator and Spoilage Organisms
2.6. Antimicrobial Susceptibility Testing
2.7. Statistical Analysis
3. Results
3.1. Incidence and Quantity of Salmonella in Retail Chicken
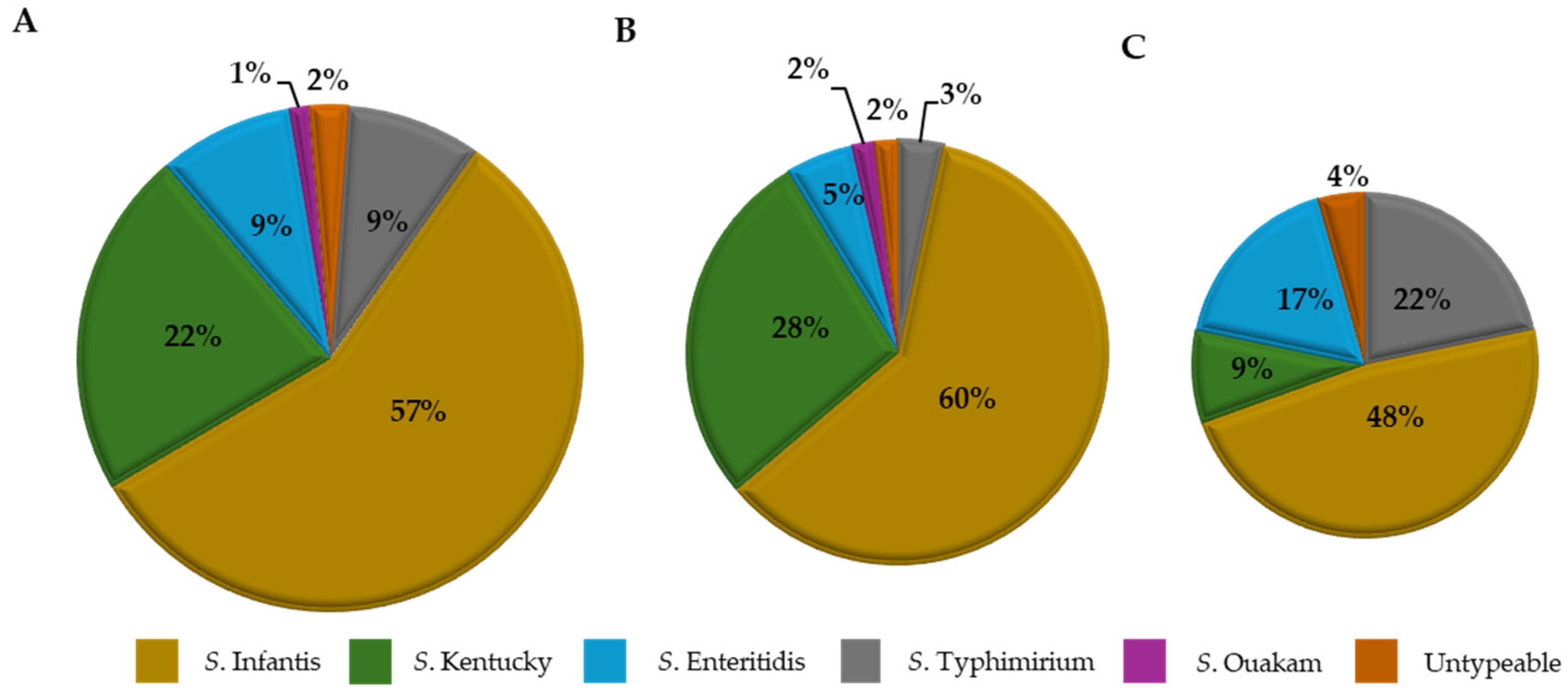
Serogroup Distribution of Salmonella Isolates
3.2. Incidence and Quantity of Campylobacter in Retail Chicken
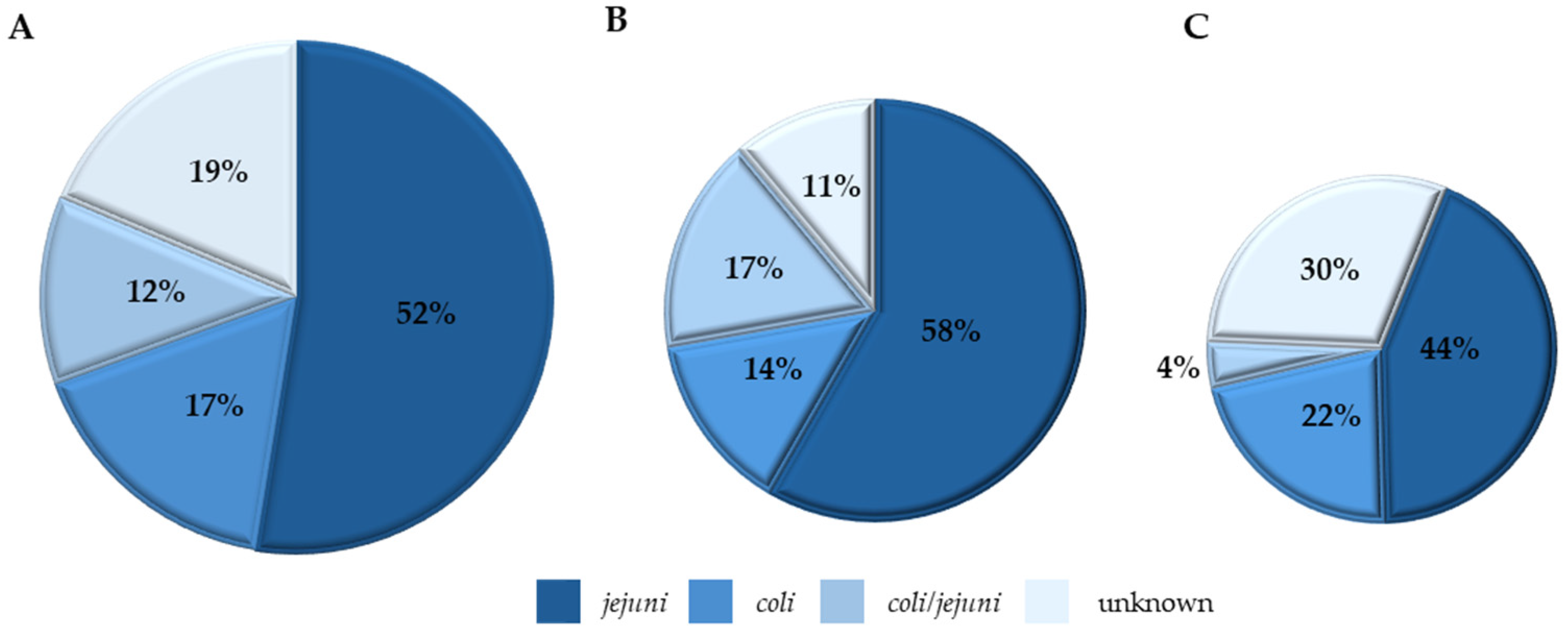
Species Distribution of Campylobacter Isolates
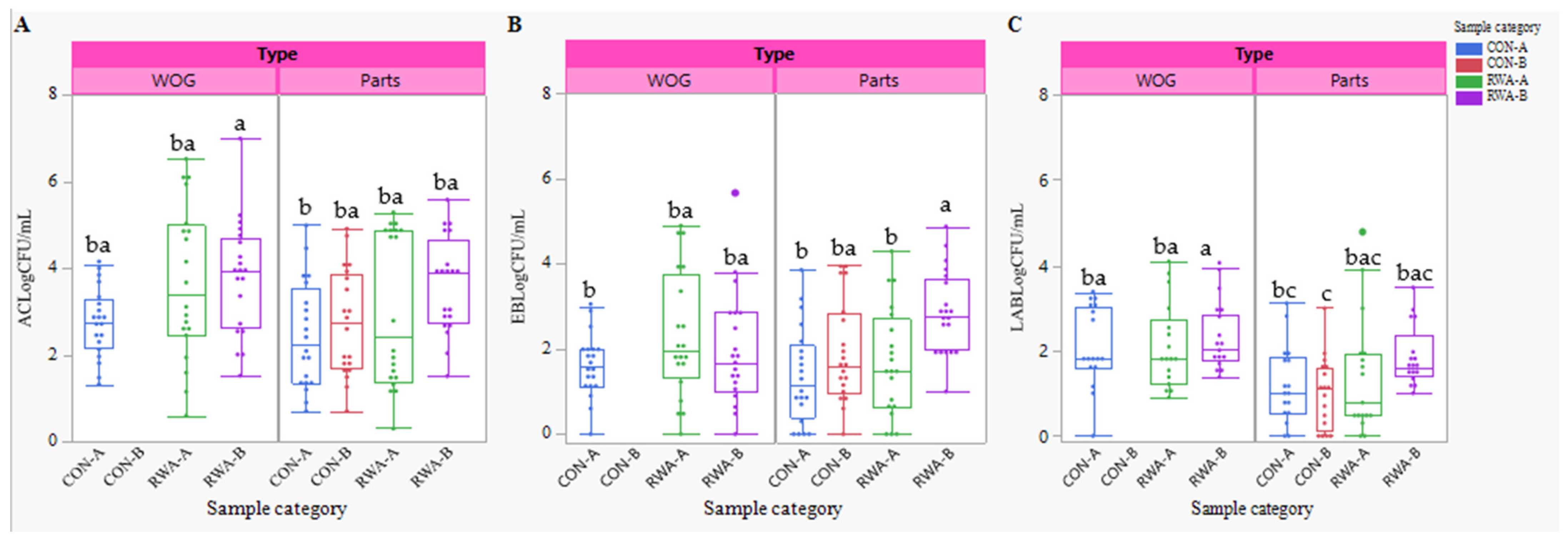
3.3. Indicator and Spoilage Organisms
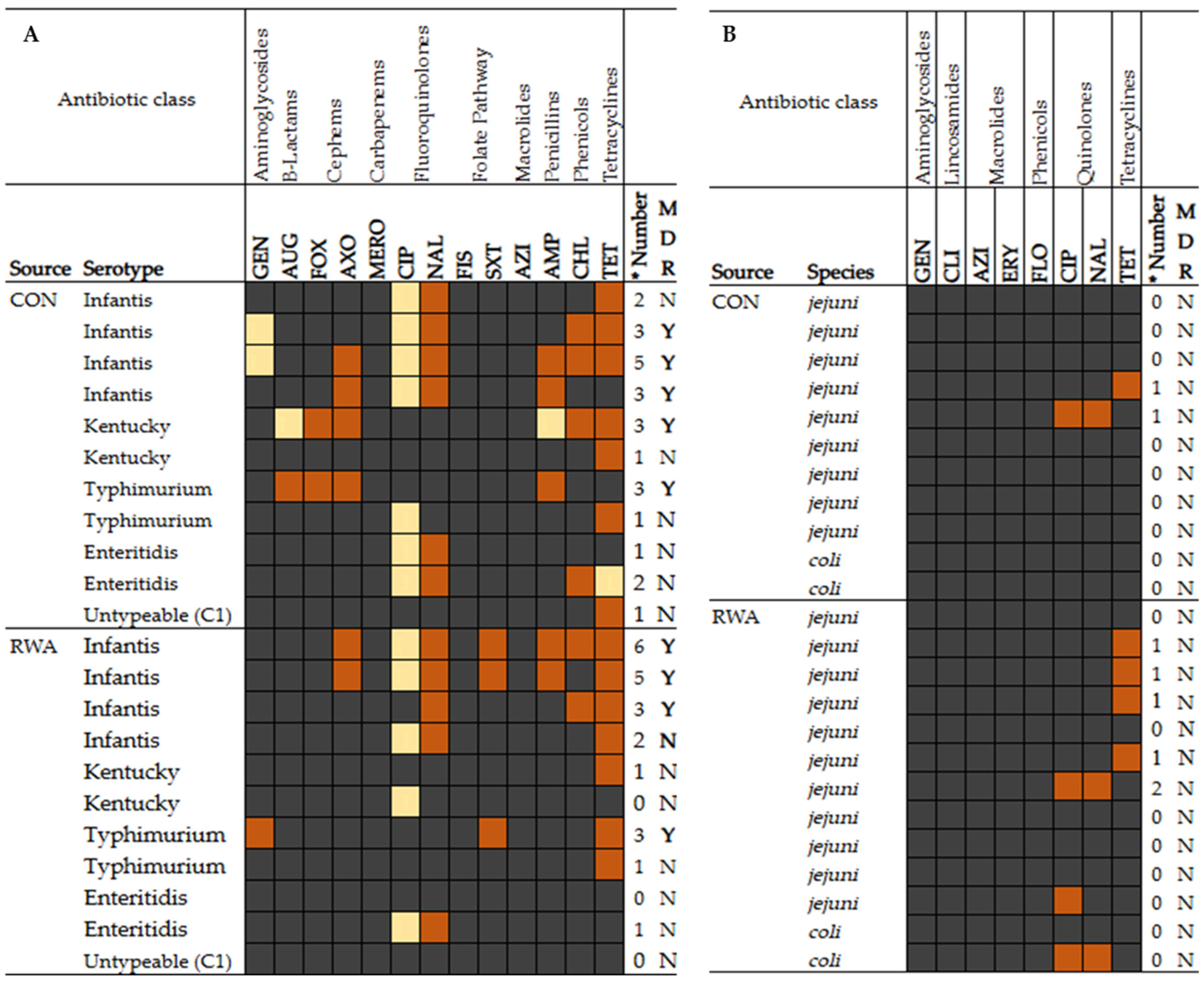
3.4. Antimicrobial Susceptibility Profile of Selected Salmonella and Campylobacter Isolates
4. Discussion
4.1. Pathogen Incidence, Quantification, and Serogroup/Species Dynamics
4.2. Indicators and Lactic Acid Bacteria
4.3. Antimicrobial Susceptibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture USDA. Poultry Expected to Continue Leading Global Meat Imports as Demand Rises. Available online: https://www.ers.usda.gov/topics/animal-products/poultry-eggs/sector-at-a-glance/ (accessed on 17 June 2024).
- National Chicken Council NCC. Per Capita Consumption of Poultry and Livestock, 1965 to Forecast 2022, in Pounds. Available online: https://www.nationalchickencouncil.org/about-the-industry/statistics/per-capita-consumption-of-poultry-and-livestock-1965-to-estimated-2012-in-pounds/ (accessed on 17 June 2024).
- Connolly, G.; Clark, C.M.; Campbell, R.E.; Byers, A.W.; Reed, J.B.; Campbell, W. Poultry Consumption and Human Health: How Much Is Really Known? A Systematically Searched Scoping Review and Research Perspective. Adv. Nutr. 2022, 13, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention CDC. Chicken and Food Poisoning. Available online: https://www.cdc.gov/food-safety/foods/chicken.html (accessed on 17 June 2024).
- Coveny, M.M. Salmonellosis and the Rare Side-Effects that Can Last Years: Illness After Salmonella Poisoning. Food poisoning news. Available online: https://www.foodpoisoningnews.com/salmonellosis-and-the-rare-side-effects-that-can-last-years-illness-after-salmonella-poisoning/ (accessed on 17 June 2024).
- Centers for Disease Control and Prevention CDC. Salmonella. Available online: https://www.cdc.gov/salmonella/signs-symptoms/?CDC_AAref_Val=https://www.cdc.gov/salmonella/general/salmonella-symptoms.html (accessed on 17 June 2024).
- Ford, L.; Buuck, S.; Eisenstein, T.; Cote, A.; McCormic, Z.D.; Kremer-Caldwell, S.; Kissler, B.; Forstner, M.; Sorenson, A.; Wise, M.E.; et al. Salmonella Outbreaks Associated with Not Ready-to-Eat Breaded, Stuffed Chicken Products-United States, 1998–2022. MMWR. 2023, 72, 484–487. Available online: https://www.cdc.gov/mmwr/volumes/72/wr/mm7218a2.htm?s_cid=mm7218a2_w (accessed on 18 June 2024). [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention CDC. Campylobacter Infection (Campylobacteriosis). Available online: https://www.cdc.gov/campylobacter/signs-symptoms/guillain-barre-syndrome.html (accessed on 18 June 2024).
- O’Bryan, C.A.; Ricke, S.C.; Marcy, J.A. Public health impact of Salmonella spp. on raw poultry. Current concepts and future prospects in the United States. Food Control 2022, 132, 108539. [Google Scholar] [CrossRef]
- Wang, J.; Vaddu, S.; Bhumanapalli, S.; Mishra, A.; Applegate, T.; Singh, M.; Thippareddi, H.A. Systematic review and meta-analysis of the sources of Salmonella in poultry production (pre-harvest) and their relative contributions to the microbial risk of poultry meat. Poult. Sci. 2023, 102, 102566. [Google Scholar] [CrossRef]
- Obe, T.; Nannapaneni, R.; Schilling, W.; Zhang, L.; McDaniel, C.; Kiess, A. Prevalence of Salmonella enterica on poultry processing equipment after completion of sanitization procedures. Poult. Sci. 2020, 99, 4539–4548. [Google Scholar] [CrossRef]
- Jol, S.; Kassainenko, A.; Wszol, K.; Oggel, J. Issues in Time and Temperature Abuse of Refrigerated Foods. Food Safety Magazine. Available online: https://www.food-safety.com/articles/4647-issues-in-time-and-temperature-abuse-of-refrigerated-foods (accessed on 18 June 2024).
- Donelan, A.K.; Chambers, D.H.; Chambers, E.; Godwin, S.L.; Cates, S.C. Consumer Poultry Handling Behavior in the Grocery Store and In-Home Storage. J. Food Prot. 2016, 79, 582–588. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention CDC. CDC and Food Safety. Available online: https://www.cdc.gov/food-safety/media/pdfs/cdc-and-food-safety.pdf (accessed on 23 June 2024).
- University of Georgia Extension. Organic Poultry Production vs. Other. Available online: https://extension.uga.edu/publications/detail.html?number=C1139&title=organic-poultry-production-vs-other-systems (accessed on 25 June 2024).
- Mak, P.H.W.; Rehman, M.A.; Kiarie, E.G.; Topp, E.; Diarra, M.S. Production systems and important antimicrobial resistant-pathogenic bacteria in poultry: A review. J. Anim. Sci. Biotechnol. 2022, 13, 148. [Google Scholar] [CrossRef]
- Golden, C.E.; Rothrock, M.J.; Mishra, A. Mapping foodborne pathogen contamination throughout the conventional and alternative poultry supply chains. Poult. Sci. 2021, 100, 101157. [Google Scholar] [CrossRef]
- United States Department of Agriculture USDA-FSIS. Meat and Poultry Labeling Terms. Available online: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/meat-and-poultry-labeling-terms (accessed on 16 August 2024).
- World Health Organization WHO. WHO Guidelines on the Use of Medically Important Antimicrobials in Food-Producing Animals. Available online: https://www.who.int/publications/i/item/9789241550130 (accessed on 5 August 2024).
- Mujahid, S.; Hansen, M.; Miranda, R.; Newsom-Stewart, K.; Rogers, J.E. Prevalence and Antibiotic Resistance of Salmonella and Campylobacter Isolates from Raw Chicken Breasts in Retail Markets in the United States and Comparison to Data from the Plant Level. Life 2023, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Ge, B.; Zheng, J.; Meng, J. Prevalence and antimicrobial resistance of Campylobacter spp. and Salmonella serovars in organic chickens from Maryland retail stores. Appl. Environ. Microbiol. 2005, 71, 4108–4111. [Google Scholar] [CrossRef] [PubMed]
- Lestari, S.I.; Han, F.; Wang, F.; Ge, B. Prevalence and Antimicrobial Resistance of Salmonella Serovars in Conventional and Organic Chickens from Louisiana Retail Stores. J. Food Prot. 2009, 72, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.E.; Mishra, A. Prevalence of Salmonella and Campylobacter spp. In alternative and conventionally produced chicken in the United States: A systematic review and meta-analysis. J. Food Prot. 2020, 83, 1181–1197. [Google Scholar] [CrossRef]
- Mollenkopf, D.F.; Cenera, J.K.; Bryant, E.M.; King, C.A.; Kashoma, I.; Kumar, A.; Funk, J.A.; Rajashekara, G.; Wittum, T.E. Organic or Antibiotic-Free Labeling Does Not Impact the Recovery of Enteric Pathogens and Antimicrobial-Resistant Escherichia coli from Fresh Retail Chicken. Foodborne Pathog. Dis. 2014, 11, 920–929. [Google Scholar] [CrossRef]
- Anuradha, J.P.; Jurgen, S.; Abdirahman, D.; John, B.; Salina, P. Prevalence and antibiotic resistance of Salmonella in organic and non-organic chickens on the Eastern Shore of Maryland, USA. Front. Microbiol. 2023, 14, 1272892. [Google Scholar] [CrossRef]
- United States Department of Agriculture USDA-FSIS. Microbiology Laboratory Guidebook. Available online: https://www.fsis.usda.gov/news-events/publications/microbiology-laboratory-guidebook (accessed on 27 August 2024).
- United States Department of Agriculture USDA-FSIS. Salmonella and Campylobacter Verification Program for Raw Poultry Products. Available online: https://www.fsis.usda.gov/policy/fsis-directives/10250.1 (accessed on 27 August 2024).
- Centers for Disease Control and Prevention CDC. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS). Available online: https://www.cdc.gov/narms/about/antibiotics-tested.html (accessed on 27 August 2024).
- Clinical & Laboratory Standards Institute CLSI. CLSI M100. Available online: https://clsi.org/shop/standards/m100/ (accessed on 27 August 2024).
- SAS Software Version 9.4. Cary, NC: SAS Institute Inc. Available online: https://www.sas.com/en_us/legal/editorial-guidelines.html (accessed on 27 August 2024).
- Novoa Rama, E.; Bailey, M.; Kumar, S.; Leone, C.; Den Bakker, H.C.; Thippareddi, H.; Singh, M. Prevalence and antimicrobial resistance of Salmonella in conventional and no antibiotics ever broiler farms in the United States. Food Control 2022, 135, 108738. [Google Scholar] [CrossRef]
- Poudel, S.; Li, T.; Chen, S.; Zhang, X.; Cheng, W.-H.; Sukumaran, A.T.; Kiess, A.S.; Zhang, L. Prevalence, Antimicrobial Resistance, and Molecular Characterization of Campylobacter Isolated from Broilers and Broiler Meat Raised without Antibiotics. Microbiol. Spectr. 2022, 10, e00251-22. [Google Scholar] [CrossRef]
- Bailey, M.A.; Taylor, R.M.; Brar, J.S.; Corkran, S.C.; Velásquez, C.; Novoa Rama, E.; Oliver, H.F.; Singh, M. Prevalence and antimicrobial resistance of Campylobacter from antibiotic-free broilers during organic and conventional processing. Poult. Sci. 2019, 98, 1447–1454. [Google Scholar] [CrossRef]
- Bailey, M.; Taylor, R.; Brar, J.; Corkran, S.; Velásquez, C.; Novoa-Rama, E.; Oliver, H.F.; Singh, M. Prevalence and antimicrobial resistance of Salmonella from antibiotic-free broilers during organic and conventional processing. J. Food Prot. 2020, 83, 491–496. [Google Scholar] [CrossRef]
- Chavez-Velado, D.R.; Vargas, D.A.; Sanchez-Plata, M.X. Bio-Mapping Salmonella and Campylobacter Loads in Three Commercial Broiler Processing Facilities in the United States to Identify Strategic Intervention Points. Foods 2024, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.A.; De Villena, J.F.; Larios, V.; Bueno López, R.; Chávez-Velado, D.R.; Casas, D.E.; Jiménez, R.L.; Blandon, S.E.; Sanchez-Plata, M.X. Data-Mining Poultry Processing Bio-Mapping Counts of Pathogens and Indicator Organisms for Food Safety Management Decision Making. Foods 2023, 12, 898. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food Drug Administration FDA. NARMS Now: Integrated Data. Available online: https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/narms-now-integrated-data (accessed on 27 July 2021).
- Ockerman, H.W.; Basu, L. Edible, for human consumption. Encycl. Meat Sci. 2014, 1, 104–111. [Google Scholar] [CrossRef]
- Holst, M.M.; Wittry, B.C.; Crisp, C.; Torres, J.; Irving, D.; Nicholas, D. Contributing Factors of Foodborne Illness Outbreaks—National Outbreak Reporting System, United States, 2014–2022. MMWR 2025, 74, 1–12. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention CDC. Serotypes and the Importance of Serotyping Salmonella. Available online: http://medbox.iiab.me/modules/en-cdc/www.cdc.gov/salmonella/reportspubs/salmonella-atlas/serotyping-importance.html (accessed on 23 June 2024).
- U.S. Food Drug Administration. FDA. Integrated Reports/Summaries. Available online: https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/integrated-reportssummaries (accessed on 27 July 2021).
- Centers for Disease Control and Prevention CDC. Multidrug-Resistant Salmonella Infantis Infections Linked to Raw Chicken Products. Available online: https://archive.cdc.gov/#/details?archive_url=https://archive.cdc.gov/www_cdc_gov/salmonella/infantis-10-18/index.html (accessed on 27 July 2021).
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.E.; Oliver, H.F.; Martin-Gonzalez, F.S.; Singh, M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef]
- Siceloff, A.T.; Waltman, D.; Shariat, N.W. Regional Salmonella Differences in United States Broiler Production from 2016 to 2020 and the Contribution of Multiserovar Populations to Salmonella Surveillance. Appl. Environ. Microbiol. 2022, 88, e00204-22. [Google Scholar] [CrossRef]
- Obe, T.; Siceloff, A.T.; Crowe, M.G.; Morgan Scott, H.; Shariat, N.W. Combined Quantification and Deep Serotyping for Salmonella Risk Profiling in Broiler Flocks. Appl. Environ. Microbiol. 2023, 89, e02035-22. [Google Scholar] [CrossRef]
- Williams, M.S.; Ebel, E.D.; Golden, N.J.; Saini, G.; Nyirabahizi, E.; Clinch, N. Assessing the effectiveness of performance standards for Salmonella contamination of chicken parts. Int. J. Food Microbiol. 2022, 378, 109801. [Google Scholar] [CrossRef]
- Parzygnat, J.L.; Dunn, R.R.; Koci, M.D.; Crespo, R.; Harden, L.; Hakur, S. Fluoroquinolone-resistant Campylobacter in backyard and commercial broiler production systems in the United States. JAC Antimicrob. Resist. 2024, 6, dlae102. [Google Scholar] [CrossRef]
- Barco, L.; Belluco, S.; Roccato, A.; Ricci, A. A systematic review of studies on Escherichia coli and Enterobacteriaceae on beef carcasses at the slaughterhouse. Int. J. Food Microbiol. 2015, 207, 30–39. [Google Scholar] [CrossRef]
- Sivanandy, P.; Yuk, L.S.; Yi, C.S.; Kaur, I.; Ern, F.H.S.; Manirajan, P. A systematic review of recent outbreaks and the efficacy and safety of drugs approved for the treatment of Salmonella infections. IJID Regions 2024, 14, 100516. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Anal, A.K. Prevalence and antibiogram study of Salmonella and Staphylococcus aureus in poultry meat. Asian Pac. J. Trop. Biomed. 2013, 3, 163–168. [Google Scholar] [CrossRef]
- Yin, X.; M’ikanatha, N.M.; Nyirabahizi, E.; McDermott, P.F.; Tate, H. Antimicrobial resistance in non-Typhoidal Salmonella from retail poultry meat by antibiotic usage-related production claims—United States, 2008–2017. Int. J. Food Microbiol. 2021, 342, 109044. [Google Scholar] [CrossRef]
- Shah, D.H.; Paul, N.C.; Sischo, W.C.; Crespo, R.; Guard, J. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 2017, 96, 687–702. [Google Scholar] [CrossRef]
- U.S. Food Drug Administration. FDA. NARMS Integrated Report. Available online: https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/2015-narms-integrated-report (accessed on 27 July 2024).
| CLSI Class | Antimicrobial Agent | Abbreviation | Range of Concentration (μg/mL) |
|---|---|---|---|
| Salmonella isolates | |||
| Aminoglycosides | Gentamicin | GEN | 0.25–16 |
| β–lactam agents | Amoxicillin-clavulanic acid | AUG | 1/0.5–32/16 |
| Cephems | Cefoxitin | FOX | 0.5–32 |
| Cephems | AXO | 0.25–64 | |
| Folate pathway antagonists | Sulfisoxazole | FIS | 16–256 |
| Trimethoprim/Sulfamethoxazole | SXT | 0.12/2.38–4/76 | |
| Macrolides | Azithromycin | AZI | 0.25–32/0.12–16 |
| Penems | Meropenem | MERO | 0.06–4 |
| Penicillins | Ampicillin | AMP | 1–32 |
| Quinolones | Ciprofloxacin | CIP | 0.015–4 |
| Nalidixic acid | NAL | 0.5–32 | |
| Phenicols | Chloramphenicol | CHL | 2–32 |
| Tetracyclines | Tetracycline | TET | 4–32 |
| Campylobacter isolates | |||
| Aminoglycosides | Gentamicin | GEN | 0.25–16 |
| Lincosamides | Clindamycin | CLI | 0.03–16/0.016–256 * |
| Macrolides | Azithromycin | AZI | 0.015–64/0.016–256 * |
| Erythromycin | ERY | 0.03–64/0.016–256 * | |
| Phenicols | Florfenicol | FLO | 0.03–64 |
| Quinolones | Ciprofloxacin | CIP | 0.015–64/0.002–32 * |
| Nalidixic acid | NAL | 4–64/ 0.016–256 * | |
| Tetracyclines | Tetracycline | TET | 0.06–64/0.016–256 * |
| Product Type | Production System | * Brand | n | Salmonella Positives | Salmonella Quantity Range Log10 CFU/mL | Campylobacter Positives | Campylobacter Quantity Range Log10 CFU/mL |
|---|---|---|---|---|---|---|---|
| WOG | CON | A | 20 | 12 (60) a | <1–2.42 | 10 (50) ba | 10 (<10–4.66) |
| B | - | - | - | - | |||
| RWA | A | 20 | 10 (50) ba | <1–0.86 | 13 (65) a | 10 (<10–2.2) | |
| B | 20 | 5 (25) bac | <1 | 5 (25) bc | - | ||
| Parts | CON | A | 20 | 3 (15) bc | <1 | 0 c | - |
| B | 20 | 5 (25) bac | <1–0.28 | 5 (25) bc | 1 (1.5) | ||
| RWA | A | 20 | 1 (5) c | <1 | 1 (5) c | - | |
| B | 20 | 2 (10) c | 0.98 | 4 (20) bc | - | ||
| Giblets | CON | A | 30 | 24 (80) | <1–3.43 | 21 (70) | 23 (<10–5.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betiku, E.; Crandall, P.G.; Obe, T. Microbial Assessment and Antibiotic Susceptibility of Isolated Pathogens in Retail Chicken. Foods 2025, 14, 1738. https://doi.org/10.3390/foods14101738
Betiku E, Crandall PG, Obe T. Microbial Assessment and Antibiotic Susceptibility of Isolated Pathogens in Retail Chicken. Foods. 2025; 14(10):1738. https://doi.org/10.3390/foods14101738
Chicago/Turabian StyleBetiku, Eniola, Philip Glen Crandall, and Tomi Obe. 2025. "Microbial Assessment and Antibiotic Susceptibility of Isolated Pathogens in Retail Chicken" Foods 14, no. 10: 1738. https://doi.org/10.3390/foods14101738
APA StyleBetiku, E., Crandall, P. G., & Obe, T. (2025). Microbial Assessment and Antibiotic Susceptibility of Isolated Pathogens in Retail Chicken. Foods, 14(10), 1738. https://doi.org/10.3390/foods14101738








