Metabolic Profiling and Stable Isotope Analysis of Wines: Pilot Study for Cross-Border Authentication
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
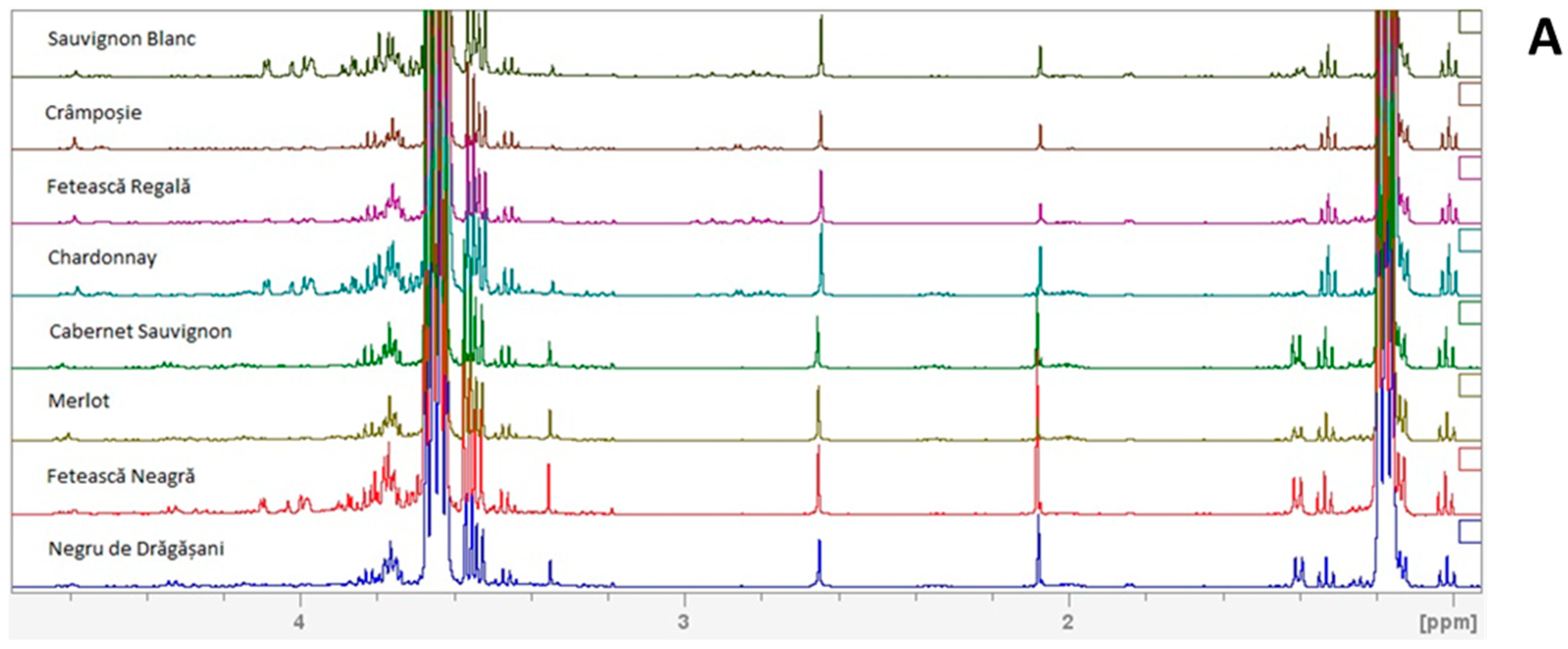
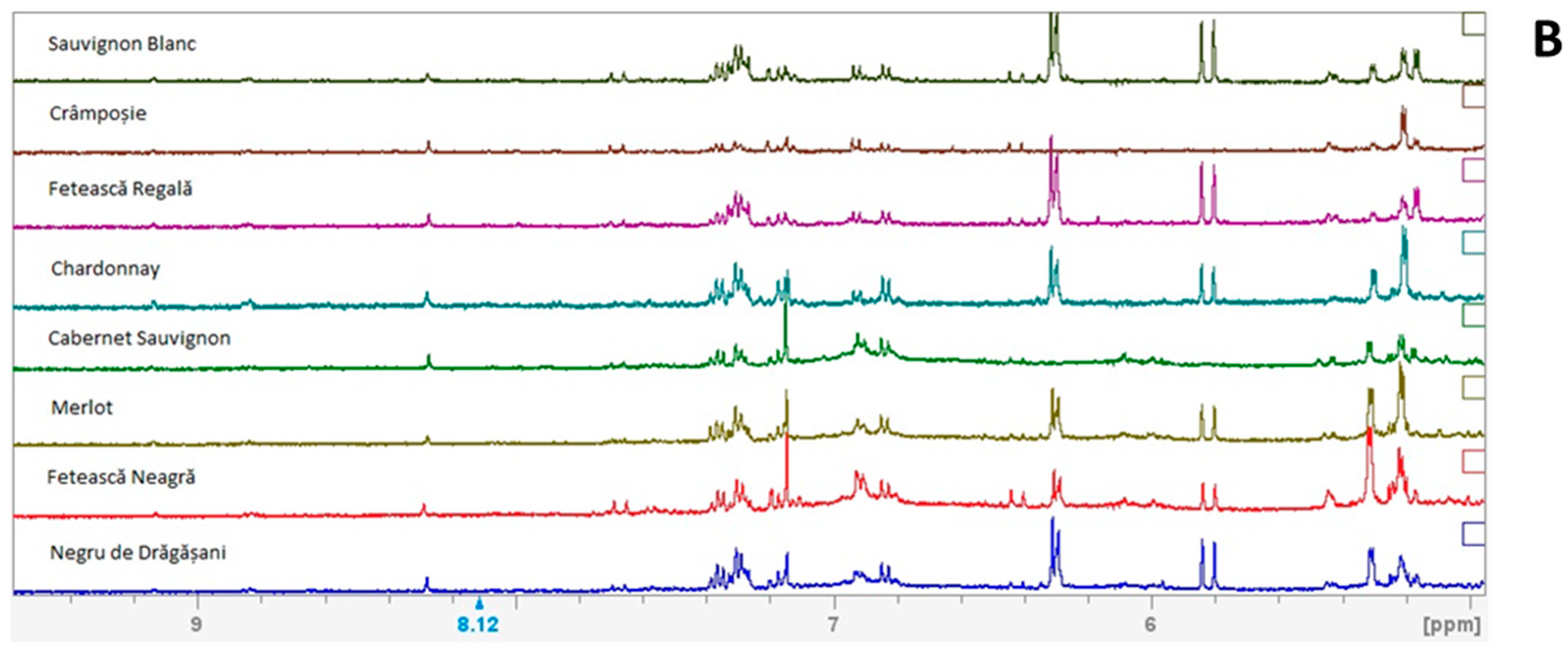
2.2. 1H NMR Metabolomic Profile
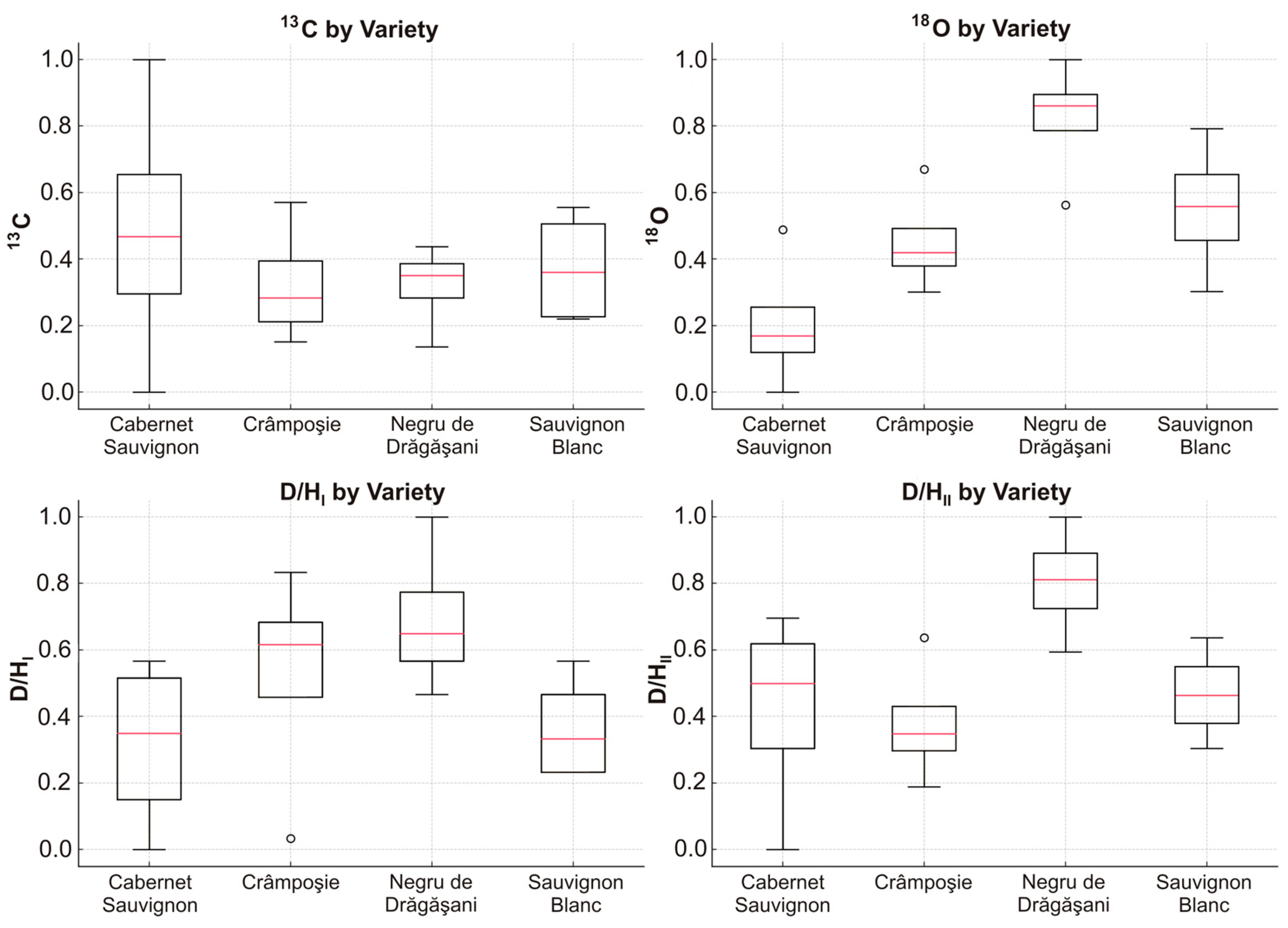
2.3. Determination of Relative Distribution of Deuterium
2.4. Determination of Carbon and Oxygen Stable Isotope Ratios
2.5. Statistical Analysis
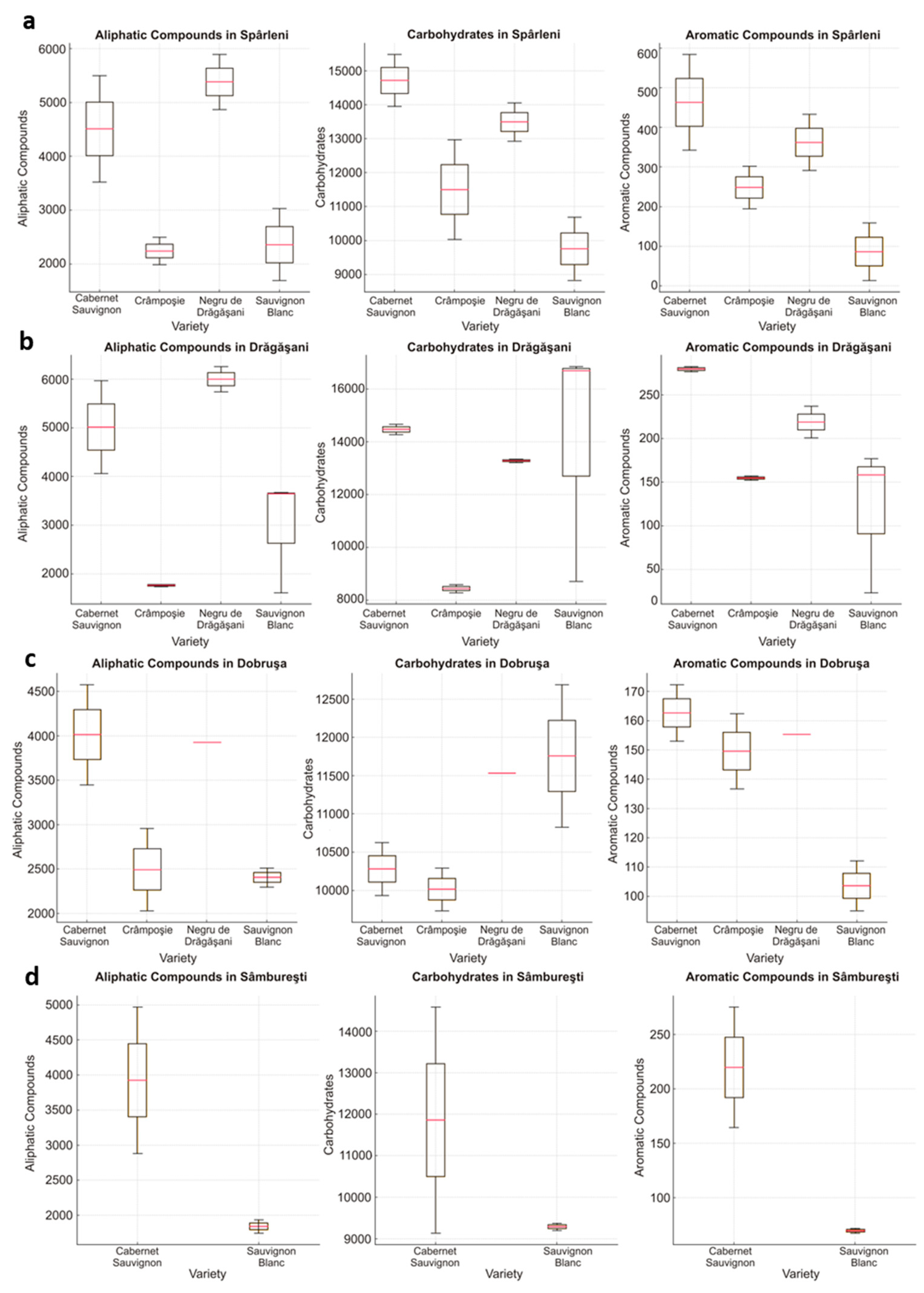
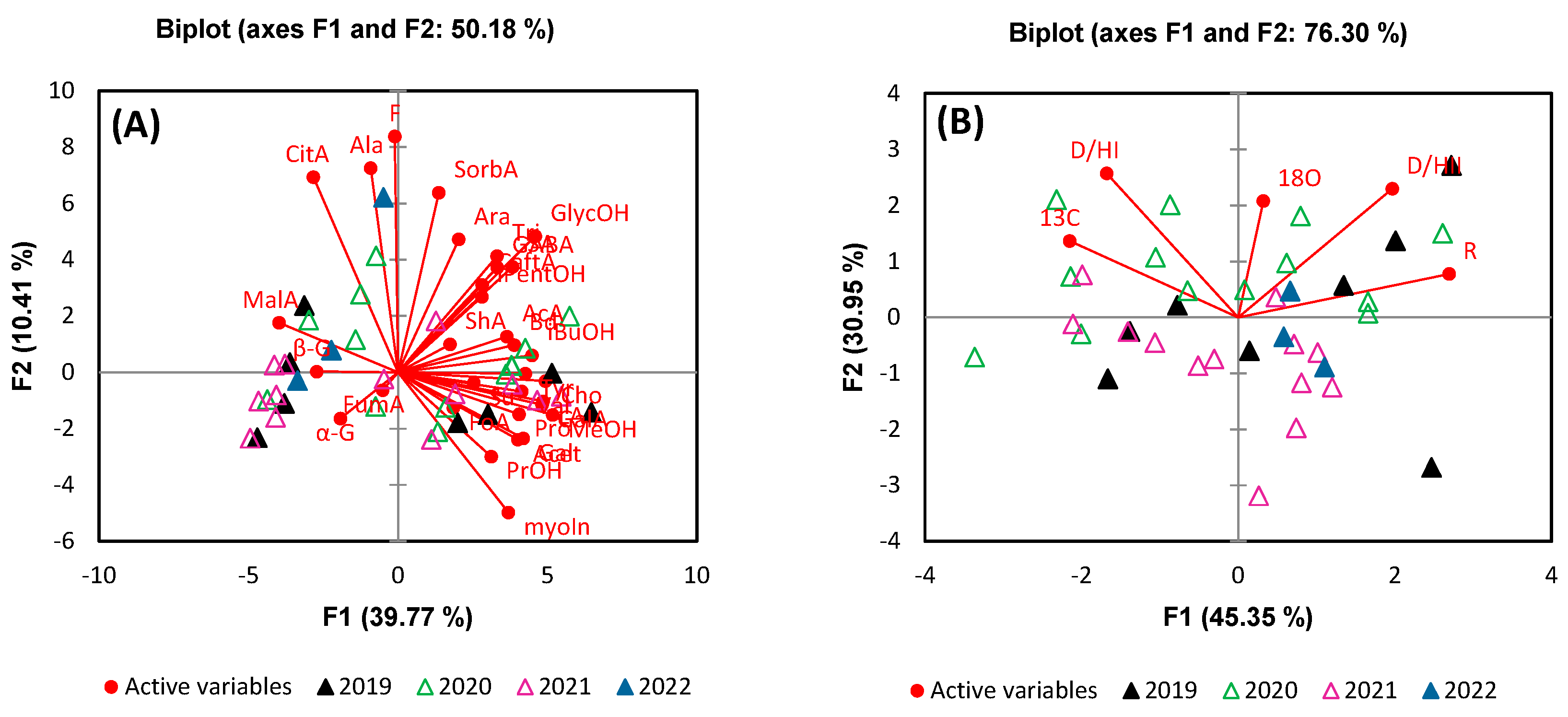
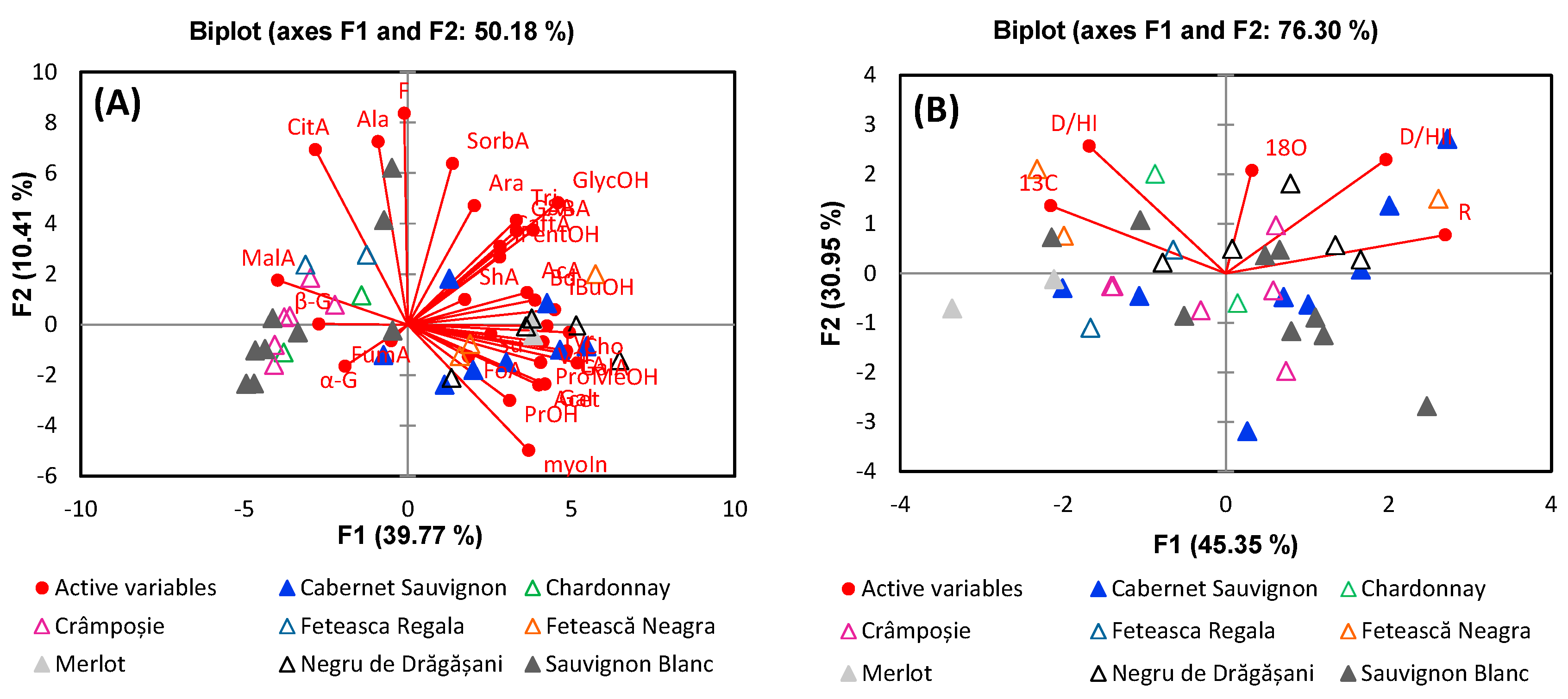
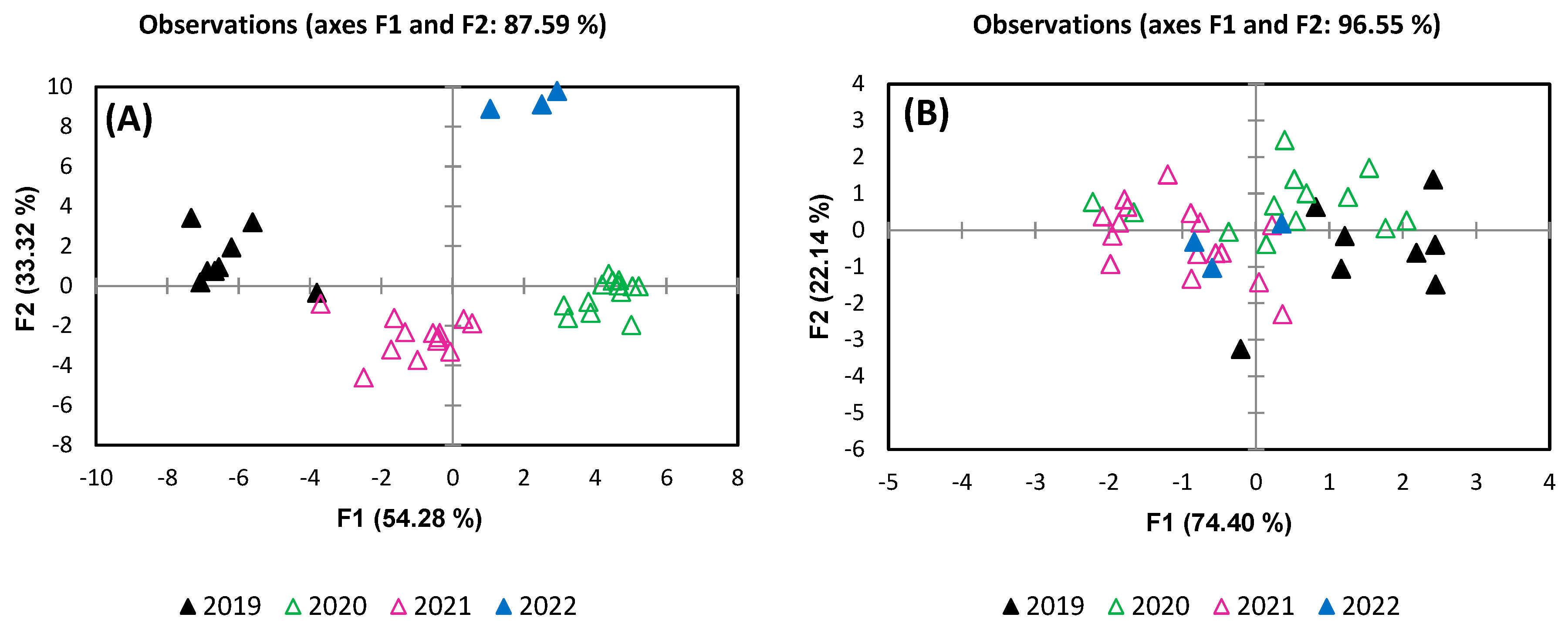
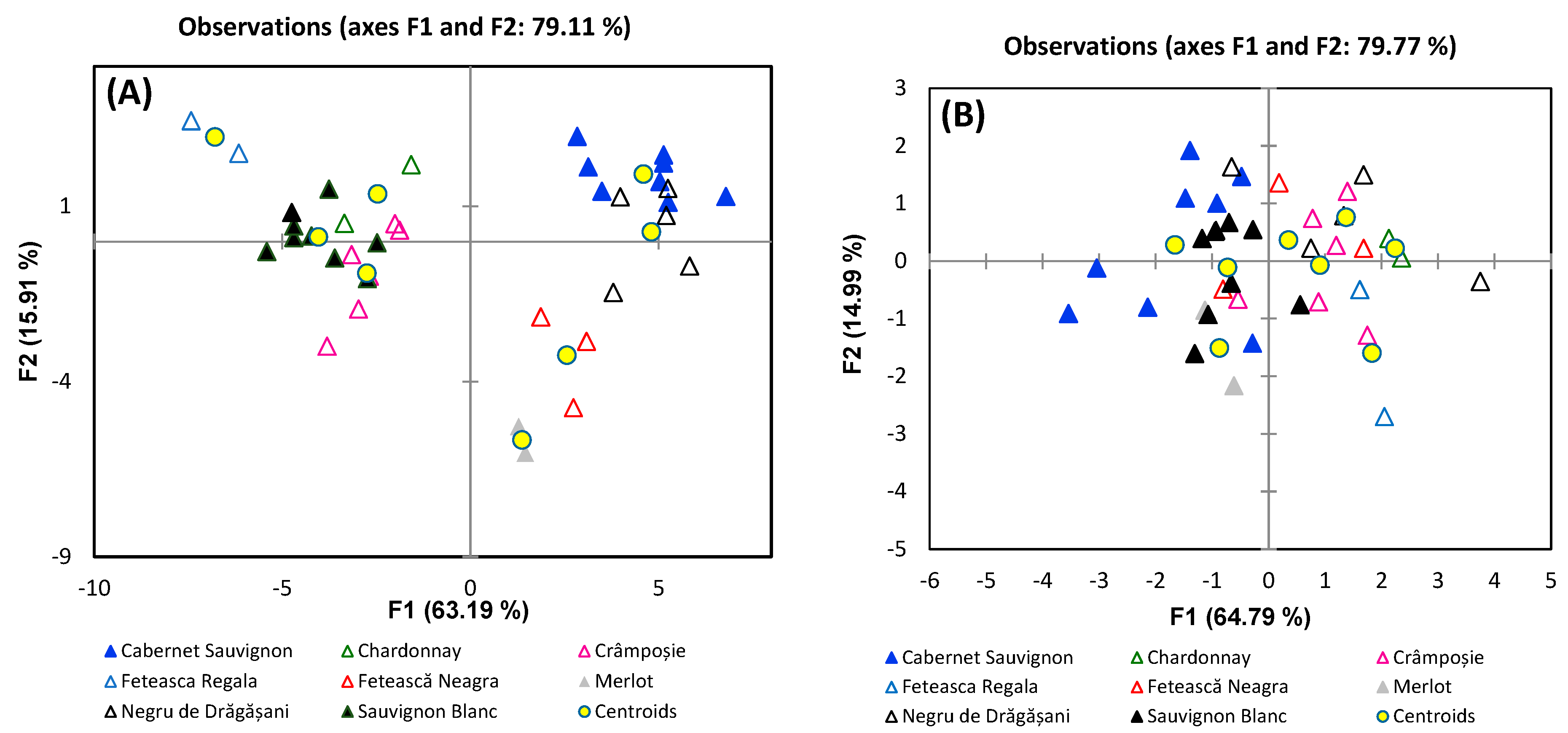
3. Results and Discussion
4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horacek, M.; Nieuwoudt, H.; Bauer, F.F.; Bagheri, B.; Setati, M.E. Differentiation of Geographic Origin of South African Wines from Austrian Wines by IRMS and SNIF-NMR. Foods 2023, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Khakimov, B.; Bakhytkyzy, I.; Fauhl-Hassek, C.; Engelsen, S.B. Non-Volatile Molecular Composition and Discrimination of Single Grape White Wines of Chardonnay, Riesling, Sauvignon Blanc and Silvaner Using Untargeted GC-MS Analysis. Food Chem. 2022, 369, 130878. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Pérez-Coello, M.S.; Marina, M.L. Wine Science in the Metabolomics Era. TrAC Trends Anal. Chem. 2015, 74, 1–20. [Google Scholar] [CrossRef]
- Barisan, L.; Galletto, L. How Do Sparkling Wine Producers Adopt a Sub-Appellation? Evidence from an Exploratory Study on Heroic Prosecco Superiore Rive. Wine Econ. Policy 2021, 10, 45–59. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, F.; Gutiérrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Fang, Y.; Ma, T. Real Wine or Not? Protecting Wine with Traceability and Authenticity for Consumers: Chemical and Technical Basis, Technique Applications, Challenge, and Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 6783–6808. [Google Scholar] [CrossRef]
- Gougeon, L.; Da Costa, G.; Richard, T.; Guyon, F. Wine Authenticity by Quantitative 1H NMR versus Multitechnique Analysis: A Case Study. Food Anal. Methods 2019, 12, 956–965. [Google Scholar] [CrossRef]
- Dordevic, N.; Camin, F.; Marianella, R.M.; Postma, G.J.; Buydens, L.M.C.; Wehrens, R. Detecting the Addition of Sugar and Water to Wine: Detecting the Addition of Sugar and Water to Wine. Aust. J. Grape Wine Res. 2013, 19, 324–330. [Google Scholar] [CrossRef]
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Stefanescu, I.; Ionete, R.E.; Bala, C. Verifying the Red Wines Adulteration through Isotopic and Chromatographic Investigations Coupled with Multivariate Statistic Interpretation of the Data. Food Control 2016, 62, 1–9. [Google Scholar] [CrossRef]
- Košir, I.J.; Kocjančič, M.; Ogrinc, N.; Kidrič, J. Use of SNIF-NMR and IRMS in Combination with Chemometric Methods for the Determination of Chaptalisation and Geographical Origin of Wines (the Example of Slovenian Wines). Anal. Chim. Acta 2001, 429, 195–206. [Google Scholar] [CrossRef]
- Holmberg, L. Wine Fraud. Int. J. Wine Res. 2010, 2, 105–113. [Google Scholar] [CrossRef]
- Viskić, M.; Bandić, L.M.; Korenika, A.-M.J.; Jeromel, A. NMR in the Service of Wine Differentiation. Foods 2021, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Aru, V.; Sørensen, K.; Khakimov, B.; Toldam-Andersen, T.; Balling Engelsen, S. Cool-Climate Red Wines—Chemical Composition and Comparison of Two Protocols for 1H–NMR Analysis. Molecules 2018, 23, 160. [Google Scholar] [CrossRef] [PubMed]
- Cuadros-Inostroza, A.; Giavalisco, P.; Hummel, J.; Eckardt, A.; Willmitzer, L.; Peña-Cortés, H. Discrimination of Wine Attributes by Metabolome Analysis. Anal. Chem. 2010, 82, 3573–3580. [Google Scholar] [CrossRef]
- Skogerson, K.; Runnebaum, R.; Wohlgemuth, G.; De Ropp, J.; Heymann, H.; Fiehn, O. Comparison of Gas Chromatography-Coupled Time-of-Flight Mass Spectrometry and 1H Nuclear Magnetic Resonance Spectroscopy Metabolite Identification in White Wines from a Sensory Study Investigating Wine Body. J. Agric. Food Chem. 2009, 57, 6899–6907. [Google Scholar] [CrossRef]
- Akamatsu, F.; Shimizu, H.; Hayashi, S.; Kamada, A.; Igi, Y.; Koyama, K.; Yamada, O.; Goto-Yamamoto, N. Chemometric Approaches for Determining the Geographical Origin of Japanese Chardonnay Wines Using Oxygen Stable Isotope and Multi-Element Analyses. Food Chem. 2022, 371, 131113. [Google Scholar] [CrossRef]
- Camin, F.; Dordevic, N.; Wehrens, R.; Neteler, M.; Delucchi, L.; Postma, G.; Buydens, L. Climatic and Geographical Dependence of the H, C and O Stable Isotope Ratios of Italian Wine. Anal. Chim. Acta 2015, 853, 384–390. [Google Scholar] [CrossRef]
- Dinca, O.R.; Ionete, R.E.; Costinel, D.; Geana, I.E.; Popescu, R.; Stefanescu, I.; Radu, G.L. Regional and Vintage Discrimination of Romanian Wines Based on Elemental and Isotopic Fingerprinting. Food Anal. Methods 2016, 9, 2406–2417. [Google Scholar] [CrossRef]
- Fan, S.; Zhong, Q.; Gao, H.; Wang, D.; Li, G.; Huang, Z. Elemental Profile and Oxygen Isotope Ratio (δ18O) for Verifying the Geographical Origin of Chinese Wines. J. Food Drug Anal. 2018, 26, 1033–1044. [Google Scholar] [CrossRef]
- Raco, B.; Dotsika, E.; Poutoukis, D.; Battaglini, R.; Chantzi, P. O–H–C Isotope Ratio Determination in Wine in Order to Be Used as a Fingerprint of Its Regional Origin. Food Chem. 2015, 168, 588–594. [Google Scholar] [CrossRef]
- Coelho, I.; Matos, A.S.; Epova, E.N.; Barre, J.; Cellier, R.; Ogrinc, N.; Castanheira, I.; Bordado, J.; Donard, O.F.X. Multi-Element and Multi-Isotopic Profiles of Port and Douro Wines as Tracers for Authenticity. J. Food Compos. Anal. 2023, 115, 104988. [Google Scholar] [CrossRef]
- Horacek, M.; Ogrinc, N.; Magdas, D.A.; Wunderlin, D.; Sucur, S.; Maras, V.; Misurovic, A.; Eder, R.; Čuš, F.; Wyhlidal, S.; et al. Isotope Analysis (13C, 18O) of Wine from Central and Eastern Europe and Argentina, 2008 and 2009 Vintages: Differentiation of Origin, Environmental Indications, and Variations Within Countries. Front. Sustain. Food Syst. 2021, 5, 638941. [Google Scholar] [CrossRef]
- Valls Fonayet, J.; Loupit, G.; Richard, T. MS- and NMR-Metabolomic Tools for the Discrimination of Wines: Applications for Authenticity. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 98, pp. 297–357. ISBN 978-0-12-821688-0. [Google Scholar]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schütz, B.; Bansbach, M.; Schäfer, H.; Spraul, M. Targeted and Nontargeted Wine Analysis by 1H NMR Spectroscopy Combined with Multivariate Statistical Analysis. Differentiation of Important Parameters: Grape Variety, Geographical Origin, Year of Vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, P.; Francesca, N.; Moschetti, G.; Piccolo, A. NMR Spectroscopy Evaluation of Direct Relationship between Soils and Molecular Composition of Red Wines from Aglianico Grapes. Anal. Chim. Acta 2010, 673, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Zira, A.; Magiatis, P.; Haroutounian, S.A.; Skaltsounis, A.L.; Mikros, E. 1H NMR-Based Metabonomics for the Classification of Greek Wines According to Variety, Region, and Vintage. Comparison with HPLC Data. J. Agric. Food Chem. 2009, 57, 11067–11074. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Hwang, G.-S.; Van Den Berg, F.; Lee, C.-H.; Hong, Y.-S. Evidence of Vintage Effects on Grape Wines Using 1H NMR-Based Metabolomic Study. Anal. Chim. Acta 2009, 648, 71–76. [Google Scholar] [CrossRef]
- Pereira, G.E.; Gaudillere, J.-P.; van Leeuwen, C.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. 1H-NMR Metabolic Profiling of Wines from Three Cultivars, Three Soil Types and Two Contrasting Vintages. IVES Conf. Ser. Terroir 2006, 1, 402–406. [Google Scholar]
- Leder, R.; Petric, I.V.; Jusup, J.; Banović, M. Geographical Discrimination of Croatian Wines by Stable Isotope Ratios and Multielemental Composition Analysis. Front. Nutr. 2021, 8, 625613. [Google Scholar] [CrossRef]
- Horacek, M.; Hola, M.; Tobolkova, B.; Kolar, K.; Vaculovic, T.; Mikes, O.; Marosanovic, B.; Philipp, C.; Lojovic, M.; Polovka, M.; et al. Investigation of Geographic Origin of Wine from Border Regions: Results from Investigation of Two Vintages. BIO Web Conf. 2019, 15, 02039. [Google Scholar] [CrossRef]
- Gerginova, D.; Simova, S. Chemical Profiling of Wines Produced in Bulgaria and Distinction from International Grape Varieties. ACS Omega 2023, 8, 18702–18713. [Google Scholar] [CrossRef]
- Solovyev, P.A.; Fauhl-Hassek, C.; Riedl, J.; Esslinger, S.; Bontempo, L.; Camin, F. NMR Spectroscopy in Wine Authentication: An Official Control Perspective. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2040–2062. [Google Scholar] [CrossRef]
- Esslinger, S.; Fauhl-Hassek, C.; Wittkowski, R. Authentication of Wine by 1H-NMR Spectroscopy: Opportunities and Challenges. In ACS Symposium Series; Ebeler, S.B., Sacks, G., Vidal, S., Winterhalter, P., Eds.; American Chemical Society: Washington, DC, USA, 2015; Volume 1203, pp. 85–108. ISBN 978-0-8412-3010-1. [Google Scholar]
- Son, H.-S.; Kim, K.M.; Van Den Berg, F.; Hwang, G.-S.; Park, W.-M.; Lee, C.-H.; Hong, Y.-S. 1H Nuclear Magnetic Resonance-Based Metabolomic Characterization of Wines by Grape Varieties and Production Areas. J. Agric. Food Chem. 2008, 56, 8007–8016. [Google Scholar] [CrossRef] [PubMed]
- Godelmann, R.; Kost, C.; Patz, C.-D.; Ristow, R.; Wachter, H. Quantitation of Compounds in Wine Using 1H NMR Spectroscopy: Description of the Method and Collaborative Study. J. AOAC Int. 2016, 99, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Cao, Y.; Zhu, J.; Xu, W.; Wu, W. Analysis of Metabolites in Chardonnay Dry White Wine with Various Inactive Yeasts by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis. AMB Express 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Le Mao, I.; Martin-Pernier, J.; Bautista, C.; Lacampagne, S.; Richard, T.; Da Costa, G. 1H-NMR Metabolomics as a Tool for Winemaking Monitoring. Molecules 2021, 26, 6771. [Google Scholar] [CrossRef]
- Pereira, G.E.; Gaudillere, J.-P.; Van Leeuwen, C.; Hilbert, G.; Lavialle, O.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. 1H NMR and Chemometrics to Characterize Mature Grape Berries in Four Wine-Growing Areas in Bordeaux, France. J. Agric. Food Chem. 2005, 53, 6382–6389. [Google Scholar] [CrossRef]
- Hanganu, A.; Todasca, M.-C.; Chira, N.-A.; Rosca, S. Influence of Common and Selected Yeasts on Wine Composition Studied Using 1H-NMR Spectroscopy. Rev. Chim. 2011, 62, 689–692. [Google Scholar]
- Fan, S.; Zhong, Q.; Fauhl-Hassek, C.; Pfister, M.K.-H.; Horn, B.; Huang, Z. Classification of Chinese Wine Varieties Using 1H NMR Spectroscopy Combined with Multivariate Statistical Analysis. Food Control 2018, 88, 113–122. [Google Scholar] [CrossRef]
- García-Aguilera, M.E.; Delgado-Altamirano, R.; Villalón, N.; Ruiz-Terán, F.; García-Garnica, M.M.; Ocaña-Ríos, I.; Rodríguez De San Miguel, E.; Esturau-Escofet, N. Study of the Stability of Wine Samples for 1H-NMR Metabolomic Profile Analysis through Chemometrics Methods. Molecules 2023, 28, 5962. [Google Scholar] [CrossRef]
- Snow, L.; Trass, M.; Klein, M.; Orlowicz, S.; Rivera, B. Fast and Robust Analysis of Organic Acids from Wine Using HPLC-UV. U.S. Patent 6,162,362, 19 December 2000. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Heidelberg, Germany, 2008; ISBN 978-0-12-373646-8. [Google Scholar]
- Palomino-Vasco, M.; Acedo-Valenzuela, M.I.; Rodríguez-Cáceres, M.I.; Mora-Díez, N. Monitoring Winemaking Process Using Tyrosine Influence in the Excitation-Emission Matrices of Wine. Food Chem. 2021, 344, 128721. [Google Scholar] [CrossRef]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and Identification of Organic Acids in Wine Samples. Problems and Challenges. TrAC Trends Anal. Chem. 2019, 120, 115630. [Google Scholar] [CrossRef]
- Valdés, M.E.; Talaverano, M.I.; Moreno, D.; Prieto, M.H.; Mancha, L.A.; Uriarte, D.; Vilanova, M. Effect of the Timing of Water Deficit on the Must Amino Acid Profile of Tempranillo Grapes Grown under the Semiarid Conditions of SW Spain. Food Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Metafa, M.; Kotseridis, Y.; Paraskevopoulos, I.; Kallithraka, S. Amino Acid Content of Agiorgitiko (Vitis vinifera L. cv.) Grape Cultivar Grown in Representative Regions of Nemea. Eur. Food Res. Technol. 2018, 244, 2041–2050. [Google Scholar] [CrossRef]
- Callejón, R.M.; Troncoso, A.M.; Morales, M.L. Determination of Amino Acids in Grape-Derived Products: A Review. Talanta 2010, 81, 1143–1152. [Google Scholar] [CrossRef]
- Rodicio, R.; Heinisch, J.J. Carbohydrate Metabolism in Wine Yeasts. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 189–213. ISBN 978-3-319-60020-8. [Google Scholar]
- Bauer, F.F. Yeast Stress Response and Fermentation Efficiency: How to Survive the Making of Wine—A Review. Afr. J. Enol. Vitic. 2000, 21, 27–51. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef]
- Koriem, K.M.M. Caftaric Acid: An Overview on Its Structure, Daily Consumption, Bioavailability and Pharmacological Effects. Biointerface Res. Appl. Chem. 2020, 10, 5616–5623. [Google Scholar] [CrossRef]
- Román, T.; Nicolini, G.; Barp, L.; Malacarne, M.; Tait, F.; Larcher, R. Shikimic Acid Concentration in White Wines Produced with Different Processing Protocols from Fungus-Resistant Grapes Growing in the Alps. VITIS-J. Grapevine Res. 2018, 57, 41–46. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Cibrario, A.; Perello, M.C.; Miot-Sertier, C.; Riquier, L.; De Revel, G.; Ballestra, P.; Dols-Lafargue, M. Carbohydrate Composition of Red Wines during Early Aging and Incidence on Spoilage by Brettanomyces bruxellensis. Food Microbiol. 2020, 92, 103577. [Google Scholar] [CrossRef]
- Jakabová, S.; Fikselová, M.; Mendelová, A.; Ševčík, M.; Jakab, I.; Aláčová, Z.; Kolačkovská, J.; Ivanova-Petropulos, V. Chemical Composition of White Wines Produced from Different Grape Varieties and Wine Regions in Slovakia. Appl. Sci. 2021, 11, 11059. [Google Scholar] [CrossRef]
- Scettri, A.; Baroldi, I.; Allari, L.; Bolognini, L.; Guardini, K.; Schievano, E. NMR Sugar-Profile in Genuine Grape Must. Food Chem. 2024, 451, 139374. [Google Scholar] [CrossRef] [PubMed]
- Jégou, S.; Hoang, D.A.; Salmon, T.; Williams, P.; Oluwa, S.; Vrigneau, C.; Doco, T.; Marchal, R. Effect of Grape Juice Press Fractioning on Polysaccharide and Oligosaccharide Compositions of Pinot Meunier and Chardonnay Champagne Base Wines. Food Chem. 2017, 232, 49–59. [Google Scholar] [CrossRef]
- Márquez, K.; Contreras, D.; Salgado, P.; Mardones, C. Production of Hydroxyl Radicals and Their Relationship with Phenolic Compounds in White Wines. Food Chem. 2019, 271, 80–86. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Shahid, M.A.; Babar, M.A. Role of Sugars, Amino Acids and Organic Acids in Improving Plant Abiotic Stress Tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Williams, P.; Doco, T. Recent Advances in the Knowledge of Wine Oligosaccharides. Food Chem. 2021, 342, 128330. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Williams, P.; Mazerolles, G.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Effect of Enzyme Additions on the Oligosaccharide Composition of Monastrell Red Wines from Four Different Wine-Growing Origins in Spain. Food Chem. 2014, 156, 151–159. [Google Scholar] [CrossRef]
- Bambina, P.; Spinella, A.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P.; Cinquanta, L.; Conte, P. 1H-NMR Spectroscopy Coupled with Chemometrics to Classify Wines According to Different Grape Varieties and Different Terroirs. Agriculture 2024, 14, 749. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Graziosi, R.; Silvestri, M.; Bertacchini, L.; Durante, C.; Plessi, M. Application of One- and Two-Dimensional NMR Spectroscopy for the Characterization of Protected Designation of Origin Lambrusco Wines of Modena. J. Agric. Food Chem. 2013, 61, 1741–1746. [Google Scholar] [CrossRef]
- Gougeon, L.; Da Costa, G.; Guyon, F.; Richard, T. 1H NMR Metabolomics Applied to Bordeaux Red Wines. Food Chem. 2019, 301, 125257. [Google Scholar] [CrossRef]
- Bambina, P.; Spinella, A.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P.; Corona, O.; Cinquanta, L.; Conte, P. 1H NMR-Based Metabolomics to Assess the Impact of Soil Type on the Chemical Composition of Nero d’Avola Red Wines. J. Agric. Food Chem. 2023, 71, 5823–5835. [Google Scholar] [CrossRef]
- Duley, G. The Impact of Temperature on “Pinot Noir” Berry and Wine Quality in a Steeply Sloping Cool Climate Vineyard in South Australia. VITIS-J. Grapevine Res. 2021, 60, 169–178. [Google Scholar] [CrossRef]
- Gougeon, L.; Da Costa, G.; Le Mao, I.; Ma, W.; Teissedre, P.-L.; Guyon, F.; Richard, T. Wine Analysis and Authenticity Using 1H-NMR Metabolomics Data: Application to Chinese Wines. Food Anal. Methods 2018, 11, 3425–3434. [Google Scholar] [CrossRef]
- Son, H.-S.; Hwang, G.-S.; Park, W.-M.; Hong, Y.-S.; Lee, C.-H. Metabolomic Characterization of Malolactic Fermentation and Fermentative Behaviors of Wine Yeasts in Grape Wine. J. Agric. Food Chem. 2009, 57, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Viggiani, L.; Morelli, M.A.C. Characterization of Wines by Nuclear Magnetic Resonance: A Work Study on Wines from the Basilicata Region in Italy. J. Agric. Food Chem. 2008, 56, 8273–8279. [Google Scholar] [CrossRef]
- Nyitrainé Sárdy, Á.D.; Ladányi, M.; Varga, Z.; Szövényi, Á.P.; Matolcsi, R. The Effect of Grapevine Variety and Wine Region on the Primer Parameters of Wine Based on 1H NMR-Spectroscopy and Machine Learning Methods. Diversity 2022, 14, 74. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Silva, L.M.A.; Ribeiro, P.R.V.; De Brito, E.S.; Zocolo, G.J.; Souza-Leão, P.C.; Marques, A.T.B.; Quintela, A.L.; Larsen, F.H.; Canuto, K.M. 1H NMR and LC-MS-Based Metabolomic Approach for Evaluation of the Seasonality and Viticultural Practices in Wines from São Francisco River Valley, a Brazilian Semi-Arid Region. Food Chem. 2019, 289, 558–567. [Google Scholar] [CrossRef]
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Ionete, R.E.; Stefanescu, I.; Artem, V.; Bala, C. Classification of Red Wines Using Suitable Markers Coupled with Multivariate Statistic Analysis. Food Chem. 2016, 192, 1015–1024. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Toepfer, R.; Choi, Y.H.; Verpoorte, R. Metabolic Characterization of Palatinate German White Wines According to Sensory Attributes, Varieties, and Vintages Using NMR Spectroscopy and Multivariate Data Analyses. J. Biomol. NMR 2011, 49, 255–266. [Google Scholar] [CrossRef]
- Le Mao, I.; Da Costa, G.; Richard, T. 1H-NMR Metabolomics for Wine Screening and Analysis. Oeno One 2023, 57, 15–31. [Google Scholar] [CrossRef]
- Duley, G.; Dujourdy, L.; Klein, S.; Werwein, A.; Spartz, C.; Gougeon, R.D.; Taylor, D.K. Regionality in Australian Pinot Noir Wines: A Study on the Use of NMR and ICP-MS on Commercial Wines. Food Chem. 2021, 340, 127906. [Google Scholar] [CrossRef]
- Mascellani, A.; Hoca, G.; Babisz, M.; Krska, P.; Kloucek, P.; Havlik, J. 1H NMR Chemometric Models for Classification of Czech Wine Type and Variety. Food Chem. 2021, 339, 127852. [Google Scholar] [CrossRef]


| Crt. No. | Grape Variety | Label Quality Indicator | Type | Colour | Site | Vintage |
|---|---|---|---|---|---|---|
| 1 | Cabernet Sauvignon | PDO | Dry | red | Dobrușa | 2019 |
| 2 | Negru de Drăgășani | PDO | Dry | red | 2020 | |
| 3 | Cabernet Sauvignon | PDO | Dry | red | 2021 | |
| 4 | Sauvignon Blanc | PDO | Semi-Dry | white | 2021 | |
| 5 | Crâmpoșie | PDO | Dry | white | 2021 | |
| 6 | Sauvignon Blanc | PDO | Semi-Dry | white | 2022 | |
| 7 | Crâmpoșie | PDO | Dry | white | 2022 | |
| 8 | Sauvignon Blanc | PDO | Semi-Dry | white | Drăgășani | 2022 |
| 9 | Negru de Drăgășani | PDO | Dry | red | 2020 | |
| 10 | Cabernet Sauvignon | PDO | Dry | red | 2019 | |
| 11 | Crâmpoșie | PDO | Dry | white | 2021 | |
| 12 | Sauvignon Blanc | PDO | Semi-Dry | white | 2021 | |
| 13 | Negru de Drăgășani | PDO | Dry | red | 2019 | |
| 14 | Crâmpoșie | PDO | Dry | white | 2019 | |
| 15 | Fetească Regală | PDO | Dry | white | 2019 | |
| 16 | Fetească Regală | PDO | Dry | white | 2020 | |
| 17 | Sauvignon Blanc | PDO | Semi-Dry | white | 2020 | |
| 18 | Cabernet Sauvignon | PDO | Dry | red | 2021 | |
| 19 | Merlot | PDO | Semi-Dry | red | Sâmburești | 2020 |
| 20 | Fetească Neagră | PDO | Dry | red | 2020 | |
| 21 | Sauvignon Blanc | PDO | Semi-Dry | white | 2020 | |
| 22 | Cabernet Sauvignon | PDO | Dry | red | 2020 | |
| 23 | Cabernet Sauvignon | PDO | Dry | red | 2021 | |
| 24 | Sauvignon Blanc | PDO | Semi-Dry | white | 2021 | |
| 25 | Fetească Neagră | PDO | Dry | red | 2021 | |
| 26 | Merlot | PDO | Semi-Dry | red | 2021 | |
| 27 | Merlot | PDO | Semi-Dry | red | 2021 | |
| 28 | Cabernet Sauvignon | PDO | Dry | red | 2021 | |
| 29 | Cabernet Sauvignon | PDO | Dry | red | 2021 | |
| 30 | Sauvignon Blanc | PDO | Semi-Dry | white | Spârleni | 2019 |
| 31 | Chardonnay | PDO | Dry | white | 2019 | |
| 32 | Negru de Drăgășani | PDO | Dry | red | 2019 | |
| 33 | Crâmpoșie | PDO | Dry | white | 2020 | |
| 34 | Chardonnay | PDO | Dry | white | 2020 | |
| 35 | Negru de Drăgășani | PDO | Dry | red | 2020 | |
| 36 | Cabernet Sauvignon | PDO | Dry | red | 2020 | |
| 37 | Fetească Neagră | PDO | Dry | red | 2020 | |
| 38 | Cabernet Sauvignon | PDO | Dry | red | 2021 | |
| 39 | Crâmpoșie | PDO | Dry | white | 2021 | |
| 40 | Sauvignon Blanc | PDO | Semi-Dry | white | 2021 |
| Crt. No. | Metabolite | Abbreviation | Chemical Shift [ppm] | Multiplicity |
|---|---|---|---|---|
| 1 | 2,3-Butanediol | Bd | 1.132 | d |
| 2 | Lactic acid | LA | 1.407 | d |
| 3 | Alanine | Ala | 1.467 | d |
| 4 | 1-Propanol | PrOH | 1.540 | m |
| 5 | isoPentanol | iPentOH | 1.652 | m |
| 6 | isoButanol | iBuOH | 1.729 | m |
| 7 | Sorbic acid | SorbA | 1.841 | d |
| 8 | Acetic acid | AcA | 2.082 | s |
| 9 | Acetoin | Acet | 2.215 | s |
| 10 | GABA | GABA | 2.269 | t |
| 11 | Proline | Pro | 2.352 | m |
| 12 | Succinic acid | SA | 2.653 | s |
| 13 | Malic acid | MalA | 2.895 | dd |
| 14 | Citric acid | CitA | 2.963 | d |
| 15 | Choline | Cho | 3.189 | s |
| 16 | Methanol | MeOH | 3.350 | s |
| 17 | Glycerol | GlycOH | 3.549 | dd |
| 18 | Fructose | F | 3.980 | m |
| 19 | myo-Inositol | myoIn | 4.046 | t |
| 20 | Arabinose | Ara | 4.497 | d |
| 21 | Glucose | G | 4.606 (β) | d |
| 5.214 (α) | d | |||
| 22 | Galactose | Gal | 5.248 | d |
| 23 | Galacturonic acid | GalA | 5.313 | d |
| 24 | Sucrose | Su | 5.430 | d |
| 25 | Caftaric acid | CaftA | 6.422 | d |
| 26 | Fumaric acid | FumA | 6.628 | s |
| 27 | Shikimic acid | ShA | 6.807 | m |
| 28 | Tyrosine | Tyr | 6.840 | d |
| 29 | Formic acid | FoA | 8.278 | s |
| 30 | Trigonelline | Tri | 9.142 | s |
| Bd | LA | Ala | PrOH | iPentOH | iBuOH | SorbA | AcA | Acet | GABA | Pro | SA | Cho | MeOH | FumA | ||
| Merlot | 826.1 a | 619.7 bc | 39.9 a | 42.5 a | 205.3 a | 88.6 a | 179.1 a | 303 abc | 16.4 a | 11.7 ab | 718.3 a | 1035.9 a | 32.8 a | 191.6 a | 1.5 a | |
| Fetească Neagra | 810.6 a | 923.8 ab | 33.6 a | 53.4 a | 135.7 a | 60.6 ab | 134.9 a | 683.5 a | 7.2 bc | 9.5 ab | 324.2 b | 809.7 a | 29.5 ab | 184.5 a | 2.0 a | |
| Negru de Drăgășani | 664.6 ab | 1300 a | 42.4 a | 65.8 a | 256.8 a | 83.8 a | 83.6 a | 615.7 ab | 8.4 b | 9.1 ab | 559.3 a | 1045.3 a | 35.8 a | 161.7 ab | 0.6 a | |
| Cabernet Sauvignon | 495.1bc | 788.5 b | 33.3 a | 56.7 a | 241.7 a | 65.2 ab | 74.0 a | 400 abc | 10.9 ab | 12.6 a | 597.4 a | 978.1 a | 34.8 a | 139.2 b | 1.1 a | |
| Feteasca Regala | 548.0 bc | 74.2 c | 52.8 a | 46.9 a | 233.0 a | 35.9 b | 176.7 a | 165.1 c | 1.2 c | 8.3 ab | 177.1 b | 849.7 a | 17.8 bc | 26.2 c | 2.1 a | |
| Sauvignon Blanc | 465.6 bc | 198.3 c | 46.9 a | 41.0 a | 202.1 a | 41.8 b | 79.7 a | 241.5 bc | 0.9 c | 8.1 b | 184.2 b | 761.1 a | 14.7 c | 32.9 c | 1.1 a | |
| Chardonnay | 428.0 c | 83.6 c | 47.7 a | 34.8 a | 157.6 a | 37.3 b | 43.8 a | 229.3 bc | 0.6 c | 6.0 b | 285.3 b | 647.5 a | 14.8 c | 45.0 c | 0.0 a | |
| Crâmpoșie | 343.4 c | 209.4 c | 48.6 a | 37.1 a | 178.3 a | 29.6 b | 47.2 a | 187.5 bc | 0.4 c | 5.5 b | 199.4 b | 712.8 a | 16.2 c | 23.1 c | 1.4 a | |
| FoA | MalA | CitA | GlycOH | F | myoIn | Ara | β-G | α-G | Gal | GalA | Su | CaftA | ShA | Tyr | Tri | |
| Merlot | 11.1 a | 0.0 c | 0.0 c | 11,579ab | 219.2 a | 295 bcd | 181.1 ab | 1083 ab | 278.5 a | 48.8 ab | 473.4 a | 55.7 a | 98.1 ab | 16.0 a | 55.6 abc | 16.9 ab |
| Fetească Neagra | 11.3 a | 0.0 c | 0.0 c | 12,134 a | 893.8 a | 351 abc | 129.1 bc | 566.4 ab | 221.3 a | 53.2 a | 480.5 a | 35.1 a | 147.5 a | 45.1 a | 67.9 a | 19.2 a |
| Negru de Drăgășani | 16.3 a | 0.0 c | 0.0 c | 11,248 abc | 323.5 a | 430.3 ab | 107.7 bc | 301.6 b | 103.9 a | 41.5 ab | 396.3 a | 63.5 a | 135.7 a | 52.1 a | 61.3 ab | 14.3 ab |
| Cabernet Sauvignon | 12.3 a | 433.3 bc | 85.2 c | 10,707 abc | 509.1 a | 494.1 a | 71.8 cd | 538.9 b | 161.1 a | 34.6 bc | 284.3 b | 30.2 a | 94.1 ab | 111.8 a | 59.1 ab | 16.2 ab |
| Feteasca Regala | 16.8 a | 1445 ab | 1036.0 a | 9450 abc | 681.0 a | 160.5 cd | 296.3 a | 1404 ab | 66.0 a | 10.0 d | 45.4 c | 33.8 a | 92.2 ab | 17.5 a | 39 abcd | 13.3 ab |
| Sauvignon Blanc | 10.6 a | 1478 ab | 596.7 b | 8566 abc | 757.0 a | 227.1 cd | 42.3 cd | 1501.3 a | 314.3 a | 16.5 d | 86.6 c | 28.8 a | 55.2 b | 7.1 a | 24.7 d | 10.3 |
| Chardonny | 13.2 a | 2181.6a | 407.0 bc | 8130 bc | 651.5 a | 297 bcd | 99.3 bcd | 699.4 ab | 208.5 a | 22.0 cd | 126.3 c | 22.7 a | 23.5 b | 28.1 a | 31.7 bcd | 18.8 a |
| Crâmpoșie | 10.3 a | 1301 ab | 432.7 b | 7788 c | 826.0 a | 160.1 d | 18.8 d | 831.5 ab | 267.2 a | 15.0 d | 57.0 c | 21.9 a | 121.1 a | 26.1 a | 27.1 cd | 9.8 b |
| Bd | LA | Ala | PrOH | iPentOH | iBuOH | SorbA | AcA | Acet | GABA | Pro | SA | Cho | MeOH | FumA | ||
| 2019 | 512.2 a | 616.2 a | 39.3 b | 58.6 a | 222.9 a | 61.9 a | 52.3 a | 374.2 a | 3.5 a | 6.5 a | 343.7 a | 838.6 a | 25.7 a | 91.8 a | 0.5 ab | |
| 2020 | 577.2 a | 678.8 a | 43.2 b | 44.8 a | 196.8 a | 57.7 a | 123.4 a | 423.7 a | 6.8 a | 9.8 a | 416.2 a | 913.5 a | 24.8 a | 106.0 a | 0.8 ab | |
| 2021 | 496.2 a | 429.6 a | 37.4 b | 47.1 a | 206.5 a | 49.6 a | 71.3 a | 274.9 a | 6.2 a | 9.5 a | 381.6 a | 771.1 a | 24.6 a | 93.7 a | 2.4 a | |
| 2022 | 507.0 a | 279.3 a | 71.1 a | 40.1 a | 226.9 a | 37.1 a | 88.7 a | 316.8 a | 1.1 a | 11.0 a | 214.8 a | 1022.2 a | 20.1 a | 36.2 a | 0.0 b | |
| FoA | MalA | CitA | GlycOH | F | myoIn | Ara | β-G | α-G | Gal | GalA | Su | CaftA | ShA | Tyr | Tri | |
| 2019 | 14.9 a | 997.6 ab | 380.9 a | 9412.6 a | 444.6 a | 393.2 a | 82.2 a | 668.3 a | 162.5 a | 27.6 a | 185.5 a | 60.1 a | 87.9 a | 23.6 a | 50.9 a | 11.9 a |
| 2020 | 11.5 ab | 383.2 b | 266.1 a | 10,645 a | 866.3 a | 290.9 a | 114.3 a | 667.7 a | 182.3 a | 31.4 a | 269.3 a | 30.7 b | 106.4 a | 44.1 a | 49.8 a | 16.0 a |
| 2021 | 11.2 b | 1015 ab | 218.7 a | 9018.8 a | 418.3 a | 312.3 a | 61.1 a | 1188.5 a | 288.3 a | 29.2 a | 223.2 a | 24.0 b | 90.0 a | 64.0 a | 37.7 a | 12.3 a |
| 2022 | 13.8 ab | 1891.8 a | 728.7 a | 9837.2 a | 996.2 a | 226.7 a | 69.9 a | 972.5 a | 198.3 a | 12.3 a | 74.8 a | 30.4 b | 89.6 a | 14.1 a | 25.8 a | 15.3 a |
| Bd | LA | Ala | PrOH | iPentOH | iBuOH | SorbA | AcA | Acet | GABA | Pro | SA | Cho | MeOH | FumA | ||
| Sâmburești | 589.6 a | 544.0 a | 36.0 a | 42.8 a | 159.4 b | 55.2 a | 155.5 a | 258.4 a | 8.6 a | 9.6 a | 486.4 a | 719.1 a | 25.3 a | 133.1 a | 2.2 a | |
| Spârleni | 561.3 a | 618.7 a | 42.9 a | 50.0 a | 219.2 ab | 53.2 a | 57.3 b | 429.9 a | 4.1 a | 9.5 a | 308.5 a | 884.4 a | 25.1 a | 100.9 a | 0.7 a | |
| Drăgășani | 485.8 a | 496.9 a | 43.4 a | 48.8 a | 259.3 a | 62.8 a | 120.4 a | 366.4 a | 3.8 a | 8.4 a | 353.3 a | 967.7 a | 24.9 a | 77.2 a | 0.3 a | |
| Dobrușa | 476.6 a | 507.5 a | 48.1 a | 50.4 a | 166.6 b | 40.3 a | 2.2 b | 314.0 a | 6.0 a | 8.5 a | 370.5 a | 792.5 a | 22.0 a | 59.0 a | 2.1 a | |
| FoA | MalA | CitA | GlycOH | F | myoIn | Ara | β-G | α-G | Gal | GalA | Su | CaftA | ShA | Tyr | Tri | |
| Sâmburești | 10.2 a | 467 a | 92.8 b | 941 a | 266 b | 341 a | 110 a | 108 ab | 279 a | 40.9 a | 330 a | 33.2 ab | 77.5 a | 37.3 a | 48.4 a | 15.1 a |
| Spârleni | 12.0 a | 1039 a | 206.6 b | 10,328 a | 948.3 a | 310.8 a | 71.0 a | 438.0 b | 267.5 a | 30.5 ab | 2725 ab | 24.5 b | 123.7 a | 78.0 a | 46.1 a | 14.7 a |
| Drăgășani | 13.5 a | 824.7 a | 587.4 a | 10,150 a | 790 ab | 291 a | 111.2 a | 923 ab | 146.4 a | 19.2 b | 150.5 b | 48.1 a | 88.9 a | 32.0 a | 47.2 a | 12.4 a |
| Dobrușa | 13.0 a | 1085 a | 294 ab | 8548 a | 286 ab | 330.8 a | 37.0 a | 1255 a | 175.4 a | 24.2 ab | 118.1 b | 30.7 ab | 80.7 a | 17.9 a | 29.5 a | 12.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miricioiu, M.G.; Ionete, R.E.; Costinel, D.; Simova, S.; Gerginova, D.; Botoran, O.R. Metabolic Profiling and Stable Isotope Analysis of Wines: Pilot Study for Cross-Border Authentication. Foods 2024, 13, 3372. https://doi.org/10.3390/foods13213372
Miricioiu MG, Ionete RE, Costinel D, Simova S, Gerginova D, Botoran OR. Metabolic Profiling and Stable Isotope Analysis of Wines: Pilot Study for Cross-Border Authentication. Foods. 2024; 13(21):3372. https://doi.org/10.3390/foods13213372
Chicago/Turabian StyleMiricioiu, Marius Gheorghe, Roxana Elena Ionete, Diana Costinel, Svetlana Simova, Dessislava Gerginova, and Oana Romina Botoran. 2024. "Metabolic Profiling and Stable Isotope Analysis of Wines: Pilot Study for Cross-Border Authentication" Foods 13, no. 21: 3372. https://doi.org/10.3390/foods13213372
APA StyleMiricioiu, M. G., Ionete, R. E., Costinel, D., Simova, S., Gerginova, D., & Botoran, O. R. (2024). Metabolic Profiling and Stable Isotope Analysis of Wines: Pilot Study for Cross-Border Authentication. Foods, 13(21), 3372. https://doi.org/10.3390/foods13213372







