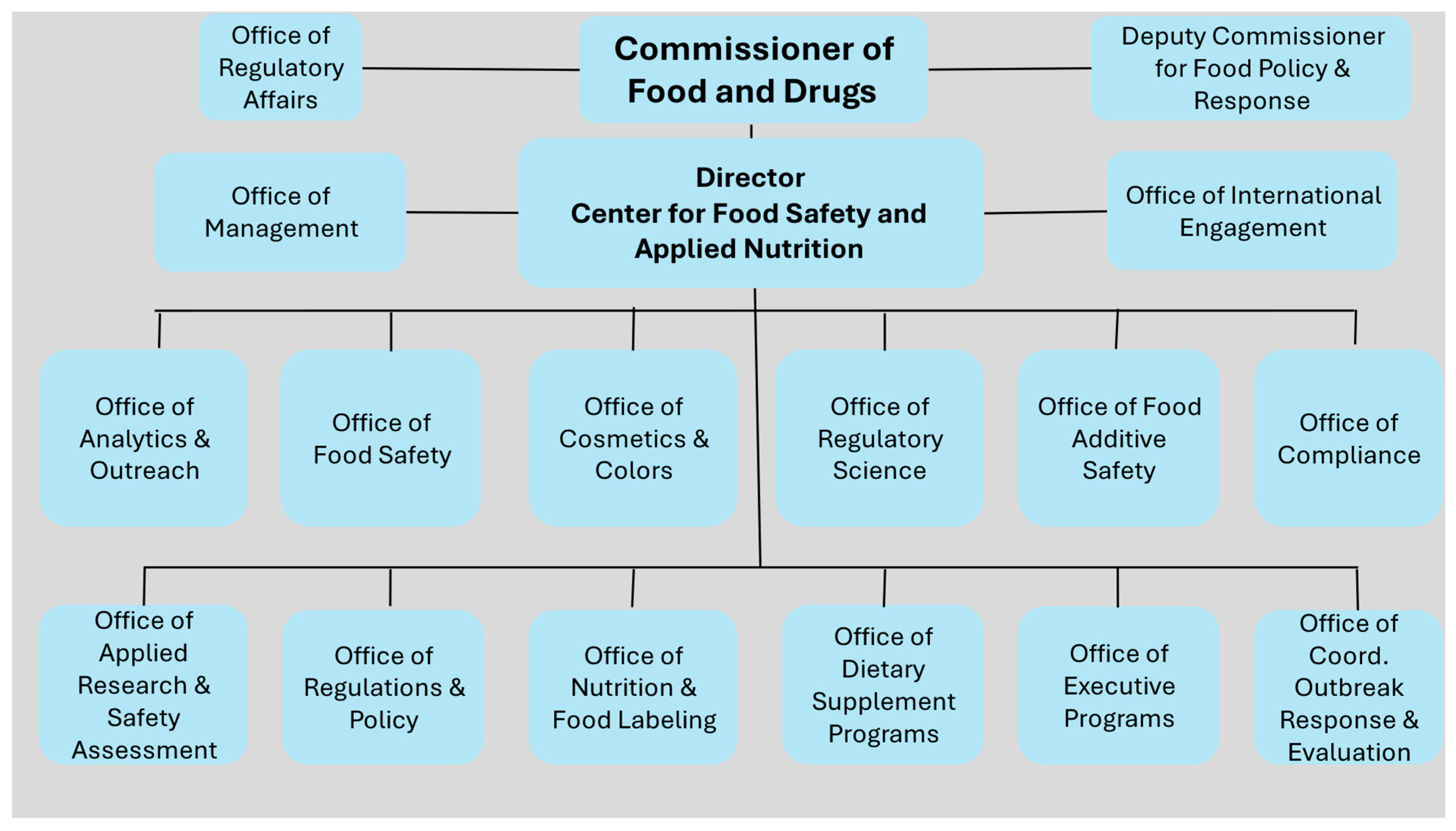
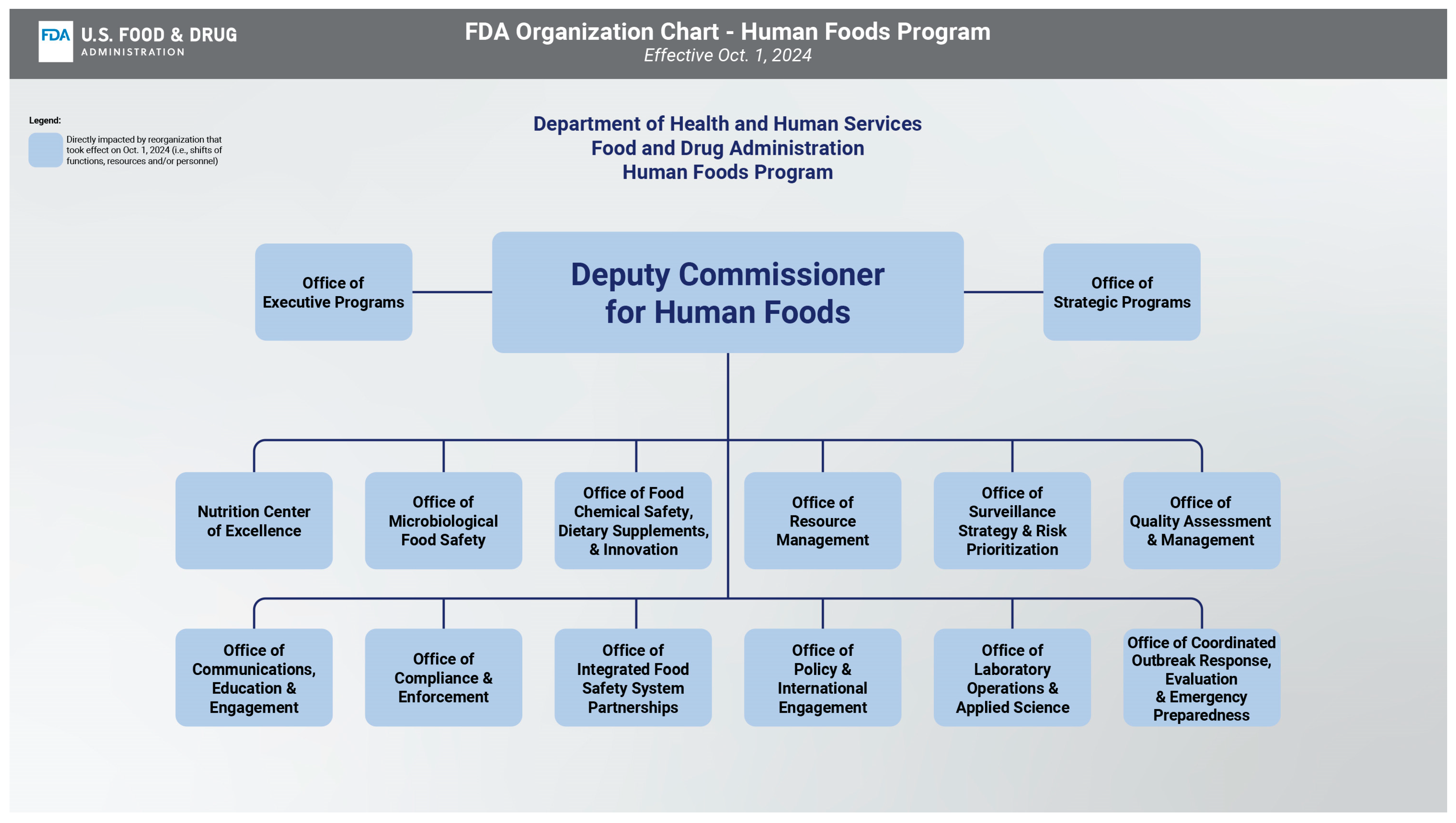
Restructuring the FDA’s Food Program: Promises and Pitfalls
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Drug Administration. News Release. FDA’s Reorganization Approved for Establishing Unified Human Foods Program, New Model for Field Operations and Other Modernization Efforts|FDA. 30 May 2024. Available online: https://www.fda.gov/news-events/press-announcements/fdas-reorganization-approved-establishing-unified-human-foods-program-new-model-field-operations-and (accessed on 27 June 2024).
- Yeung, B.; Grabell, M.; Simon, M. The Low-and-Slow Approach to Food Safety Reform Keeps Going Up in Smoke. ProPublica, 23 December 2021. Available online: https://www.propublica.org/article/the-low-and-slow-approach-to-food-safety-reform-keeps-going-up-in-smoke (accessed on 6 August 2024).
- Centers for Disease Control and Prevention. Cronobacter Outbreak Linked to Powdered Infant Formula. 13 May 2024. Available online: https://www.cdc.gov/cronobacter/outbreaks/source-date/index.html (accessed on 27 June 2024).
- Food and Drug Administration. Inspection of Abbott Laboratories Plant in Sturgis, MI (Form FDA 483), 18 March 2022. Available online: https://www.fda.gov/media/157073/download (accessed on 27 June 2024).
- Food and Drug Administration. FDA Evaluation of Infant Formula Response. September 2022. Available online: https://www.fda.gov/media/161689/download (accessed on 27 June 2024).
- Food and Nutrition Board. Challenges in Supply, Market Competition, and Regulation of Infant Formula in the United States. National Academies of Science, Engineering, and Medicine, 25 July 2024. Available online: https://nap.nationalacademies.org/catalog/27765/challenges-in-supply-market-competition-and-regulation-of-infant-formula-in-the-united-states (accessed on 6 August 2024).
- Reagan-Udall Foundation. Operational Evaluation of the FDA Human Foods Program. December 2022. Available online: https://reaganudall.org/sites/default/files/2022-12/Human%20Foods%20Program%20Independent%20Expert%20Panel%20Final%20Report%20120622.pdf (accessed on 27 June 2024).
- Department of Health and Human Services, Food and Drug Administration. Statement of Organization, Functions, and Delegations of Authority. 89 Fed Reg 47567-76 (2 June 2024). Available online: https://www.federalregister.gov/documents/2024/06/03/2024-11893/statement-of-organization-functions-and-delegations-of-authority (accessed on 27 June 2024).
- Al-Faruque, F. FDA Leaders Detail Reorg Plans, Say 1500 ORA Staff Will Be Reassigned. Regulatory Focus, 19 January 2024. Available online: https://www.raps.org/News-and-Articles/News-Articles/2024/1/FDA-leaders-detail-reorg-plans,-say-1,500-ORA-staf (accessed on 6 August 2024).
- Daniells, S. Sens. Durbin & Blumenthal Express Concerns over Proposed Changes to FDA’s Supplement Office. Nutraingredients, 25 August 2021. Available online: https://www.nutraingredients-usa.com/Article/2023/08/26/Sens.-Durbin-Blumenthal-express-concerns-over-proposed-changes-to-FDA-s-Supplement-Office (accessed on 6 August 2024).
- Food and Drug Administration; Center for Food Safety and Applied Nutrition. Presentation to Association of Food and Drug Officials, College Park, MD, 29 August 2024. Available online: https://www.cspinet.org/resource/safe-food-coalition-slides (accessed on 10 October 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzouk, S.; Rumalla, K.C.; Lurie, P.G. Restructuring the FDA’s Food Program: Promises and Pitfalls. Foods 2024, 13, 3334. https://doi.org/10.3390/foods13203334
Marzouk S, Rumalla KC, Lurie PG. Restructuring the FDA’s Food Program: Promises and Pitfalls. Foods. 2024; 13(20):3334. https://doi.org/10.3390/foods13203334
Chicago/Turabian StyleMarzouk, Sammer, Kranti C. Rumalla, and Peter G. Lurie. 2024. "Restructuring the FDA’s Food Program: Promises and Pitfalls" Foods 13, no. 20: 3334. https://doi.org/10.3390/foods13203334
APA StyleMarzouk, S., Rumalla, K. C., & Lurie, P. G. (2024). Restructuring the FDA’s Food Program: Promises and Pitfalls. Foods, 13(20), 3334. https://doi.org/10.3390/foods13203334





