Antibiotic Resistance Genes in Global Food Transformation System: Edible Insects vs. Livestock
Abstract
1. Introduction
2. Prevalent ARGs in Insects and Livestock
3. Probable Reasons for ARG Abundance: Edible Insects vs. Livestock
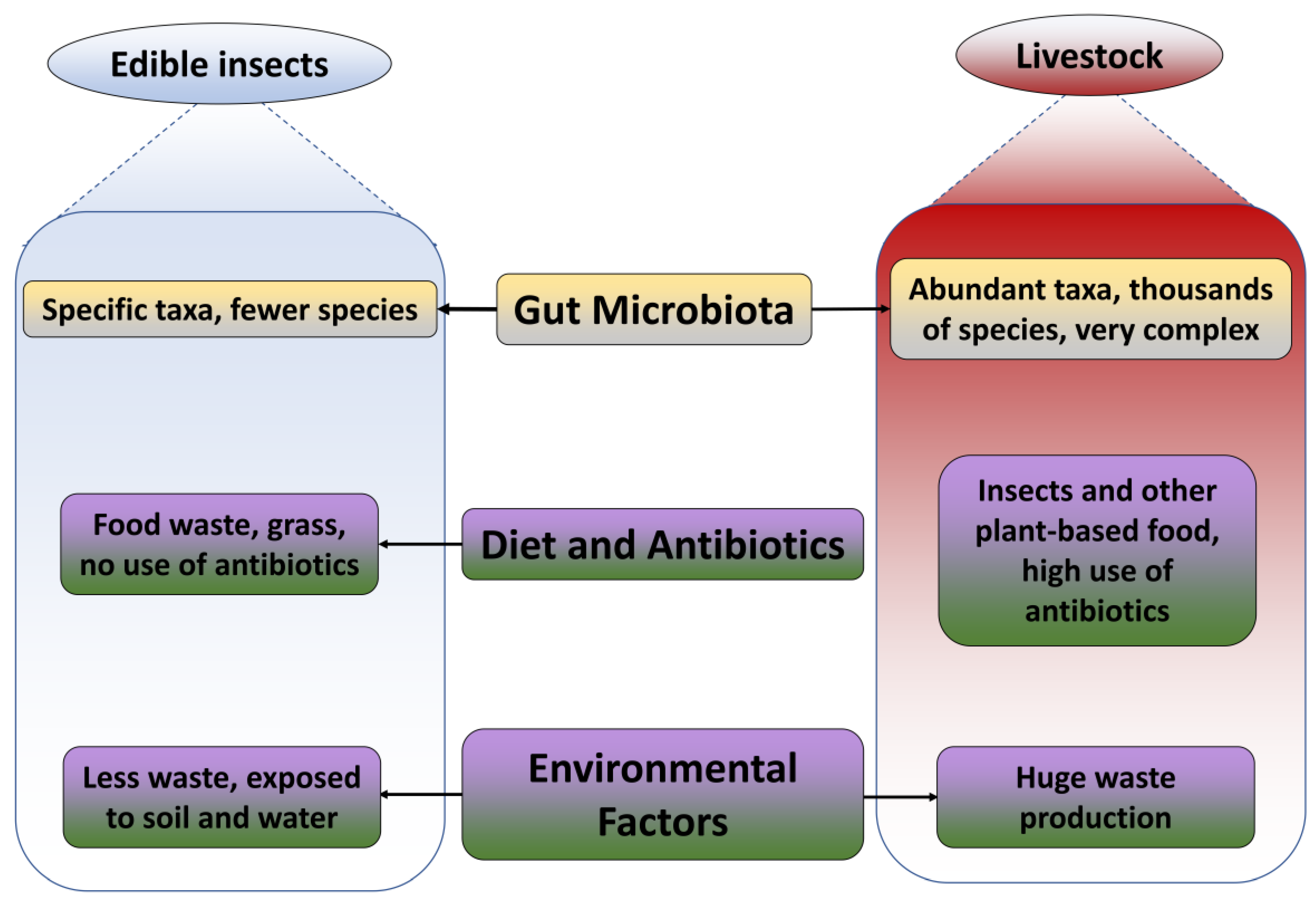
3.1. Gut Microbiome
3.2. Diet and Antibiotics
3.3. Environmental Factors
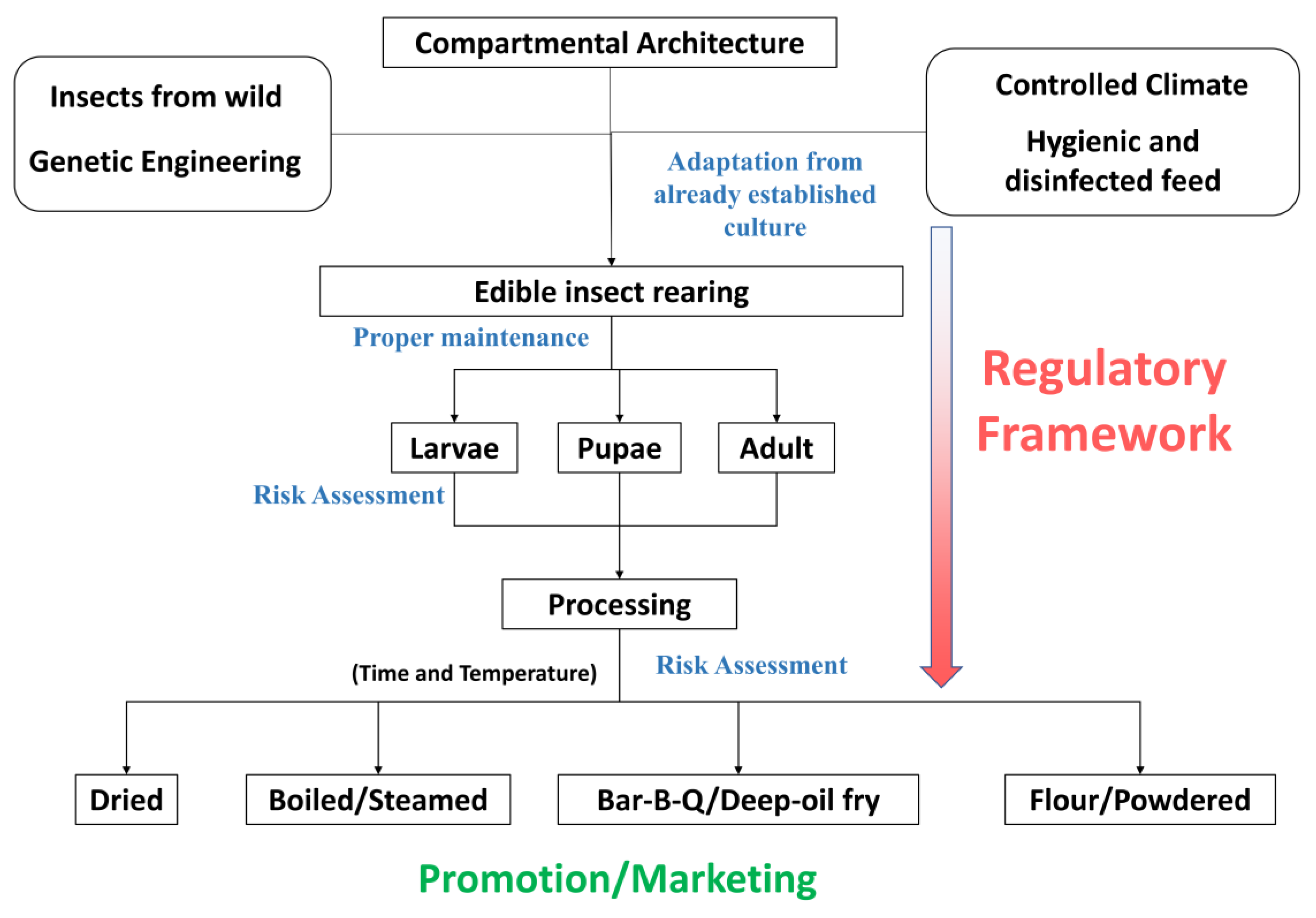
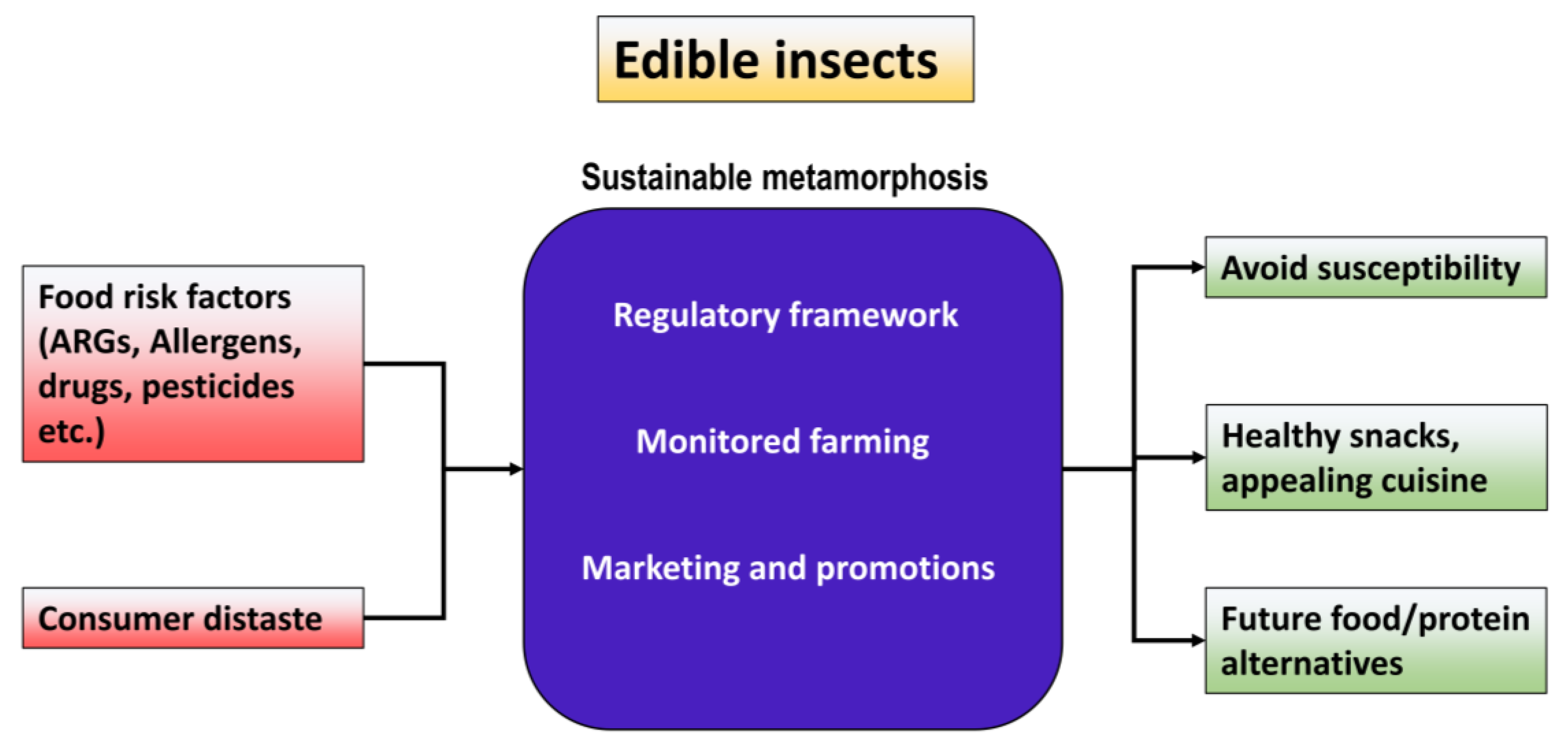
4. Pertinence of Edible Insects over Livestock: Our Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pandey, S.; Doo, H.; Keum, G.B.; Kim, E.S.; Kwak, J.; Ryu, S.; Choi, Y.; Kang, J.; Kim, S.; Lee, N.R.; et al. Antibiotic Resistance in Livestock, Environment and Humans: One Health Perspective. J. Anim. Sci. Technol. 2024, 66, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Stålsby Lundborg, C.; Sun, X.; Zhu, N.; Gu, S.; Dong, H. Economic Burden of Antibiotic Resistance in China: A National Level Estimate for Inpatients. Antimicrob. Resist. Infect. Control 2021, 10, 5. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, X.; Liu, Y.; Zhao, H.; Wang, G. Burden of Infectious Diseases and Bacterial Antimicrobial Resistance in China: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Reg. Health West. Pac. 2024, 43, 100972. [Google Scholar] [CrossRef] [PubMed]
- Tzachor, A.; Richards, C.E.; Holt, L. Future Foods for Risk-Resilient Diets. Nat. Food 2021, 2, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.J.; Weir, T.L. Chitin and Omega-3 Fatty Acids in Edible Insects Have Underexplored Benefits for the Gut Microbiome and Human Health. Nat. Food 2023, 4, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Lee, J.H.; Kim, T.K.; Shin, D.M. Edible Insects in Food. Adv. Food Nutr. Res. 2024, 108, 223–264. [Google Scholar] [CrossRef]
- Rivas-Navia, D.M.; Dueñas-Rivadeneira, A.A.; Dueñas-Rivadeneira, J.P.; Aransiola, S.A.; Maddela, N.R.; Prasad, R. Bioactive Compounds of Insects for Food Use: Potentialities and Risks. J. Agric. Food Res. 2023, 14, 100807. [Google Scholar] [CrossRef]
- Milanović, V.; Osimani, A.; Pasquini, M.; Aquilanti, L.; Garofalo, C.; Taccari, M.; Cardinali, F.; Riolo, P.; Clementi, F. Getting Insight into the Prevalence of Antibiotic Resistance Genes in Specimens of Marketed Edible Insects. Int. J. Food Microbiol. 2016, 227, 22–28. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Muisa-Zikali, N.; Teta, C.; Musvuugwa, T.; Rzymski, P.; Abia, A.L.K. Insects, Rodents, and Pets as Reservoirs, Vectors, and Sentinels of Antimicrobial Resistance. Antibiotics 2021, 10, 68. [Google Scholar] [CrossRef]
- Li, M.; Mao, C.; Li, X.; Jiang, L.; Zhang, W.; Li, M.; Liu, H.; Fang, Y.; Liu, S.; Yang, G.; et al. Edible Insects: A New Sustainable Nutritional Resource Worth Promoting. Foods 2023, 12, 4073. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.J.; Jandová, A.; Jeffs, C.T.; Higgie, M.; Nováková, E.; Lewis, O.T.; Hrček, J. Microbiome Structure of a Wild Drosophila Community along Tropical Elevational Gradients and Comparison to Laboratory Lines. Appl. Env. Microbiol. 2023, 89, e0009923. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.C.; Bletz, M.C.; Becker, C.G.; Bender, H.A.; Buitrago-Rosas, D.; Diebboll, H.; Huynh, R.; Kearns, P.J.; Kueneman, J.; Kurosawa, E.; et al. Publisher Correction: Host-Associated Microbiomes Are Predicted by Immune System Complexity and Climate. Genome Biol. 2020, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Ignasiak, K.; Maxwell, A. Antibiotic-Resistant Bacteria in the Guts of Insects Feeding on Plants: Prospects for Discovering Plant-Derived Antibiotics. BMC Microbiol. 2017, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Ruschioni, S.; Riolo, P.; Isidoro, N.; Loreto, N.; Galarini, R.; et al. Distribution of Transferable Antibiotic Resistance Genes in Laboratory-Reared Edible Mealworms (Tenebrio molitor L.). Front. Microbiol. 2018, 9, 2702. [Google Scholar] [CrossRef] [PubMed]
- Roncolini, A.; Cardinali, F.; Aquilanti, L.; Milanović, V.; Garofalo, C.; Sabbatini, R.; Abaker, M.S.S.; Pandolfi, M.; Pasquini, M.; Tavoletti, S.; et al. Investigating Antibiotic Resistance Genes in Marketed Ready-to-Eat Small Crickets (Acheta domesticus). J. Food Sci. 2019, 84, 3222–3232. [Google Scholar] [CrossRef]
- Osimani, A.; Cardinali, F.; Aquilanti, L.; Garofalo, C.; Roncolini, A.; Milanović, V.; Pasquini, M.; Tavoletti, S.; Clementi, F. Occurrence of Transferable Antibiotic Resistances in Commercialized Ready-to-Eat Mealworms (Tenebrio molitor L.). Int. J. Food Microbiol. 2017, 263, 38–46. [Google Scholar] [CrossRef]
- Grispoldi, L.; Karama, M.; El-Ashram, S.; Saraiva, C.M.; García-Díez, J.; Chalias, A.; Barbera, S.; Cenci-Goga, B.T. Hygienic Characteristics and Detection of Antibiotic Resistance Genes in Crickets (Acheta domesticus) Breed for Flour Production. Microbiol. Res. 2021, 12, 503–512. [Google Scholar] [CrossRef]
- Milanović, V.; Osimani, A.; Roncolini, A.; Garofalo, C.; Aquilanti, L.; Pasquini, M.; Tavoletti, S.; Vignaroli, C.; Canonico, L.; Ciani, M.; et al. Investigation of the Dominant Microbiota in Ready-to-Eat Grasshoppers and Mealworms and Quantification of Carbapenem Resistance Genes by QPCR. Front. Microbiol. 2018, 9, 3036. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Milanović, V.; Garofalo, C.; Osimani, A.; Clementi, F.; Van Campenhout, L.; Aquilanti, L. Real-Time PCR Detection and Quantification of Selected Transferable Antibiotic Resistance Genes in Fresh Edible Insects from Belgium and the Netherlands. Int. J. Food Microbiol. 2019, 290, 288–295. [Google Scholar] [CrossRef]
- Yang, J.; Ju, Z.; Yang, Y.; Zhao, X.; Jiang, Z.; Sun, S. Serotype, Antimicrobial Susceptibility and Genotype Profiles of Salmonella Isolated from Duck Farms and a Slaughterhouse in Shandong Province, China. BMC Microbiol. 2019, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Diana, J. Risk Factors Associated with the Abundance of Antimicrobial Resistance Gene Tet(W) and Class 1 Integron (Intl1) in Pigs in the Netherlands. Utrecht University Student Theses Repository. 2023. Available online: https://studenttheses.uu.nl/handle/20.500.12932/45525 (accessed on 10 September 2024).
- Osimani, A.; Garofalo, C.; Aquilanti, L.; Milanović, V.; Cardinali, F.; Taccari, M.; Pasquini, M.; Tavoletti, S.; Clementi, F. Transferable Antibiotic Resistances in Marketed Edible Grasshoppers (Locusta migratoria migratorioides). J. Food Sci. 2017, 82, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Jin, C.X.; Wei, S.; Kumar, R.R.; Zhou, Q.X. Occurrence of Antibiotic Resistance Genes in Livestock Farms of Different Scales in North China. J. Agro-Environ. Sci. 2020, 39, 2640–2652. [Google Scholar] [CrossRef]
- Xiong, W.; Yang, J.; Zeng, J.; Xiao, D.; Tong, C.; Zeng, Z. Metagenomic Analysis of Antimicrobial Resistance in Ducks, Workers, and the Environment in Duck Farms, Southern China. Ecotoxicol. Env. Saf. 2023, 262, 115191. [Google Scholar] [CrossRef]
- Pholwat, S.; Pongpan, T.; Chinli, R.; Rogawski McQuade, E.T.; Thaipisuttikul, I.; Ratanakorn, P.; Liu, J.; Taniuchi, M.; Houpt, E.R.; Foongladda, S. Antimicrobial Resistance in Swine Fecal Specimens across Different Farm Management Systems. Front. Microbiol. 2020, 11, 1238. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Zhang, Y.; Xia, L.; Zhao, L.; Guo, C.; Liu, X.; Qin, L.; Hao, Z. High Prevalence and Diversity Characteristics of Bla NDM, Mcr, and Bla ESBLs Harboring Multidrug-Resistant Escherichia Coli from Chicken, Pig, and Cattle in China. Front. Cell. Infect. Microbiol. 2022, 11, 755545. [Google Scholar] [CrossRef]
- Lim, S.K.; Kim, D.; Moon, D.C.; Cho, Y.; Rho, M. Antibiotic Resistomes Discovered in the Gut Microbiomes of Korean Swine and Cattle. Gigascience 2020, 9, giaa043. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Wang, M.; Luo, M.; Peng, Y.; Li, Z.; Xu, J.; Ou, M.; Kan, B.; Li, X.; et al. The Prevalence and Distribution of Aminoglycoside Resistance Genes. Biosaf. Health 2023, 5, 14–20. [Google Scholar] [CrossRef]
- Dong, W.L.; Kong, L.C.; Wang, Y.; Gou, C.L.; Xu, B.; Ma, H.X.; Gao, Y.H. Aminoglycoside Resistance of Trueperella Pyogenes Isolated from Pigs in China. J. Vet. Med. Sci. 2017, 79, 1836–1839. [Google Scholar] [CrossRef]
- Luiken, R.E.C.; Van Gompel, L.; Munk, P.; Sarrazin, S.; Joosten, P.; Dorado-García, A.; Borup Hansen, R.; Knudsen, B.E.; Bossers, A.; Wagenaar, J.A.; et al. Associations between Antimicrobial Use and the Faecal Resistome on Broiler Farms from Nine European Countries. J. Antimicrob. Chemother. 2019, 74, 2596–2604. [Google Scholar] [CrossRef]
- Garofalo, C.; Milanović, V.; Cardinali, F.; Aquilanti, L.; Clementi, F.; Osimani, A. Current Knowledge on the Microbiota of Edible Insects Intended for Human Consumption: A State-of-the-Art Review. Food Res. Int. 2019, 125, 108527. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible Insects in a Food Safety and Nutritional Perspective: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Sun, J.; Liao, X.P.; D’Souza, A.W.; Boolchandani, M.; Li, S.H.; Cheng, K.; Luis Martínez, J.; Li, L.; Feng, Y.J.; Fang, L.X.; et al. Environmental Remodeling of Human Gut Microbiota and Antibiotic Resistome in Livestock Farms. Nat. Commun. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.; Pérez-Pardal, L.; Carvalheira, J.; Beja-Pereira, A. Gut Microbiome Studies in Livestock: Achievements, Challenges, and Perspectives. Animals 2022, 12, 3375. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Thomas, B.J.; Friedersdorff, J.C.A.; Ougham, H.; Creevey, C.J. Deep Sequence Analysis Reveals the Ovine Rumen as a Reservoir of Antibiotic Resistance Genes. Environ. Pollut. 2018, 235, 571–575. [Google Scholar] [CrossRef]
- Ma, T.; McAllister, T.A.; Guan, L.L. A Review of the Resistome within the Digestive Tract of Livestock. J. Anim. Sci. Biotechnol. 2021, 12, 121. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Wales, A.D.; Davies, R.H. Co-Selection of Resistance to Antibiotics, Biocides and Heavy Metals, and Its Relevance to Foodborne Pathogens. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef]
- Ferro, G.; Guarino, F.; Castiglione, S.; Rizzo, L. Antibiotic Resistance Spread Potential in Urban Wastewater Effluents Disinfected by UV/H2O2 Process. Sci. Total Environ. 2016, 560–561, 29–35. [Google Scholar] [CrossRef]
- Ma, Y.; Metch, J.W.; Yang, Y.; Pruden, A.; Zhang, T. Shift in Antibiotic Resistance Gene Profiles Associated with Nanosilver during Wastewater Treatment. FEMS Microbiol. Ecol. 2016, 92, fiw022. [Google Scholar] [CrossRef]
- Xia, J.; Sun, H.; Zhang, X.; Zhang, T.; Ren, H.; Ye, L. Aromatic Compounds Lead to Increased Abundance of Antibiotic Resistance Genes in Wastewater Treatment Bioreactors. Water Res. 2019, 166, 115073. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, X.; Xia, J.; Sun, H.; Zhang, X.; Ye, L. Phenolic Compounds Promote the Horizontal Transfer of Antibiotic Resistance Genes in Activated Sludge. Sci. Total Environ. 2021, 800, 149549. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and Network Analysis Reveal Wide Distribution and Co-Occurrence of Environmental Antibiotic Resistance Genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L.; Senehi, N.; Sun, R.; Alvarez, P.J.J. Antibiotic Resistance Genes from Livestock Waste: Occurrence, Dissemination, and Treatment. NPJ Clean. Water 2020, 3, 4. [Google Scholar] [CrossRef]
- Teixeira, C.S.S.; Villa, C.; Costa, J.; Ferreira, I.M.P.L.V.O.; Mafra, I. Edible Insects as a Novel Source of Bioactive Peptides: A Systematic Review. Foods 2023, 12, 2026. [Google Scholar] [CrossRef]
- Chioma, N.; Onoriode, C.E.; Abiye, A.I. Occurrence of Virulent and Antibiotic Resistant Staphylococcus Species in Ready-to-Eat Rhynchophorus Phoenicis and Archachatina Marginata Vended along the Port Harcourt-Bayelsa Route. Afr. J. Microbiol. Res. 2021, 15, 107–119. [Google Scholar] [CrossRef]

| Insect | Scientific Name | Country | Bacteria | Methods | ARGs | Antibiotics | Ref. |
|---|---|---|---|---|---|---|---|
| Small crickets | Acheta domesticus | Netherlands Market | Streptococcus pyogenes 7008 | nested PCR | tet (O) | [8] | |
| Small crickets | Acheta domesticus | Netherlands Market | Lactobacillus casei/paracasei ILC2279 | PCR | tet (M) | [8] | |
| Locusts | Locusta migratoria | Netherlands Market | Staphylococcus aureus COL. | PCR | tet(K) | [8] | |
| Mealworm larvae | Tenebrio molitor | Netherlands Market | Staphylococcus aureus COL.; Enterococcus italicus 1102; Staphylococcus aureus COL. | PCR | tet(M), tet(S), tet(K) | [8] | |
| Giant waterbugs | Belostomatidae | Thailand Market | Enterococcus hirae Api 2.16; Staphylococcus aureus COL.; Staphylococcus aureus ATCC 2921 | nested PCR | erm(B); tet(K); blaZ | [8] | |
| Black ants | Polyrhachis | Thailand Market | Staphylococcus aureus COL. | PCR | tet(K) | [8] | |
| Black ants | Polyrhachis | Thailand Market | Staphylococcus aureus COL. | nested PCR | erm(B); erm(C); blaZ | [8] | |
| Winged termite alates | Termitoidae | Thailand Market | Enterococcus hirae Api 2.16; Staphylococcus spp. SE12; Enterococcus italicus 1102; Staphylococcus aureus COL.; Staphylococcus aureus ATCC 2921 | nested PCR | erm(B); erm(C); tet(S), tet(K); blaZ | [8] | |
| Rhino beetles | Hyboschema contractum | Thailand Market | Enterococcus hirae Api 2.16; Enterococcus italicus 1102; Staphylococcus aureus COL.; Staphylococcus aureus ATCC 2921 | nested PCR | erm(B); tet(S), tet(K); blaZ | [8] | |
| Mole crickets | Gryllotalpidae | Thailand Market | Enterococcus italicus 1102; Staphylococcus aureus COL. | PCR | erm(B); tet(S), tet(K) | [8] | |
| Mole crickets | Gryllotalpidae | Thailand Market | Staphylococcus aureus ATCC 2921 | nested PCR | blaZ | [8] | |
| Silkworm pupae | Bombyx mori | Thailand Market | Enterococcus italicus 1102; Staphylococcus aureus COL.; Staphylococcus aureus ATCC 2921 | nested PCR | tet(S), tet(K); blaZ | [8] | |
| Black scorpions | Heterometrus longimanus | Thailand Market | Enterococcus italicus 1102; Staphylococcus aureus COL. | PCR | tet(S), tet(K) | [8] | |
| Giant Line Green Stick insect | Diaphroedes gigantea | Sphingobacterium multivorum | broth microdilution assay | Tetracycline | [14] | ||
| Giant Line Green Stick insect | Diaphroedes gigantea | Microbacterium oxydans, | broth microdilution assay | Ciprofloxacin; Tetracycline | [14] | ||
| Giant Line Green Stick insect | Diaphroedes gigantea | Bacillus amyloliquefaciens | broth microdilution assay | Ciprofloxacin; Tetracycline | [14] | ||
| Diamondback moth | Plutella xylostella | Sanguibacter keddieii | broth microdilution assay | Ampicillin; Chloramphenicol; Ciprofloxacin; Kanamycin | [14] | ||
| Diamondback moth | Plutella xylostella | Raoultella terrigena | broth microdilution assay | Ampicillin; Chloramphenicol; Kanamycin; Rifampicin | [14] | ||
| Cinnabar moth | Tyria jacobeae | Bacillus licheniformis | broth microdilution assay | Ampicillin; Chloramphenicol; Tetracycline | [14] | ||
| Cinnabar moth | Tyria jacobeae | Staphylococcus epidermidis | broth microdilution assay | Ampicillin; Kanamycin | [14] | ||
| Cinnabar moth | Tyria jacobeae | Staphylococcus warneri | broth microdilution assay | Ciprofloxacin; Rifampicin; Tetracycline | [14] | ||
| Cinnabar moth | Tyria jacobeae | Burkholderia fungorum | broth microdilution assay | Ciprofloxacin; Kanamycin | [14] | ||
| Death’s-head Hawkmoth | Acherontia atropos | Enterobacter asburiae | broth microdilution assay | Ampicilin; Chloramphenicol; Ciprofloxacin; Kanamycin; Rifamplcin; Tetracycline | [14] | ||
| Death’s-head Hawkmoth | Acherontia atropos | Pseudomonas putida | broth microdilution assay | Chloramphenicol; Kanamycin; Rifampicin; Tetracycline | [14] | ||
| Death’s-head Hawkmoth | Acherontia atropos | Roultella terrigena | broth microdilution assay | Ampicillin; Chloramphenicol; Kanamycin | [14] | ||
| Beet Armyworm | Spodoptera exigua | Microbacterium paraoxydans | broth microdilution assay | Ampicillin; Ciprofloxacin | [14] | ||
| Beet Armyworm | Spodoptera exigua | Bacillus aquimaris | broth microdilution assay | Rifampicin; Chloramphenicol; Kanamycin | |||
| Beet Armyworm | Spodoptera exigua | Bacillus vietnamensis | broth microdilution assay | Ampicillin; Chloramphenicol; Ciprofloxacin; Kanamycin; Rifampicin | [14] | ||
| Beet Armyworm | Spodoptera exigua | Rhizobium pusense | broth microdilution assay | Ciprofloxacin; Kanamycin; Tetracycline | [14] | ||
| Rosemary beetle | Chrysolina americana | Staphylococcus epidermidis | broth microdilution assay | Ampicillin; Ciprofloxacin; Kanamycin | [14] | ||
| Mealworm larvae | Tenebrio molitor L. | Ampicillin-resistant lactic acid bacteria | pour plating | Ampicillin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Vancomycin-resistant lactic acid bacteria | pour plating | Vancomycin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Ampicillin-resistant enterococci | speard plating | Ampicillin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | HLAR enterococci | spread plating | Gentamicin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Vancomycin-resistant enterococci | spread plating | Vancomycin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Gentamicin-resistant staphylococci | spread plating | Gentamicin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Erythromycin-resistant staphylococci | spread plating | Erythromycin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Tetracycline-resistant staphylococci | spread plating | Tetracycline | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Vancomycin-resistant coagulase-positive staphylococci | spread plating | Vancomycin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Ampicillin-resistant Enterobacteriaceae | pour plating | Ampicillin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Gentamicin-resistant Enterobacteriaceae | pour plating | Gentamicin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Gentamicin-resistant Pseudomonadaceae | pour plating | Gentamicin | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Enterococcus italicus | PCR | tet(S) | [15] | ||
| Mealworm larvae | Tenebrio molitor L. | Enterococcus hirae Api 2.16; Lactobacillus casei/paracasei ILC2279; Staphylococcus aureus COL | N-pcr | erm(B); tet(M); tet (K) | [15] | ||
| Crickets | Acheta domesticus | Austria, Belgium, France, and the Netherlands | Lactic acid bacteria; Enterobacteriaceae; Pseudomonadaceae; Listeria monocytogenes | PCR | erm(B); erm(C); tet(S), tet(K); tet(S), tet(K); blaZ; aac-aph | [16] | |
| Mealworms | Tenebrio molitor L. | Belgium | Total mesophilic aerobes; spore-forming bacteria; lactic acid bacteria; Enterobacteriaceae | PCR; N-pcr | erm(B); vanA; tet(M); tet(S); tet(K); aac-aph | [16] | |
| Mealworms | Tenebrio molitor L. | Netherlands | Total mesophilic aerobes; spore-forming bacteria; lactic acid bacteria; Enterobacteriaceae | PCR; N-pcr | erm(B); erm(C); tet(M); tet(S); tet(K); mecA; aac-aph | [16] | |
| Mealworms | Tenebrio molitor L. | Thailand | Total mesophilic aerobes; spore-forming bacteria; lactic acid bacteria; Enterobacteriaceae | PCR; N-pcr | erm(B); erm (C); tet(O); tet(S); tet(K); mecA; aac-aph | [17] | |
| Mealworms | Tenebrio molitor L. | France | Total mesophilic aerobes; spore-forming bacteria; lactic acid bacteria; Enterobacteriaceae | PCR; N-pcr | erm(A); erm(B); erm©; vanA; vanB; tet(M); tet(O); tet(S); tet(K); mecA; blaZ; aac-aph | [17] | |
| House cricket | Acheta domesticus | Europe | total aerobic mesophilic microbiota, lactobacilli, Enterobacteriaceae, total coliforms, staphylococci, and enterococci | PCR; N-pcr | tetM | [18] | |
| Mealworms | Tenebrio molitor L. | Belgium, the Netherlands, Thailand | Staphylococcus sp., Bacillus sp., Weissella sp., Eikenella sp., Exiguobacterium sp. | PCR; N-pcr | bla(OXA−48); bla(NDM−1); tet(M), tet(K), tet(S); erm(B), erm(C); aac-aph | [19] | |
| Grasshopper | Locusta migratoria migratorioides | Thailand | Staphylococcus sp., Bacillus sp., Weissella sp., Eikenella sp., Exiguobacterium sp. | PCR; N-pcr | blaVIM | [19] | |
| Grasshopper | Locusta migratoria migratorioides | Belgium, the Netherlands, Thailand | Staphylococcus sp., Bacillus sp., Weissella sp., Eikenella sp., Exiguobacterium sp. | PCR; N-pcr | bla(OXA−48); bla(NDM−1); tet(M), tet(K), tet(S); erm(B), erm(C); aac-aph; blaZ | [19] | |
| Crickets | Acheta domesticus | Belgium and the Netherlands | PCR; N-pcr | tet(O), tet(K), tet (M), tet(S), erm(B) | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raka, R.N.; Zhang, L.; Chen, R.; Xue, X. Antibiotic Resistance Genes in Global Food Transformation System: Edible Insects vs. Livestock. Foods 2024, 13, 3257. https://doi.org/10.3390/foods13203257
Raka RN, Zhang L, Chen R, Xue X. Antibiotic Resistance Genes in Global Food Transformation System: Edible Insects vs. Livestock. Foods. 2024; 13(20):3257. https://doi.org/10.3390/foods13203257
Chicago/Turabian StyleRaka, Rifat Nowshin, Lin Zhang, Rui Chen, and Xiaofeng Xue. 2024. "Antibiotic Resistance Genes in Global Food Transformation System: Edible Insects vs. Livestock" Foods 13, no. 20: 3257. https://doi.org/10.3390/foods13203257
APA StyleRaka, R. N., Zhang, L., Chen, R., & Xue, X. (2024). Antibiotic Resistance Genes in Global Food Transformation System: Edible Insects vs. Livestock. Foods, 13(20), 3257. https://doi.org/10.3390/foods13203257





