Bifidobacterium animalis Supplementation Improves Intestinal Barrier Function and Alleviates Antibiotic-Associated Diarrhea in Mice
Abstract
1. Introduction
2. Materials and Methods
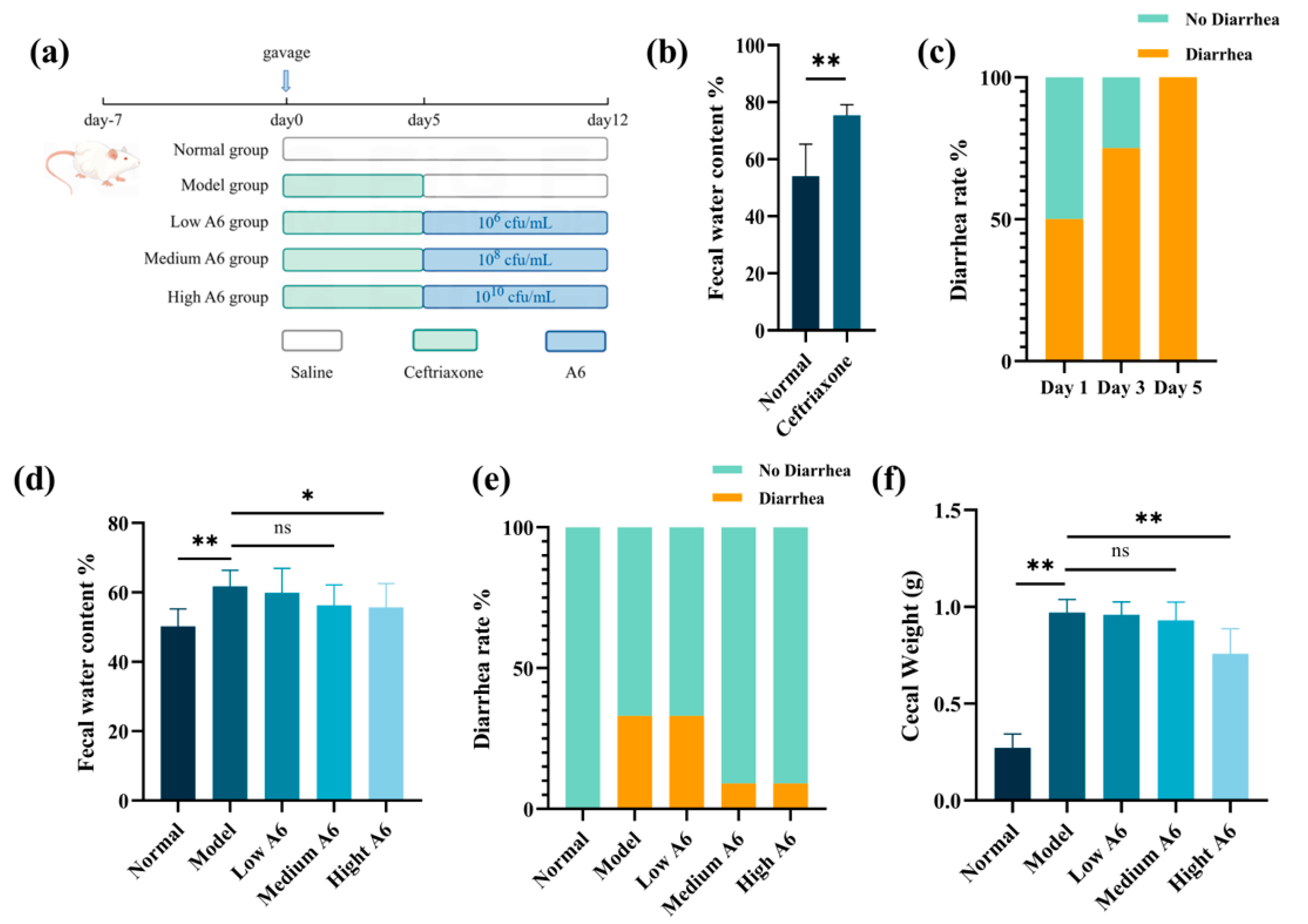
2.1. Mouse Model of AAD
2.2. Histological Analysis of Colon Tissue
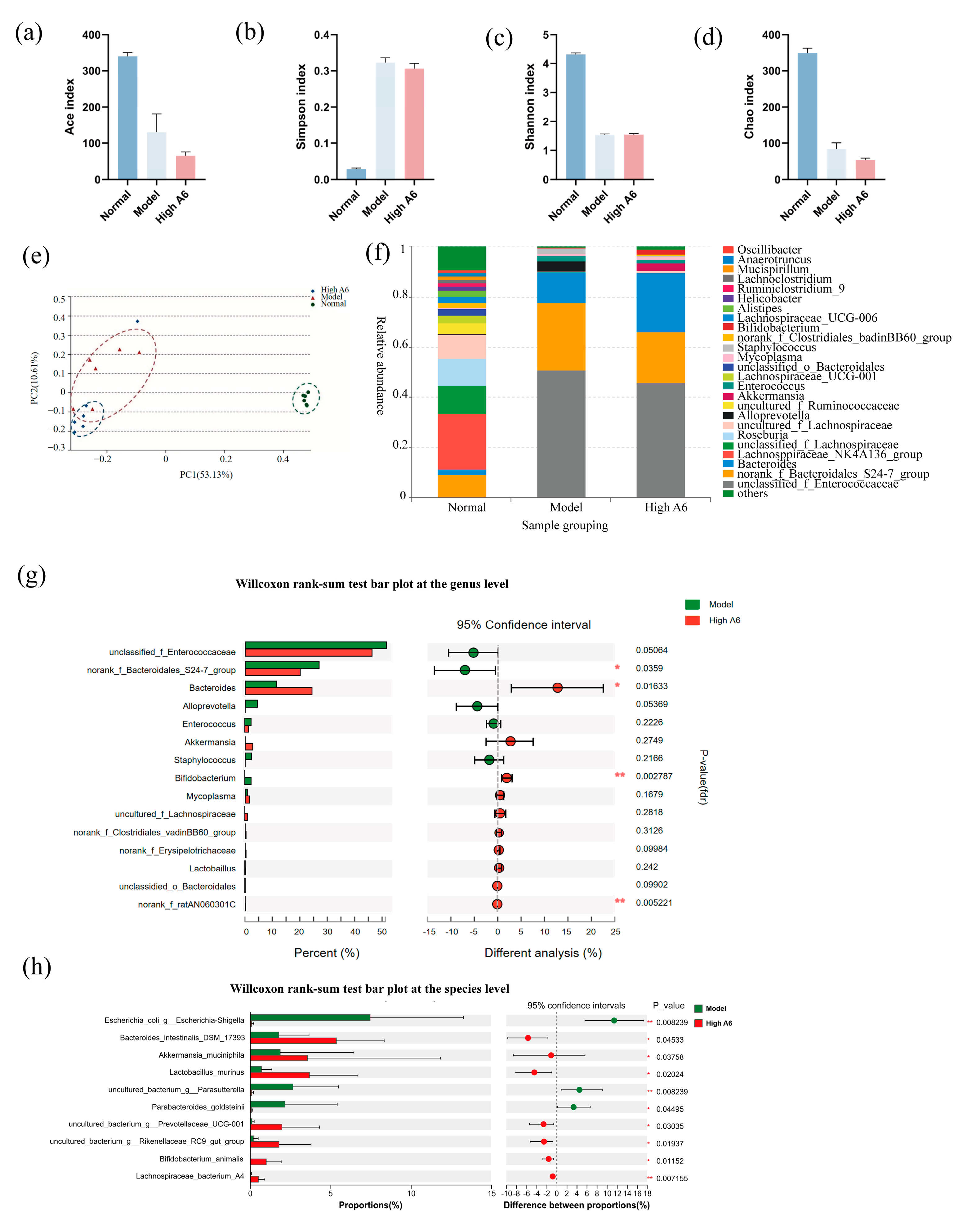
2.3. Analysis Using 16S rRNA Gene Sequencing
2.4. Determination of Fecal SCFAs
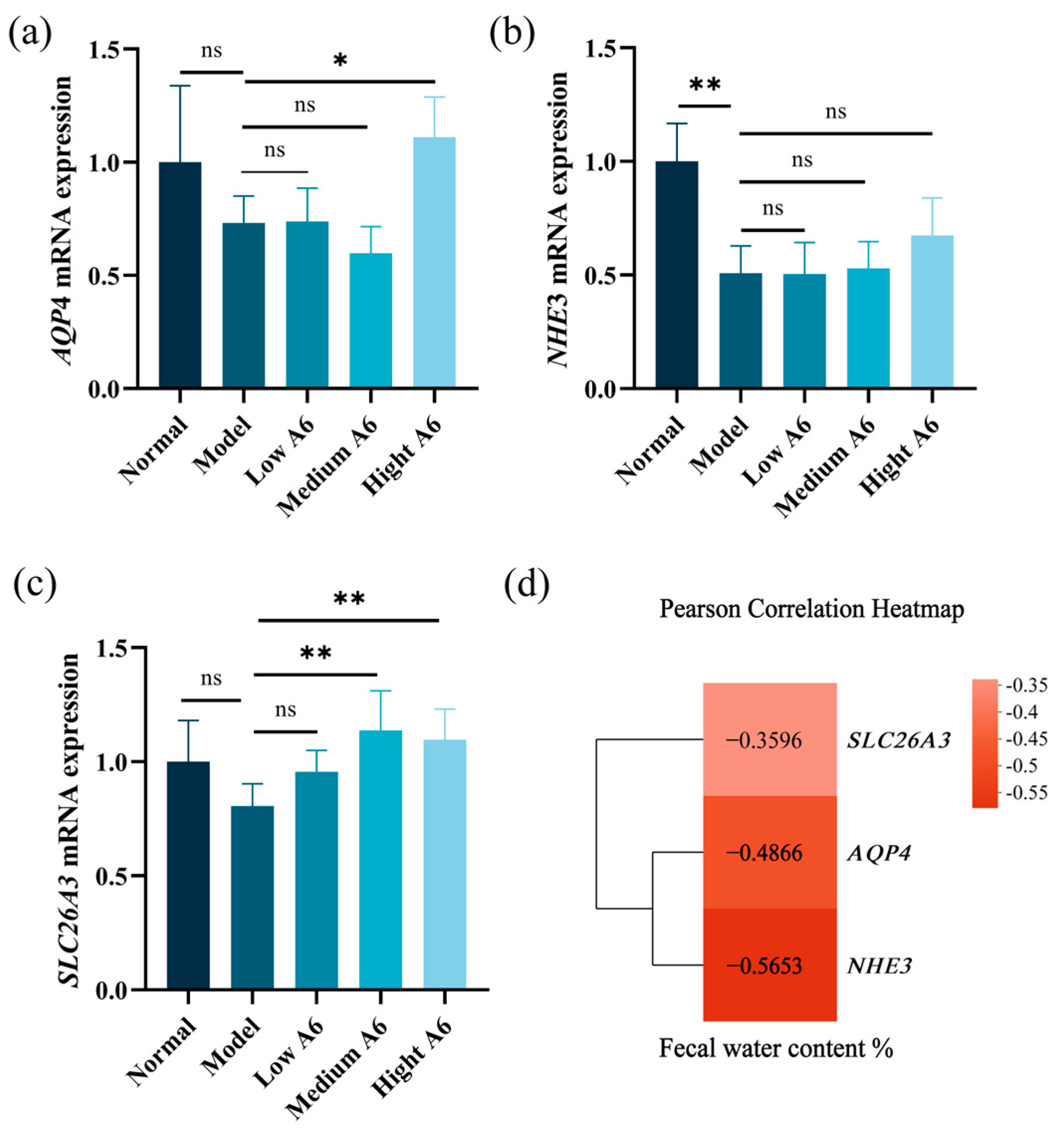
2.5. RT-qPCR for Intestinal Gene Expression
2.6. Statistical Analysis of Data
3. Results
3.1. A6 Supplementation Improved Diarrhea Status in AAD Mice
3.2. A6 Intervention Enhanced the Intestinal Mucus Barrier in AAD Mice
3.3. A6 Intervention Modulated the Mucin-Forming Gene in AAD Mice
3.4. A6 Supplementation Restored Gut Microbial Dysbiosis in AAD Mice
3.5. A6 Supplementation Upregulated SCFAs in AAD Mice
3.6. A6 Supplementation Recovered Water and Electrolyte Transport in AAD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Li, L.; Wu, M.; Liu, Z.; Zhao, Y.; Peng, J.; Ren, X.; Chen, S. Antibiotics and antibiotic-associated diarrhea: A real-world disproportionality study of the FDA adverse event reporting system from 2004 to 2022. BMC Pharmacol. Toxicol. 2023, 24, 73. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G. Clinical practice. Antibiotic-associated diarrhea. Beth Isr. Hosp. Semin. Med. 2002, 346, 334–339. [Google Scholar]
- Tanır Basaranoğlu, S.; Karaaslan, A.; Salı, E.; Çiftçi, E.; Gayretli Aydın, Z.G.; Aldemir Kocabaş, B.; Kaya, C.; Şen Bayturan, S.; Kara, S.S.; Yılmaz Çiftdoğan, D.; et al. Antibiotic associated diarrhea in outpatient pediatric antibiotic therapy. BMC Pediatr. 2023, 23, 121. [Google Scholar] [CrossRef]
- Lv, Z.; Peng, G.L.; Su, J.R. Factors associated with Clostridium difficile diarrhea in a hospital in Beijing, China. Braz. J. Med. Biol. Res. 2014, 47, 1085–1090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, Y.; Lan, X.; Qin, Y.; Wei, Y.; Li, X.; Feng, J.; Jiang, J. Polysaccharides of natural products alleviate antibiotic-associated diarrhea by regulating gut microbiota: A review. Arch. Microbiol. 2024, 206, 461. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, J.; Dong, Z.; Cao, Q.; Ye, H.; Feng, D.; Zhang, C.; Zuo, J.; Wang, W. Supplemental glucose oxidase as an antibiotic substitute alleviates diarrhea and improves intestinal health in weaned piglets. Vet. Q. 2025, 45, 1–9. [Google Scholar] [CrossRef]
- Jones, K. Probiotics: Preventing antibiotic-associated diarrhea. J. Spec. Pediatr. Nurs. 2010, 15, 160–162. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, B.; Xu, J.; Liu, Y.; Qiu, E.; Li, Z.; Li, Z.; He, Y.; Zhou, H.; Bai, Y.; et al. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front. Immunol. 2018, 9, 1040. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, H.; Kang, Y.; Tian, Y.; Li, L.; Kang, X.; Yang, H.; Wang, Y.; Tian, J.; Zhang, F.; et al. Probiotics alleviate autoimmune hepatitis in mice through modulation of gut microbiota and intestinal permeability. J. Nutr. Biochem. 2021, 98, 108863. [Google Scholar] [CrossRef]
- Ghyselinck, J.; Verstrepen, L.; Moens, F.; Van den Abbeele, P.; Said, J.; Smith, B.; Bjarnason, I.; Basit, A.W.; Gaisford, S. A 4-strain probiotic supplement influences gut microbiota composition and gut wall function in patients with ulcerative colitis. Int. J. Pharm. 2020, 587, 119648. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Kong, M.; Cheng, X.; Lin, A.; Liu, H. Modulation of gut microbiota and intestinal metabolites by lactulose improves loperamide-induced constipation in mice. Eur. J. Pharm. Sci. 2021, 158, 105676. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Bubnov, R.V.; Babenko, L.P.; Lazarenko, L.M.; Mokrozub, V.V.; Spivak, M.Y. Specific properties of probiotic strains: Relevance and benefits for the host. EPMA J. 2018, 9, 205–223. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, X.; Wang, J.; Gao, P.; Zhou, Z.; Ren, Y.; Sun, T.; Wang, L.; Meng, H.; Chen, W.; et al. Complete genome sequence of probiotic Bifidobacterium animalis subsp. lactis strain V9. J. Bacteriol. 2010, 192, 4080–4081. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, F.; Wuri, G.; Fang, B.; Shi, M.; Zhang, M.; Zhao, L. Alleviative mechanism and effect of Bifidobacterium animalis A6 on dextran sodium sulfate-induced ulcerative colitis in mice. Food Sci. Nutr. 2023, 11, 892–902. [Google Scholar] [CrossRef]
- Huo, Y.; Zhao, G.; Li, J.; Wang, R.; Ren, F.; Li, Y.; Wang, X. Bifidobacterium animalis subsp. lactis A6 enhances fatty acid β-oxidation of adipose tissue to ameliorate the development of obesity in mice. Nutrients 2022, 14, 598. [Google Scholar] [CrossRef]
- Yu, H.; Feng, T.; Huang, X.; Cheng, D.; Liu, J.; Di, W.; Hao, Y.; Yin, P.; Tang, P. Bifidobacterium animalis subsp. lactis A6 ameliorates bone and muscle loss via modulating gut microbiota composition and enhancing butyrate production. Bone Res. 2025, 13, 28. [Google Scholar]
- Wu, Y.; Liu, M.; Fang, B.; Zhang, M. Alleviating ability of different heat-treated egg yolk lipids on learning cognitive decline in mice. Sci. Technol. Food Ind. 2025, 46, 367–376. [Google Scholar]
- Tang, J.; Yue, H.; Yang, F.; Tang, C. Establishment of mouse β-actin mRNA fluorescence quantitative PCR method. Chin. J. Vet. Med. 2011, 38, 127–130. [Google Scholar]
- Bergstrom, K.S.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- Hoebler, C.; Gaudier, E.; De Coppet, P.; Rival, M.; Cherbut, C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig. Dis. Sci. 2006, 51, 381–389. [Google Scholar] [CrossRef]
- Hardin, J.A.; Wallace, L.E.; Wong, J.F.; O’Loughlin, E.V.; Urbanski, S.J.; Gall, D.G.; MacNaughton, W.K.; Beck, P.L. Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn’s disease and infectious colitis. Cell Tissue Res. 2004, 318, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.; Nagy, L.E.; Ganapathy, V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN J. Parenter. Enter. Nutr. 2013, 37, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, Y.; Hou, Y.; Liu, Y.; Nie, H. Regulation of Cl- Electrolyte permeability in epithelia by active traditional Chinese medicine monomers for diarrhea. Curr. Drug Targets 2020, 21, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, S.; Hutton, M.L.; Lyras, D. Involvement of bacteria other than Clostridium difficile in antibiotic-associated diarrhoea. Trends Microbiol. 2016, 24, 463–476. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, C.; Xiao, N.; Tan, Z. Gut microbiota characteristics in mice with antibiotic-associated diarrhea. BMC Microbiol. 2020, 20, 313. [Google Scholar] [CrossRef]
- Freeman, C.D.; Nightingale, C.H.; Nicolau, D.P.; Belliveau, P.P.; Quintiliani, R. Serum bactericidal activity of ceftriaxone plus metronidazole against common intra-abdominal pathogens. Am. J. Hosp. Pharm. 1994, 51, 1782–1787. [Google Scholar] [CrossRef]
- Richards, D.M.; Heel, R.C.; Brogden, R.N.; Speight, T.M.; Avery, G.S. Ceftriaxone. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 1984, 27, 469–527. [Google Scholar] [CrossRef]
- da Trindade, M.T.; Salgado, H.R.N. A critical review of analytical methods for determination of ceftriaxone sodium. Crit. Rev. Anal. Chem. 2018, 48, 95–101. [Google Scholar] [CrossRef]
- Selinger, C.P.; Bell, A.; Cairns, A.; Lockett, M.; Sebastian, S.; Haslam, N. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J. Hosp. Infect. 2013, 84, 159–165. [Google Scholar]
- Chai, J.; Chang, H.; Li, L. Meta-analysis of bifidobacterium tetrad in the treatment of antibiotic-associated diarrhea in infants and young children in China. WCMJ 2017, 32, 395–399. [Google Scholar]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef]
- Chen, B.; Yang, X.; Zhan, M.; Chen, Y.; Xu, J.; Xiao, J.; Xiao, H.; Song, M. Dietary tangeretin improved antibiotic-associated diarrhea in mice by enhancing the intestinal barrier function, regulating the gut microbiota, and metabolic homeostasis. Food Funct. 2023, 14, 10731–10746. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Hattrup, C.L.; Gendler, S.J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef]
- Gendler, S.J.; Spicer, A.P.; Lalani, E.N.; Duhig, T.; Peat, N.; Burchell, J.; Pemberton, L.; Boshell, M.; Taylor-Papadimitriou, J. Structure and biology of a carcinoma-associated mucin, MUC1. Am. Rev. Respir. Dis. 1991, 144, S42–S47. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, L.P.; Wang, B.G.; Liu, J.W.; Li, P.; He, C.Y.; Yuan, Y. The co-expression of functional gastric proteins in dynamic gastric diseases and its clinical significance. BMC Clin. Pathol. 2013, 13, 21. [Google Scholar] [CrossRef]
- Zhang, J.; Yasin, M.; Carraway, C.A.; Carraway, K.L. MUC4 expression and localization in gastrointestinal tract and skin of human embryos. Tissue Cell 2006, 38, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Buisine, M.P.; Desreumaux, P.; Leteurtre, E.; Copin, M.C.; Colombel, J.F.; Porchet, N.; Aubert, J.P. Mucin gene expression in intestinal epithelial cells in Crohn’s disease. Gut 2001, 49, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Xia, S.; Xiao, S.; Yu, Q. Short-chain fatty acids affect the development of inflammatory bowel disease through intestinal barrier, immunology, and microbiota: A promising therapy? J. Gastroenterol. Hepatol. 2022, 37, 1710–1718. [Google Scholar] [CrossRef]
- Binder, H.J. Role of colonic short-chain fatty acid transport in diarrhea. Annu. Rev. Physiol. 2010, 72, 297–313. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Liu, L.; Jia, R.; Chen, W.; Chen, W.; Wang, X.; Guo, Z. The lotus seed starch-EGCG complex modulates obesity in C57BL/6J mice through the regulation of the gut microbiota. Int. J. Biol. Macromol. 2015, 310, 143256. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Sabater, C.; Delgado, S.; Ruas-Madiedo, P.; Rupérez-García, A.; Montilla, A.; Javier Moreno, F.; Margolles, A.; Ruiz, L. Arabinoxylan-based substrate preferences and predicted metabolic properties of Bifidobacterium longum subspecies as a basis to design differential media. Food Res. Int. 2023, 167, 112711. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Arboleya, S.; Hernandez-Barranco, A.M.; Alvarez-Buylla, J.R.; Ruas-Madiedo, P.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Interactions between Bifidobacterium and Bacteroides species in cofermentations are affected by carbon sources, including exopolysaccharides produced by Bifidobacteria. Appl. Environ. Microbiol. 2013, 79, 7518–7524. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Xia, Z.; Han, Y.; Wang, K.; Guo, S.; Wu, D.; Huang, X.; Li, Z.; Zhu, L. Oral administration of propionic acid during lactation enhances the colonic barrier function. Lipids Health Dis. 2017, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Sellin, J.H. SCFAs: The enigma of weak electrolyte transport in the colon. News Physiol. Sci. 1999, 14, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Ma, T.; Filiz, F.; Verkman, A.S.; Bastidas, J.A. Colon water transport in transgenic mice lacking aquaporin-4 water channels. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G463–G470. [Google Scholar] [CrossRef]
- Cresci, G.A.; Thangaraju, M.; Mellinger, J.D.; Liu, K.; Ganapathy, V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 2010, 14, 449–461. [Google Scholar] [CrossRef]
- Kiela, P.R.; Xu, H.; Ghishan, F.K. Apical NA+/H+ exchangers in the mammalian gastrointestinal tract. J. Physiol. Pharmacol. 2006, 57 (Suppl. 7), 51–79. [Google Scholar] [PubMed]
- Matas-Rico, E.; García-Diaz, B.; Llebrez-Zayas, P.; López-Barroso, D.; Santín, L.; Pedraza, C.; Smith-Fernández, A.; Fernández-Llebrez, P.; Tellez, T.; Redondo, M.; et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol. Cell Neurosci. 2008, 39, 342–355. [Google Scholar] [CrossRef]
- Hendrickx, A.P.; Top, J.; Bayjanov, J.R.; Kemperman, H.; Rogers, M.R.; Paganelli, F.L.; Bonten, M.J.; Willems, R.J. Antibiotic-driven dysbiosis mediates intraluminal agglutination and alternative segregation of Enterococcus faecium from the intestinal epithelium. mBio 2015, 6, e01346-15. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Xiong, L. Research progress on the effects and mechanisms of short-chain fatty acids on intestinal tract. JPEN 2012, 19, 56–58. [Google Scholar]
- Martins, F.H.; Rosay, T.; Rajan, A.; Carter, H.E.; Turocy, T.; Mejia, A.; Crawford, J.M.; Maresso, A.W.; Sperandio, V. Enterococcus faecalis-derived adenine enhances enterohaemorrhagic Escherichia coli Type 3 Secretion System-dependent virulence. Nat. Microbiol. 2024, 9, 2448–2461. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, S.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 2020, 27, 659–670. [Google Scholar] [CrossRef]
- Peng, L.; Chen, X.; Wang, Z.; Yi, L.; Jin, Z. Maternal puerperal infection caused by Parabacteroides goldsteinii: A case report. Front. Med. 2024, 11, 1450931. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, L.; Gilkes, A.; Ashworth, M.; Rowland, V.; Harries, T.H.; Armstrong, D.; White, P. Association between antibiotics and gut microbiome dysbiosis in children: Systematic review and meta-analysis. Gut Microbes 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Praharaj, I.; John, J.; Kaliappan, S.P.; Kampmann, B.; Kang, G.; Grassly, N.C. Changes in the intestinal microbiota following the administration of azithromycin in a randomised placebo-controlled trial among infants in south India. Sci. Rep. 2017, 7, 9168. [Google Scholar] [CrossRef] [PubMed]
- Harlow, B.E.; Lawrence, L.M.; Flythe, M.D. Diarrhea-associated pathogens, lactobacilli and cellulolytic bacteria in equine feces: Responses to antibiotic challenge. Vet. Microbiol. 2013, 166, 225–232. [Google Scholar] [CrossRef]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The gut microbiota of healthy chilean subjects reveals a high abundance of the phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 1221. [Google Scholar] [CrossRef]
- Salem, M.B.; El-Lakkany, N.M.; El-Din, S.H.S.; Hammam, O.A.; Samir, S. Diosmin alleviates ulcerative colitis in mice by increasing Akkermansia muciniphila abundance, improving intestinal barrier function, and modulating the NF-κB and Nrf2 pathways. Heliyon 2024, 10, e27527. [Google Scholar] [CrossRef]
- Kumar, A.; Anbazhagan, A.N.; Coffing, H.; Chatterjee, I.; Priyamvada, S.; Gujral, T.; Saksena, S.; Gill, R.K.; Alrefai, W.A.; Borthakur, A.; et al. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G817–G826. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, L.; Qiu, D.; Yao, R.; Jia, H.; Wang, H.; Zhou, L.; Zhang, J.; Zhang, N. Lactobacillus murinus ZNL-13 Modulates Intestinal Barrier Damage and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Foods 2025, 14, 1416. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, Z.; Chen, Q.; Du, Y.; Sun, H.; Liu, H.; Feng, Y.; Li, Z.; Teng, T.; Shi, B. Protective effect of the branched short-chain fatty acid isobutyrate on intestinal damage in weaned piglets through intestinal microbiota remodeling. J. Sci. Food Agric. 2025, 105, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Du, H.; Zhang, M.; Xu, H.; Pu, X.; Chen, Q.; Luo, R.; Hu, Y.; Wang, Y.; Tu, H.; et al. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/MTOR signaling pathway and regulating gut microbiota. Phytother. Res. 2022, 36, 2081–2094. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, C.; Duan, H.; Narbad, A.; Zhao, J.; Chen, W.; Zhai, Q.; Yu, L.; Tian, F. Cross-feeding of bifidobacteria promotes intestinal homeostasis: A lifelong perspective on the host health. NPJ Biofilms Microbiomes 2024, 10, 47. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Mahony, J.; van Sinderen, D.; Ventura, M. Glycan utilization and cross-feeding activities by Bifidobacteria. Trends Microbiol. 2018, 26, 339–350. [Google Scholar] [CrossRef]


| Genetics | Primer Sequence | Bibliography |
|---|---|---|
| β-actin | F: GGCTGTATTCCCCTCCATCG R: CCAGTTGGTAACAATGCCATGT | (Tang J, 2011) [19] |
| Mucin1 | F: CTGTTCACCACCACCATGAC R: CTTGGAAGGGCAAGAAAACC | (Krik S, 2010) [20] |
| Mucin4 | F: CAGCAGCCAGTGGGGGACAG R: CTCAGACACAGCCAGGGAACTC | (Hoebler E, 2006) [21] |
| AQP4 | F: AGCATCGCTAAGTCCGTCTTC R: TCCTCCACCTCCATGTAGCTC | (Hardin JA, 2004) [22] |
| NHE3 | F: TGGCCGGGCTTTCGACCACA R: GGGACCCACGGCGCTCTCCCT | (Gail C, 2013) [23] |
| SLC26A3 | F: CACAAATTCAGAAGACGAACA R: GCATCAGCATTCCCTTTAAGTT | (Gail C, 2013) [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Liu, M.; Li, J.; Liu, Y.; Ge, S.; Gao, H.; Zhang, M. Bifidobacterium animalis Supplementation Improves Intestinal Barrier Function and Alleviates Antibiotic-Associated Diarrhea in Mice. Foods 2025, 14, 1704. https://doi.org/10.3390/foods14101704
Du X, Liu M, Li J, Liu Y, Ge S, Gao H, Zhang M. Bifidobacterium animalis Supplementation Improves Intestinal Barrier Function and Alleviates Antibiotic-Associated Diarrhea in Mice. Foods. 2025; 14(10):1704. https://doi.org/10.3390/foods14101704
Chicago/Turabian StyleDu, Xiaoyu, Mingkun Liu, Jingyu Li, Yue Liu, Shaoyang Ge, Haina Gao, and Ming Zhang. 2025. "Bifidobacterium animalis Supplementation Improves Intestinal Barrier Function and Alleviates Antibiotic-Associated Diarrhea in Mice" Foods 14, no. 10: 1704. https://doi.org/10.3390/foods14101704
APA StyleDu, X., Liu, M., Li, J., Liu, Y., Ge, S., Gao, H., & Zhang, M. (2025). Bifidobacterium animalis Supplementation Improves Intestinal Barrier Function and Alleviates Antibiotic-Associated Diarrhea in Mice. Foods, 14(10), 1704. https://doi.org/10.3390/foods14101704





