The Impact of Cooking on Antioxidant and Enzyme Activities in Ruichang Yam Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cooked Chinese Yam
2.2. Chemicals and Reagents
2.3. Extraction of Polyphenols
2.3.1. Extraction of Soluble-Free and Soluble-Conjugated Phenolic Fractions of Yam
2.3.2. Extraction of Insoluble Bound Phenolic Fractions of Yam
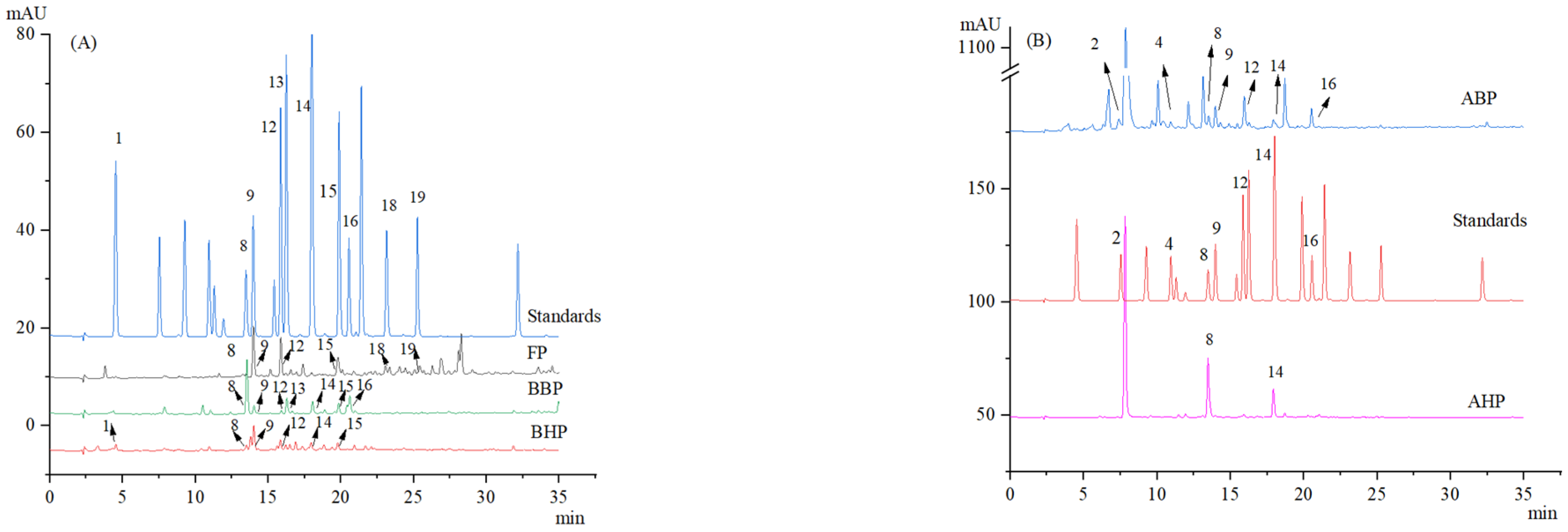
2.4. HPLC Analysis of Chinese Yams
2.5. Analysis of Bioactive Components of Chinese Yams
2.5.1. Determination of Total Phenolic Content
2.5.2. Determination of the Total Flavonoid Content
2.6. Evaluation of Antioxidant Activities of Ruichang Yam
2.6.1. Radical Scavenging Activity (DPPH) Assay
2.6.2. Hydroxyl Radical Activity (OH) Assay
2.6.3. Total Antioxidant Activity (T-AOC) Assay
2.6.4. Total Reducing Activity (T-RC) Assay
2.7. Enzyme Inhibition
2.7.1. α-Glucosidase Inhibition Assay
2.7.2. Pancreatic Lipase Inhibition Assay
2.8. Data Analysis
3. Results and Analysis
3.1. Chemical Composition of Polyphenols in Chinese Yams
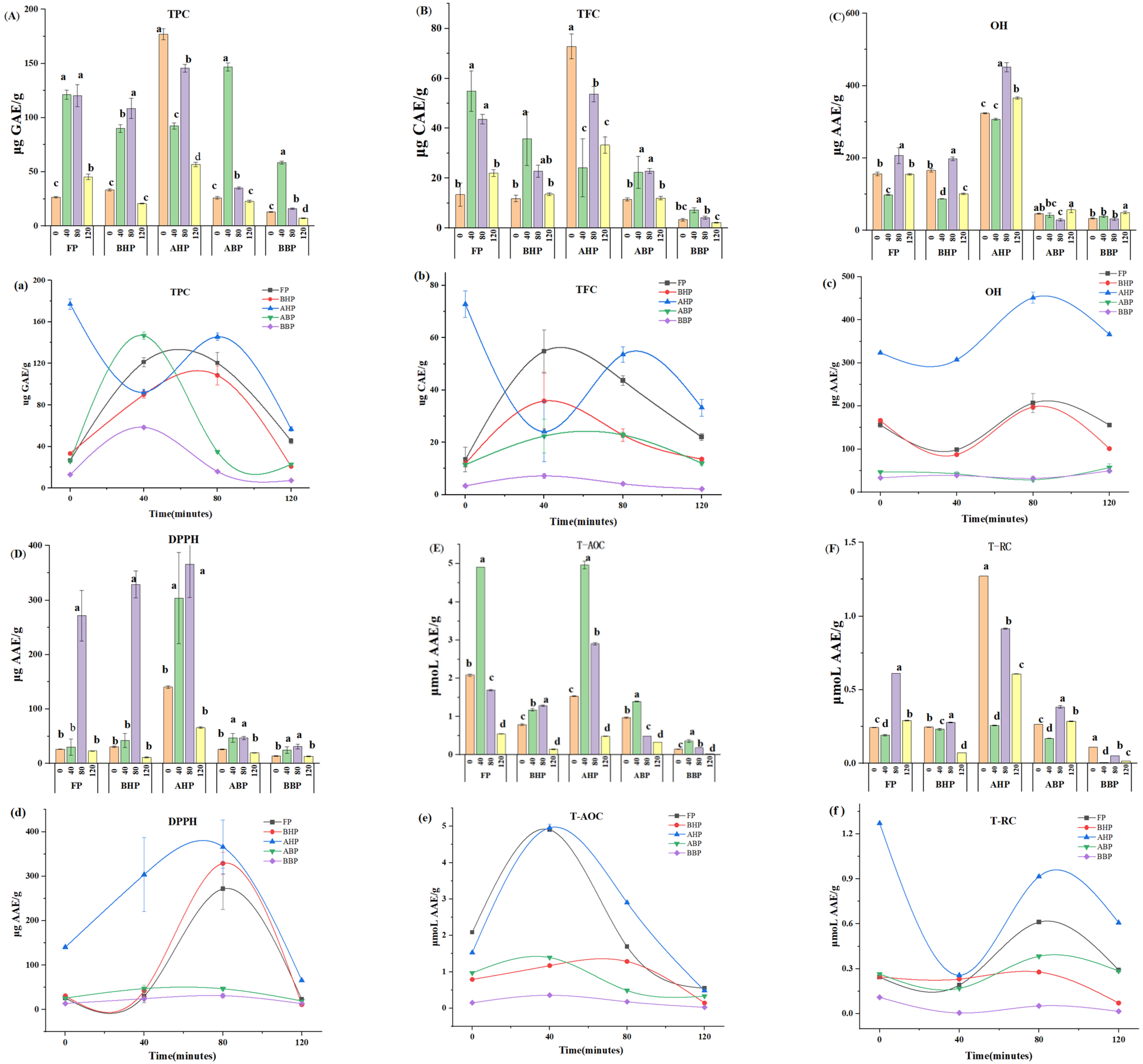
3.2. Effect of Cooking Time on Polyphenols and Flavonoids in Chinese Yams
3.3. Effect of Cooking Time on the Antioxidant Activity of Chinese Yam Polyphenols
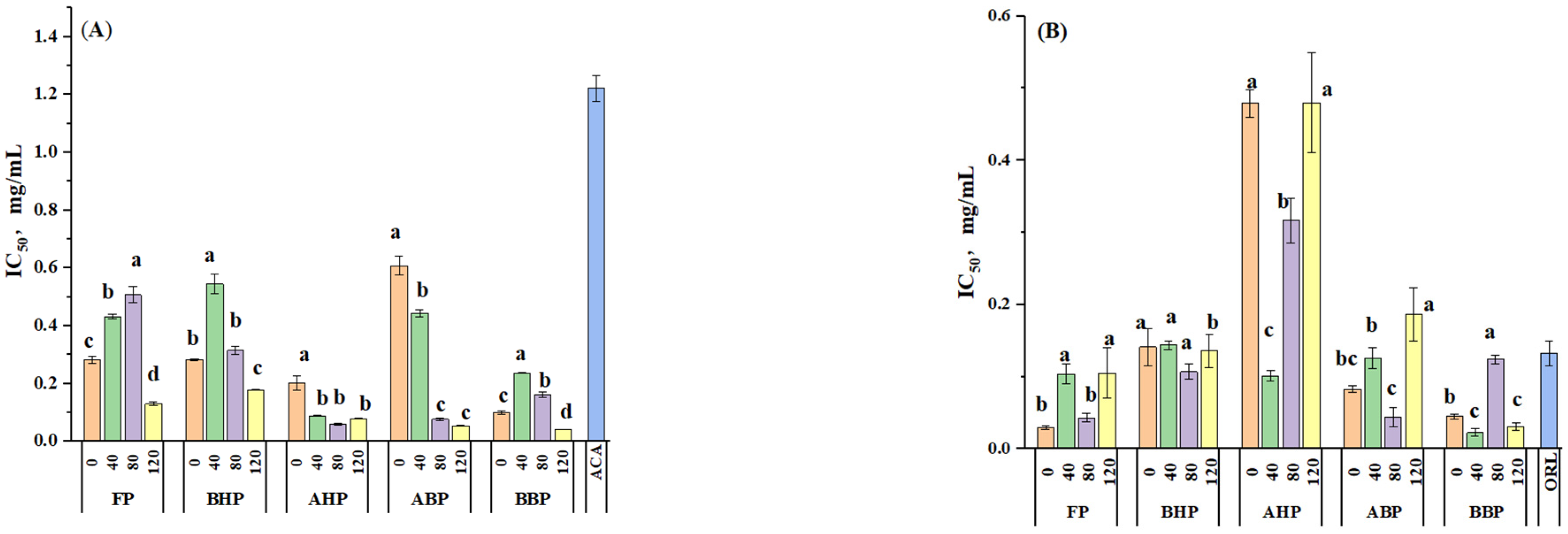
3.4. In Vitro α-Glucosidase and Pancreatic Lipase Activity
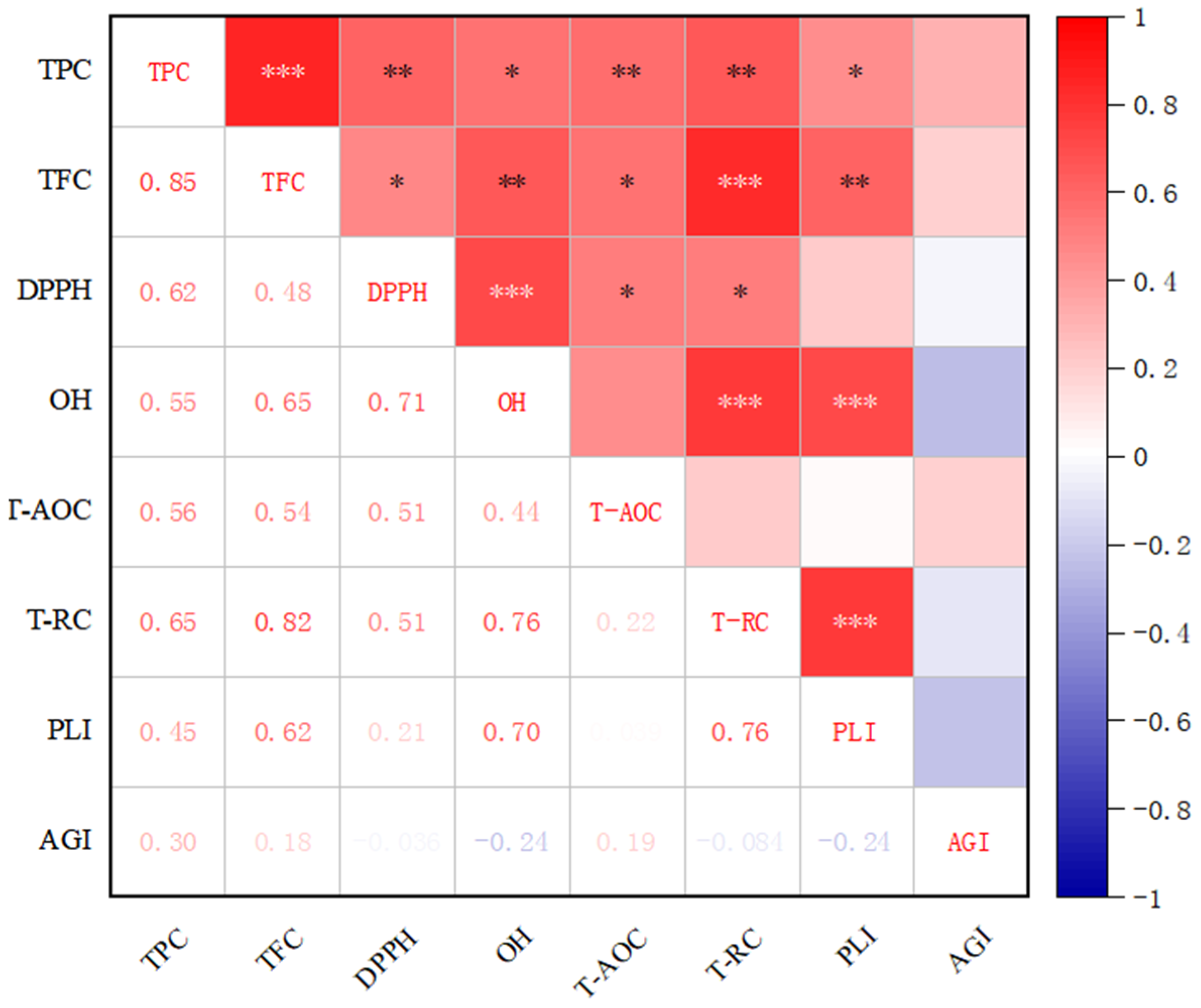
3.5. Pearson Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, L.; Liu, Z.; Xu, X.; Wang, F.; Yu, G.; Yao, Y. Effects of different planting years on soil enzyme activity and nutrient content in yam field. Zhejiang Agric. Sci. 2020, 61, 920–923. [Google Scholar] [CrossRef]
- Khol, M.; Ma, F.; Lei, L.; Liu, W.; Liu, X. A Frontier Review of Nutraceutical Chinese Yam. Foods 2024, 13, 1426. [Google Scholar] [CrossRef] [PubMed]
- Epping, J.; Laibach, N. An underutilized orphan tuber crop-Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef]
- Liu, K.; Shu, C.; Shi, X.; Liu, D.; Liu, H. Analysis of Biochemical Ingredients in Rhizoma Dioscoreae from Different Producing Areas. Hubei Agric. Sci. 2011, 50, 2108–2110. [Google Scholar] [CrossRef]
- Ting, H. Preliminary Study on Pathogen of Ruichang yam Anthracnose and Pathogenic Identification of the Virus and Nematode Diseases. Master Thesis, Jiangxi Agricultural University, Jianggxi, China, 2015. [Google Scholar]
- Zhou, P.; Wang, L.; Huang, G. Research on Development Status, Problems and Countermeasures of Ruichang Yam. Acta Agric. Univ. Jiangxiensis 2024, 36, 27–33. [Google Scholar] [CrossRef]
- Gui, L. Analysis on Production Status of Ruichang Yam in Jiangxi Province and Technologies of Preventing Ruichang Yam from Gloeosporium Pestis. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]
- Zhou, Q.; Li, B.; Tan, M.; Hong, Y.; Zhou, Z.; Yu, X. Key points of Ruichang yam planting technology. Agric. Dev. Equip. 2019, 12, 167. [Google Scholar]
- Yan, Y.; Wang, T.; Wang, Y.; Chen, B.; Peng, W. Analysis of the development strategy and current situation of Ruichang Yam. Pract. Tech. Rural Areas 2023, 08, 18–20. [Google Scholar]
- Shan, N.; Wang, P.-T.; Zhu, Q.-L.; Sun, J.-Y.; Zhang, H.-Y.; Liu, X.-Y.; Cao, T.-X.; Chen, X.; Huang, Y.-J.; Zhou, Q.-H. Comprehensive characterization of yam tuber nutrition and medicinal quality of Dioscorea opposita and D. alata from different geographic groups in China. J. Integr. Agric. 2020, 19, 2839–2848. [Google Scholar] [CrossRef]
- Xiong, A.; Li, J.; Zhou, Q.; Ke, Y.; Zhai, C. Discussion on the basic characteristics and application prospects of Ruichang yam. South China Agric. 2019, 13, 125+130. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Mastrodi Salgado, J.; de Oliveira Rios, A.; dos Santos, P.R.; Jablonski, A. Stability of carotenoids, total phenolics and in vitro antioxidant capacity in the thermal processing of orange-fleshed sweet potato (Ipomoea batatas Lam.) cultivars grown in Brazil. Plant Foods Hum. Nutr. 2012, 67, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.C.; Kaaya, A.N.; Tumuhimbise, G.A.; Katungisa, A.; Amagloh, F.K.; Yada, B. Household Processing Methods and Their Impact on Bioactive Compounds and Antioxidant Activities of Sweetpotato Genotypes of Varying Storage Root Flesh Colours. Antioxidants 2022, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- AlJuhaimi, F.; Mohamed Ahmed, I.A.; Özcan, M.M.; Uslu, N.; Albakry, Z. Quantitative Determination of Biogenic Element Contents and Phytochemicals of Broccoli (Brassica oleracea var. italica) Cooked Using Different Techniques. Plants 2024, 13, 1283. [Google Scholar] [CrossRef] [PubMed]
- Kido, M.; Yoshimoto, M.; Sakao, K.; Wada, K.; Hou, D.X. Effects of Cooking Methods on Caffeoylquinic Acids and Radical Scavenging Activity of Sweet Potato. Foods 2024, 13, 1101. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Gong, L.; Hu, L.; Feng, D.; Chi, J.; Wang, B.; Wang, J. Effects of different household cooking methods on the biological properties of Chinese yam. Food Chem. 2021, 363, 130246. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Concepción Alvarez, A.; Arias-Santé, M.F.; Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Núñez Pizarro, P.; Bustos, S.M.; Schwember, A.R.; Shahidi, F.; et al. Soluble Free, Esterified and Insoluble-Bound Phenolic Antioxidants from Chickpeas Prevent Cytotoxicity in Human Hepatoma HuH-7 Cells Induced by Peroxyl Radicals. Antioxidants 2022, 11, 1139. [Google Scholar] [CrossRef]
- Gao, Y.; Ping, H.; Li, B.; Li, Y.; Zhao, F.; Ma, Z. Characterization of free, conjugated, and bound phenolics in early and late ripening kiwifruit cultivars. J. Sci. Food Agric. 2021, 101, 4743–4750. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Jiang, N.; Wang, Y.; Feng, X. Use of ultra-performance liquid chromatography-tandem mass spectrometry on sweet cherries to determine phenolic compounds in peel and flesh. J. Sci. Food Agric. 2019, 99, 3555–3562. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Shengyu, W.; Mei, Y.; Heyu, H.; Caiqi, Z.; Huanhaun, D.; Yonngmei, G.; Weifeng, Z. Research Progress on Release Patterns of Conjugated Phenolics During Plant Growth, Food Processing and Human Digestion. Sci. Technol. Food Ind. 2024, 45, 408–417. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.; Tsao, R. Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and α-Glucosidase and Pancreatic Lipase Inhibitory Effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- de la Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Medica 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Ereifej, K.; Al-Karaki, G.; Al-Duais, M.; Andrade, J.E.; Tranchant, C.C.; Kubow, S.; et al. Profiles of free and bound phenolics extracted from Citrus fruits and their roles in biological systems: Content, and antioxidant, anti-diabetic and anti-hypertensive properties. Food Funct. 2017, 8, 3187–3197. [Google Scholar] [CrossRef]
- Lavelli, V.; Sri Harsha, P.S.; Ferranti, P.; Scarafoni, A.; Iametti, S. Grape skin phenolics as inhibitors of mammalian α-glucosidase and α-amylase--effect of food matrix and processing on efficacy. Food Funct. 2016, 7, 1655–1663. [Google Scholar] [CrossRef]
- Dong, Q.; Hu, N.; Yue, H.; Wang, H. Inhibitory Activity and Mechanism Investigation of Hypericin as a Novel α-Glucosidase Inhibitor. Molecules 2021, 26, 4566. [Google Scholar] [CrossRef]
- Alpha Glucosidase Inhibitors. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Lim, S.Y.; Steiner, J.M.; Cridge, H. Lipases: It’s not just pancreatic lipase! Am. J. Vet. Res. 2022, 83. [Google Scholar] [CrossRef]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 2000, 20, 270–279. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Tamia, G.M.; He, X.; Sun, J.; Chen, P.; Lee, S.H.; Wang, T.T.Y.; Gao, B.; Xie, Z.; et al. Soluble Free, Soluble Conjugated, and Insoluble Bound Phenolics in Tomato Seeds and Their Radical Scavenging and Antiproliferative Activities. J. Agric. Food Chem. 2022, 70, 9039–9047. [Google Scholar] [CrossRef]
- Kim, J.S. Antioxidant Activities of Selected Berries and Their Free, Esterified, and Insoluble-Bound Phenolic Acid Contents. Prev. Nutr. Food Sci. 2018, 23, 35–45. [Google Scholar] [CrossRef]
- Wu, S.; Mo, R.; Wang, R.; Li, Q.; Shen, D.; Liu, Y. Identification of Key Antioxidants of Free, Esterified, and Bound Phenolics in Walnut Kernel and Skin. Foods 2023, 12, 825. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, J.; Zhang, R.; Xu, X.; Huang, W.; Yang, D.; Zhao, Z. Determination of total phenolic and flavonoid content and study on antioxidant activity of Amomum villosum from various producing areas. China J. Tradit. Chin. Med. Pharm. 2021, 36, 122–126. [Google Scholar]
- Chen, S.; Rao, G.; Wang, L.; Sheng, Y. Optimization of Microwave-assisted Deep Eutectic Solvent Extractionof Total Flavonoids fromPaeonia rockii Seeds andIts Antioxidant Activity. Sci. Technol. Food Ind. 2024, 45, 161–168. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, C.; Zhang, F. Study on Antioxidant Activity of Extracts of Gynura procumbens. Feed Rev. 2023, 05, 12–17. [Google Scholar] [CrossRef]
- Guo, F.; An, J.; Wang, M.; Zhang, W.; Chen, C.; Mao, X.; Liu, S.; Wang, P.; Ren, F. Inhibitory Mechanism of Quercimeritrin as a Novel α-Glucosidase Selective Inhibitor. Foods 2023, 12, 3415. [Google Scholar] [CrossRef]
- Sultana, R.; Alashi, A.M.; Islam, K.; Saifullah, M.; Haque, C.E.; Aluko, R.E. Inhibitory Activities of Polyphenolic Extracts of Bangladeshi Vegetables against α-Amylase, α-Glucosidase, Pancreatic Lipase, Renin, and Angiotensin-Converting Enzyme. Foods 2020, 9, 844. [Google Scholar] [CrossRef]
- Silva, A.D.; Ávila, S.; Küster, R.T.; Dos Santos, M.P.; Grassi, M.T.; de Queiroz Pereira Pinto, C.; Miguel, O.G.; Ferreira, S.M.R. In vitro Bioaccessibility of Proteins, Phenolics, Flavonoids and Antioxidant Activity of Amaranthus viridis. Plant Foods Hum. Nutr. 2021, 76, 478–486. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Pastoriza, S.; Jiménez-Hernández, N.; D’Auria, G.; Francino, M.P.; Rufián-Henares, J.A. Effect of Food Thermal Processing on the Composition of the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 11500–11509. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.-h.; Chen, X.-m.; Huang, A.-l.; Hu, J. Effect of Heat Treatment on Antioxidant Capacity of Purple Sweet Potato. J. Chang. Norm. Univ. 2023, 42, 94–102. [Google Scholar]
- Liu, L.; Gao, C.; Jing, T.; Duan, H.; Yuan, J.; Zhang, F. Effects of Cooking Methods and Conditions on Nutritional Content of Potato Tubers. J. Chin. Cereals Oils 2023, 38, 61–70. [Google Scholar]
- Chen, P.X.; Tang, Y.; Zhang, B.; Liu, R.; Marcone, M.F.; Li, X.; Tsao, R. 5-hydroxymethyl-2-furfural and derivatives formed during acid hydrolysis of conjugated and bound phenolics in plant foods and the effects on phenolic content and antioxidant capacity. J. Agric. Food Chem. 2014, 62, 4754–4761. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Yan, W.; Wen-jun, Z.; Yu-ting, Z.; Jawharay, B.; Yan-pin, L.; Jian-bao, D.; Rao-rao, L.; Jin, Y. Comparison of alkaline and acid hydrolysis extraction of non-extractable phenolic compounds in Lycii Fructus and hydrolysates analysis. Nat. Prod. Res. Dev. 2024, 36, 632–643. [Google Scholar] [CrossRef]
- Altay, M. Acarbose is again on the stage. World J. Diabetes 2022, 13, 1–4. [Google Scholar] [CrossRef]
- Zhou, Y.; Shu, Q.; Tang, X.; Zeng, Z.; Yang, J. To Analysis the Significance of the Clinical Compatiblity Application between Shanyao and Huangqi. J. Basic Chin. Med. 2017, 23, 1332–1333. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Kumar, S.; Singla, B.; Singh, U.P. Polyphenols: Role in Modulating Immune Function and Obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Leangnim, N.; Unban, K.; Thangsunan, P.; Tateing, S.; Khanongnuch, C.; Kanpiengjai, A. Ultrasonic-assisted enzymatic improvement of polyphenol content, antioxidant potential, and in vitro inhibitory effect on digestive enzymes of Miang extracts. Ultrason. Sonochem. 2023, 94, 106351. [Google Scholar] [CrossRef]
- Liu, Y.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E.M. Composition of Phenolic Acids and Antioxidant Properties of Selected Pulses Cooked with Different Heating Conditions. Foods 2020, 9, 908. [Google Scholar] [CrossRef]
- Mallet, J.F.; Shahbazi, R.; Alsadi, N.; Saleem, A.; Sobiesiak, A.; Arnason, J.T.; Matar, C. Role of a Mixture of Polyphenol Compounds Released After Blueberry Fermentation in Chemoprevention of Mammary Carcinoma: In Vivo Involvement of miR-145. Int. J. Mol. Sci. 2023, 24, 3677. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Zhang, Y.; Chen, Y.; Ge, X.; Sui, W.; Zhu, Q.; Geng, J.; Zhang, M. Release of bound polyphenols from wheat bran soluble dietary fiber during simulated gastrointestinal digestion and colonic fermentation in vitro. Food Chem. 2023, 402, 134111. [Google Scholar] [CrossRef]
- Liao, W.; Liu, S.; Dong, R.; Xie, J.; Chen, Y.; Hu, X.; Xie, J.; Xue, P.; Feng, L.; Yu, Q. Mixed solid-state fermentation for releasing bound polyphenols from insoluble dietary fiber in carrots via Trichoderma viride and Aspergillus niger. Food Funct. 2022, 13, 2044–2056. [Google Scholar] [CrossRef]
- Meng, Y.; Meng, X.; Fu, J.; Qin, Z.; Zhai, C. Extraction and Antioxidant Activity of Total Polyphenols from Chinese Yam by Ultrasound-Assisted Enzymatic Hydrolysis. J. Liaoning Univ. Tradit. 2020, 22, 63–66. [Google Scholar] [CrossRef]
- Huang, M.; Hu, A.; Zhang, Z.; Yang, C. Comparative Study on Functional Components of Yam from Different Habitats. J. Hubei Eng. Univ. 2018, 38, 30–34. [Google Scholar]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef]
- Li, S.; Wang, Q.; Li, Z.; Wu, J.; Chen, W.; Xiao, Y.; Zheng, Y. Progress on the interaction mechanism between cellulose and polyphenols and its influences on the functional properties of polyphenols. Food Ferment. Ind. 2022, 48, 283–289. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Gao, Z.; Li, M.; Wu, M.; Zhou, Q. Research Progress on the Interaction Mechanism of Pectin and Polyphenol and Their Effect on Food Processing Characteristics. Sci. Technol. Food Ind. 2024, 45, 378–386. [Google Scholar] [CrossRef]
- Li, K.; Gao, Y.; Xuan, C.; Lin, X. Comparison of Antioxidant Content and Antioxidant Activity of Different Potato Cultivars. J. Jilin Agric. Univ. 2014, 36, 56–60. [Google Scholar] [CrossRef]
- Ding, H.Y. Extracts and constituents of Rubus chingii with 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity. Int. J. Mol. Sci. 2011, 12, 3941–3949. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wei, M.; Qian, S.; Zhao, S. Purification and α-glucosidase inhibitory activity of polyphenols from purple yam. Food Ferment. Ind. 2022, 48, 182–187. [Google Scholar] [CrossRef]
- Yang, M.H.; Chin, Y.W.; Yoon, K.D.; Kim, J. Phenolic compounds with pancreatic lipase inhibitory activity from Korean yam (Dioscorea opposita). J. Enzym. Inhib. Med. Chem. 2014, 29, 1–6. [Google Scholar] [CrossRef]
- Li, M.M.; Chen, Y.T.; Ruan, J.C.; Wang, W.J.; Chen, J.G.; Zhang, Q.F. Structure-activity relationship of dietary flavonoids on pancreatic lipase. Curr. Res. Food Sci. 2023, 6, 100424. [Google Scholar] [CrossRef]
- Rahim, A.; Takahashi, Y.; Yamaki, K. Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Res. Int. 2015, 75, 289–294. [Google Scholar] [CrossRef]
| TPC (μg GAE/g) | TFC (μg CAE/g) | Radical Scavenging Activity (μg AAE/g) | Radical Scavenging Activity (μmoL AAE/g) | ||||
|---|---|---|---|---|---|---|---|
| DPPH | OH | T-AOC | T-RC | ||||
| 0 | FPs | 26.60 ± 0.65 a | 13.43 ± 4.65 a | 26.01 ± 0.38 | 156.05 ± 5.53 a | 2.08 ± 0.03 a | 0.24 ± 0.00 a |
| BHPs | 33.29 ± 0.99 a | 11.87 ± 1.30 a | 30.66 ± 1.11 | 166.16 ± 5.31 a | 0.79 ± 0.03 a | 0.25 ± 0.00 a | |
| AHPs | 177.06 ± 5.13 a | 72.85 ± 5.02 a | 140.53 ± 2.42 a | 323.67 ± 1.08 | 1.53 ± 0.01 a | 1.27 ± 0.00 a | |
| BHPs+AHPs | 210.35 ± 6.12 | 84.72 ± 6.33 | 171.2 ± 3.53 | 489.84 ± 6.38 | 2.32 ± 0.04 | 1.52 ± 0.00 | |
| ABPs | 25.94 ± 1.15 a | 11.43 ± 0.59 a | 25.84 ± 0.47 a | 46.53 ± 1.50 | 0.97 ± 0.01 a | 0.27 ± 0.00 a | |
| BBPs | 12.96 ± 0.26 a | 3.33 ± 0.52 a | 13.95 ± 0.56 a | 33.18 ± 1.49 | 0.15 ± 0.01 a | 0.11 ± 0.00 a | |
| ABPs+BBPs | 38.90 ± 1.41 | 14.77 ± 1.11 | 39.79 ± 1.03 | 79.71 ± 3.00 | 1.12 ± 0.02 | 0.38 ± 0.00 | |
| Total | 275.85 ± 8.18 | 112.92 ± 12.09 | 236.99 ± 4.94 | 725.60 ± 14.91 | 5.52 ± 0.08 | 2.14 ± 0.00 | |
| 40 | FPs | 121.18 ± 4.24 * | 54.86 ± 8.06 * | 30.06 ± 14.77 | 98.44 ± 1.30 * | 4.90 ± 0.00 * | 0.19 ± 0.01 * |
| BHPs | 89.92 ± 3.49 * | 35.8 ± 10.66 * | 42.21 ± 12.69 | 87.22 ± 0.18 * | 1.17 ± 0.03 * | 0.23 ± 0.01 * | |
| AHPs | 92.32 ± 2.78 * | 24.14 ± 11.58 * | 303.77 ± 83.6 * | 307.38 ± 2.49 | 4.96 ± 0.10 * | 0.26 ± 0.00 * | |
| BHPs+AHPs | 182.24 ± 6.27 | 59.95 ± 22.24 | 345.98 ± 96.29 | 394.60 ± 2.67 | 6.13 ± 0.14 | 0.49 ± 0.01 | |
| ABPs | 146.67 ± 3.66 * | 22.39 ± 6.39 * | 47.16 ± 8.08 * | 42.30 ± 6.10 | 1.39 ± 0.01 * | 0.17 ± 0.00 * | |
| BBPs | 58.54 ± 1.27 * | 7.15 ± 0.94 * | 24.54 ± 6.36 * | 38.73 ± 2.80 | 0.36 ± 0.04 * | 0.01 ± 0.00 * | |
| ABPs+BBPs | 205.21 ± 4.93 | 29.54 ± 7.34 | 71.71 ± 14.43 | 81.04 ± 8.90 | 1.75 ± 0.05 | 0.17 ± 0.00 | |
| Total | 508.62 ± 15.45 | 144.35 ± 37.65 | 447.75 ± 125.48 | 574.07 ± 12.87 | 12.78 ± 0.18 | 0.85 ± 0.02 | |
| 80 | FPs | 120.34 ± 10.18 * | 43.66 ± 1.85 * | 271.57 ± 46.31 *,a | 206.79 ± 21.81 *,a | 1.69 ± 0.02 *,a | 0.61 ± 0.00 *,a |
| BHPs | 108.47 ± 9.32 *,a | 22.80 ± 2.44 | 328.98 ± 24.69 *,a | 197.55 ± 5.31 *,a | 1.28 ± 0.02 *,a | 0.28 ± 0.00 *,a | |
| AHPs | 145.63 ± 3.55 *,a | 53.62 ± 3.02 *,a | 365.89 ± 60.82 * | 451.42 ± 12.73 *,a | 2.90 ± 0.03 *,a | 0.92 ± 0.00 *,a | |
| BHPs+AHPs | 254.09 ± 12.88 | 76.42 ± 5.47 | 694.87 ± 85.51 | 648.97 ± 18.04 | 4.19 ± 0.05 | 1.19 ± 0.01 | |
| ABPs | 35.01 ± 0.99 *,a | 22.84 ± 1.02 * | 46.83 ± 2.96 * | 28.87 ± 3.66 * | 0.49 ± 0.00 *,a | 0.38 ± 0.01 *,a | |
| BBPs | 15.98 ± 0.64 *,a | 4.14 ± 0.58 a | 31.20 ± 4.59 * | 32.19 ± 4.76 | 0.18 ± 0.00 a | 0.05 ± 0.00 *,a | |
| ABPs+BBPs | 50.99 ± 1.63 | 26.98 ± 1.60 | 78.02 ± 7.55 | 61.06 ± 8.43 | 0.66 ± 0.00 | 0.44 ± 0.01 | |
| Total | 425.42 ± 24.7 | 147.06 ± 8.92 | 1044.47 ± 139.38 | 916.81 ± 48.28 | 6.54 ± 0.07 | 2.24 ± 0.02 | |
| 120 | FPs | 45.37 ± 2.45 *,a | 22.05 ± 1.35 a | 22.95 ± 0.34 | 155.50 ± 2.05 a | 0.55 ± 0.00 *,a | 0.29 ± 0.00 *,a |
| BHPs | 20.73 ± 0.23 a | 13.58 ± 0.51 a | 11.19 ± 0.91 | 101.06 ± 2.19 *,a | 0.14 ± 0.01 *,a | 0.07 ± 0.00 *,a | |
| AHPs | 56.78 ± 2.04 *,a | 33.29 ± 3.23 * | 65.78 ± 1.40 a | 366.18 ± 3.14 *,a | 0.48 ± 0.01 *,a | 0.61 ± 0.00 *,a | |
| BHPs+AHPs | 77.50 ± 2.27 | 46.86 ± 3.74 | 76.98 ± 2.30 | 467.24 ± 5.33 | 0.63 ± 0.01 | 0.68 ± 0.00 | |
| ABPs | 22.80 ± 1.02 a | 12.02 ± 0.66 a | 19.57 ± 0.36 a | 57.02 ± 8.39 a | 0.33 ± 0.00 *,a | 0.29 ± 0.00 *,a | |
| BBPs | 7.36 ± 0.33 *,a | 2.18 ± 0.17 a | 13.36 ± 0.67 a | 49.55 ± 3.75 *,a | 0.02 ± 0.00 *,a | 0.02 ± 0.00 *,a | |
| ABPs+BBPs | 30.16 ± 1.35 | 14.20 ± 0.84 | 32.93 ± 1.04 | 106.57 ± 12.15 | 0.35 ± 0.00 | 0.3 ± 0.00 | |
| Total | 153.04 ± 6.08 | 83.12 ± 5.92 | 132.86 ± 3.68 | 729.32 ± 19.52 | 1.53 ± 0.02 | 1.27 ± 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, H.; Geng, M.; Shi, D.; Liu, D.; Jiao, Y.; Lei, Z.; Peng, Y. The Impact of Cooking on Antioxidant and Enzyme Activities in Ruichang Yam Polyphenols. Foods 2025, 14, 14. https://doi.org/10.3390/foods14010014
Liu H, Zhang H, Geng M, Shi D, Liu D, Jiao Y, Lei Z, Peng Y. The Impact of Cooking on Antioxidant and Enzyme Activities in Ruichang Yam Polyphenols. Foods. 2025; 14(1):14. https://doi.org/10.3390/foods14010014
Chicago/Turabian StyleLiu, Haoping, Hua Zhang, Mengting Geng, Dingxin Shi, Dongsheng Liu, Yanxiao Jiao, Zhiqiang Lei, and You Peng. 2025. "The Impact of Cooking on Antioxidant and Enzyme Activities in Ruichang Yam Polyphenols" Foods 14, no. 1: 14. https://doi.org/10.3390/foods14010014
APA StyleLiu, H., Zhang, H., Geng, M., Shi, D., Liu, D., Jiao, Y., Lei, Z., & Peng, Y. (2025). The Impact of Cooking on Antioxidant and Enzyme Activities in Ruichang Yam Polyphenols. Foods, 14(1), 14. https://doi.org/10.3390/foods14010014






